Abstract
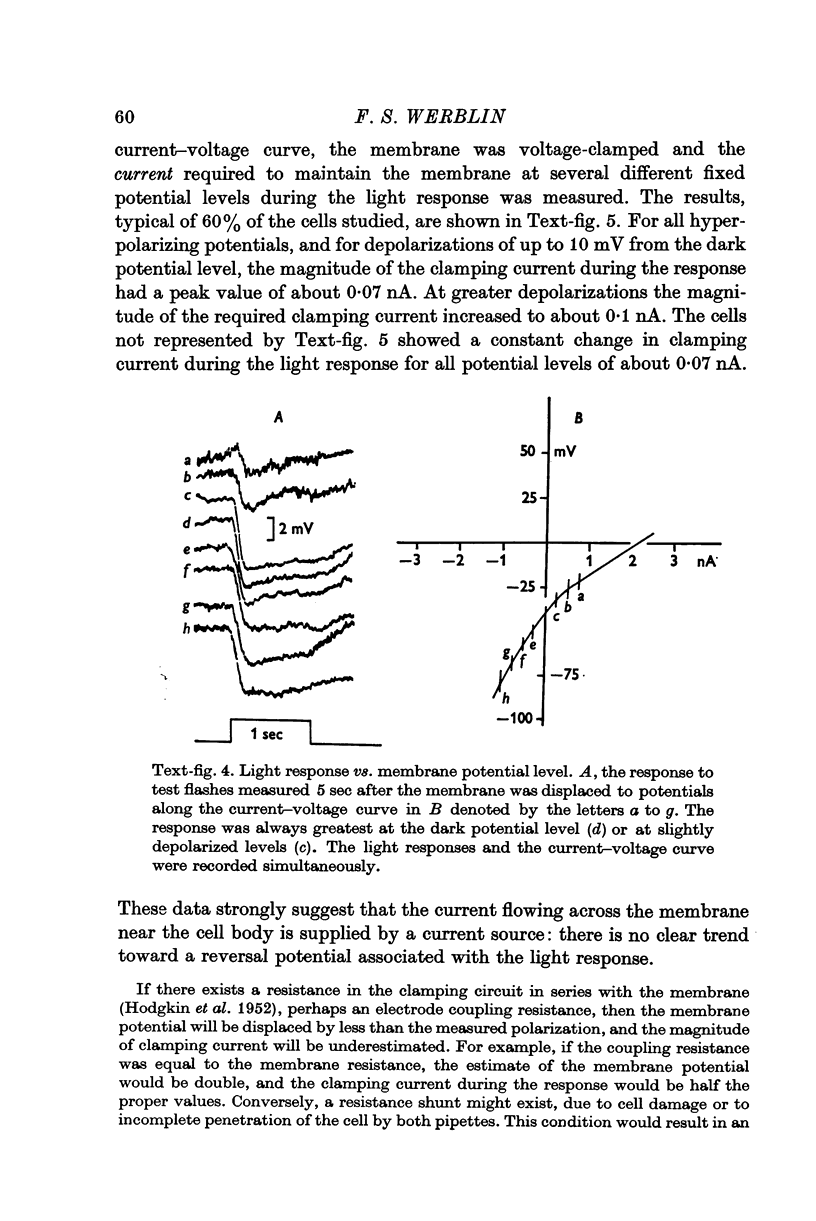
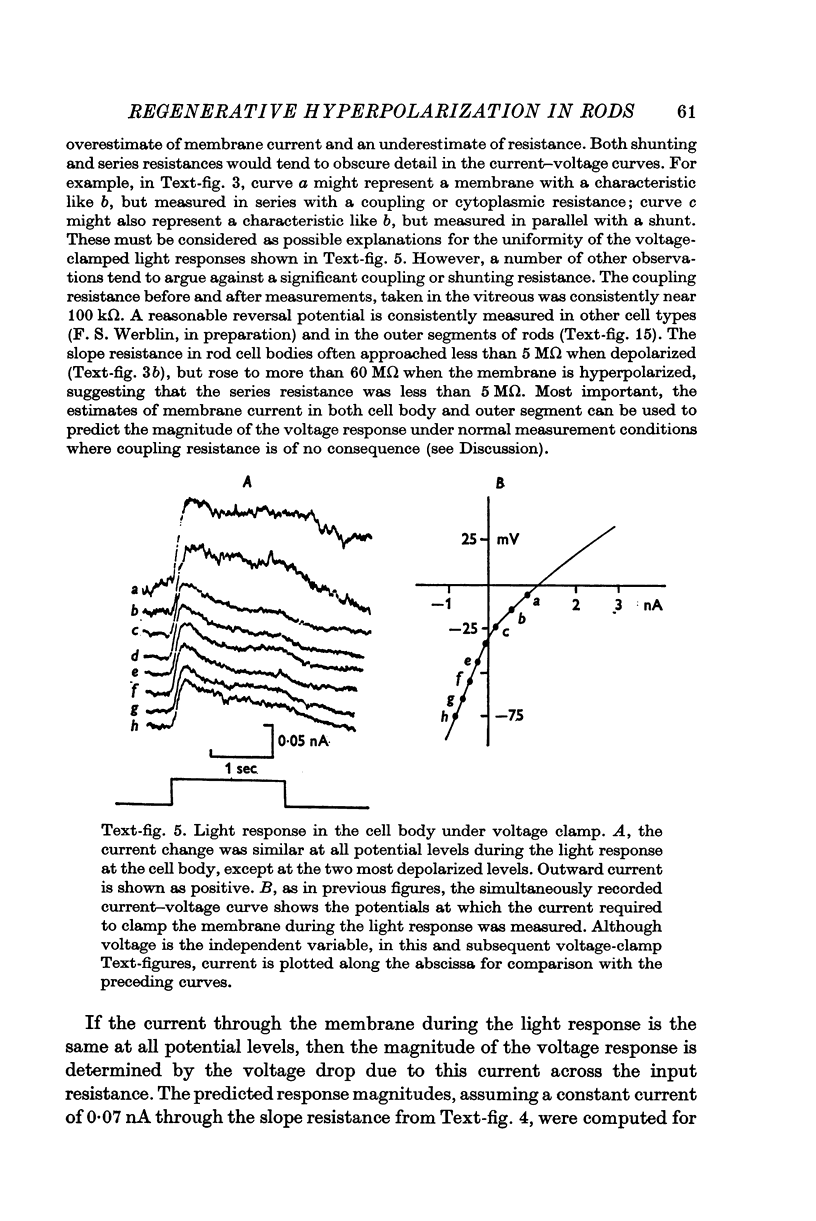
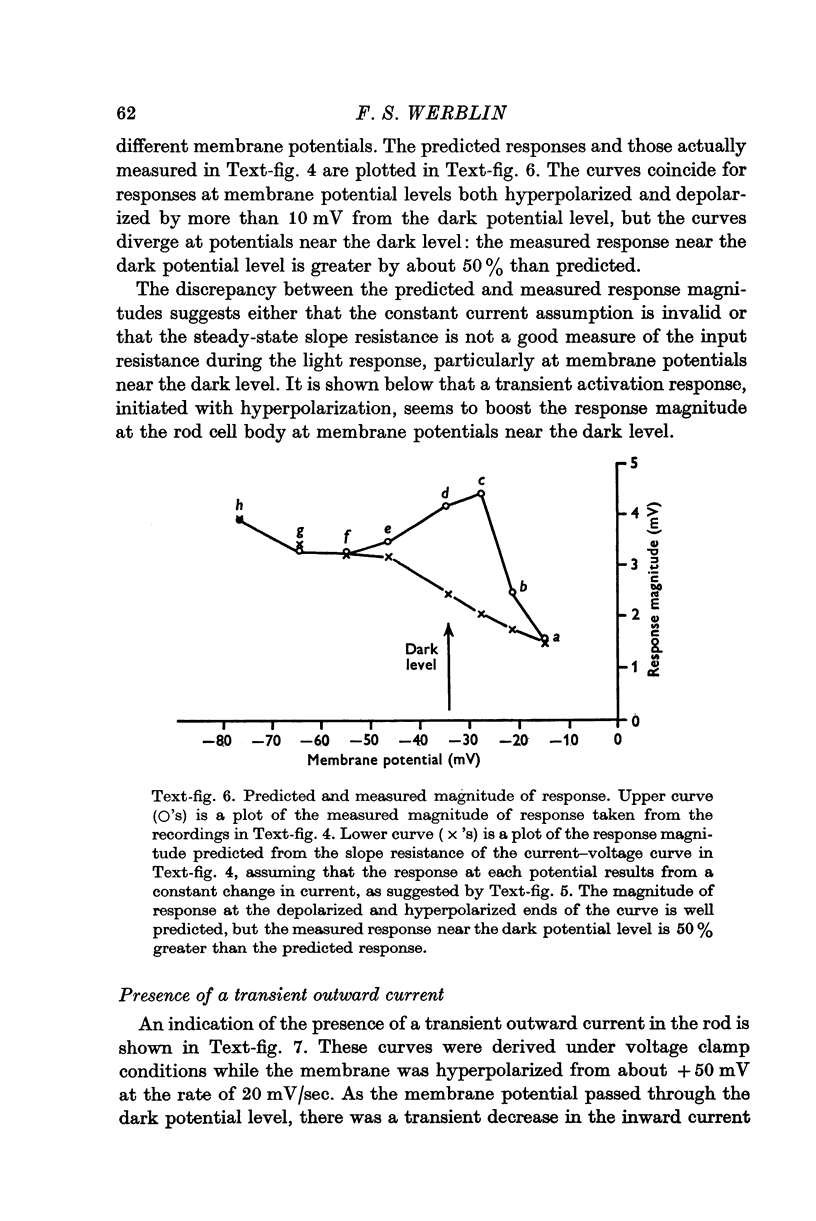
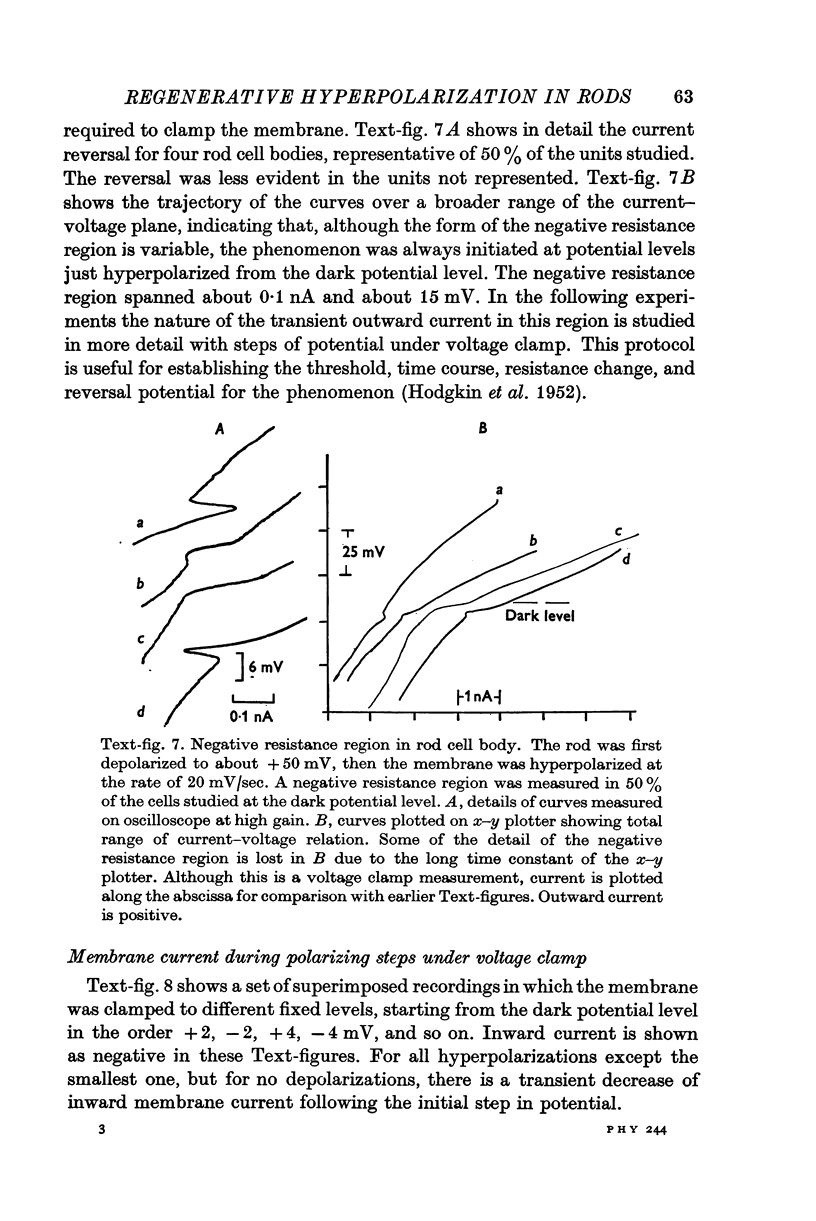
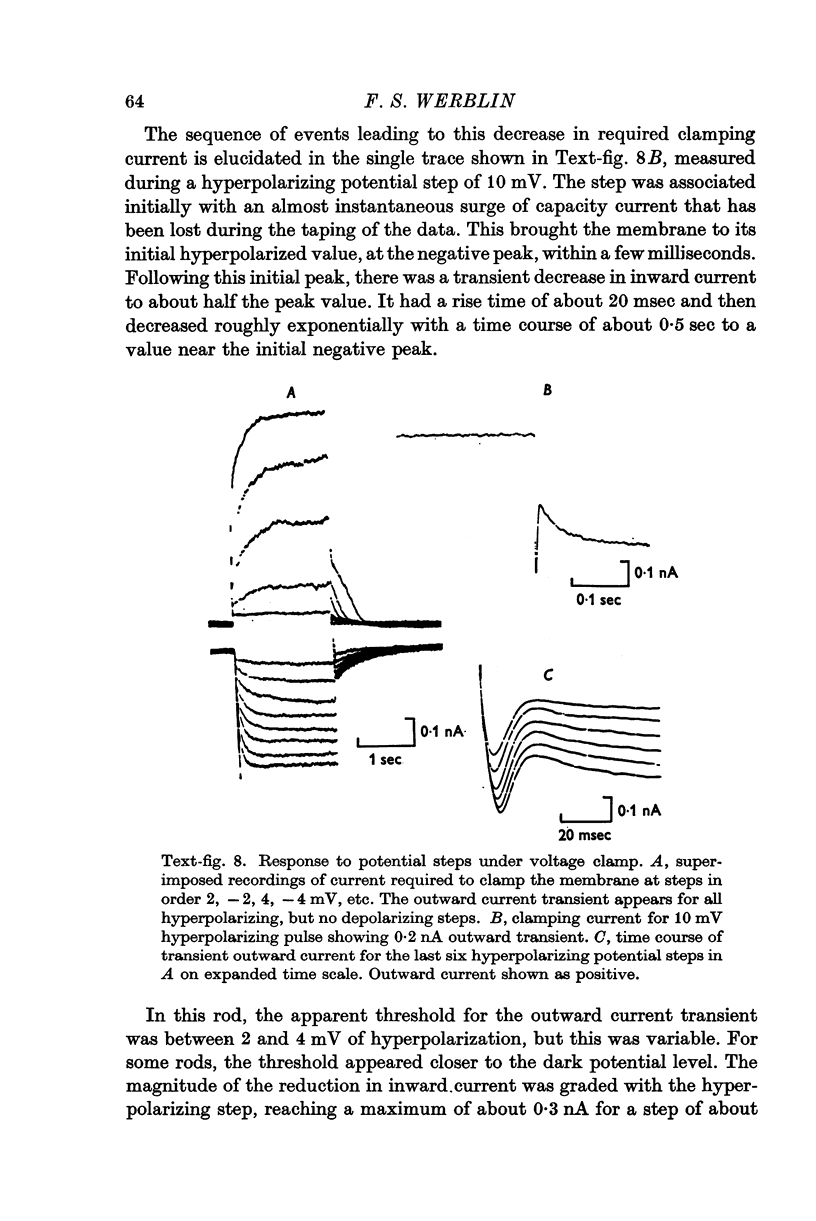
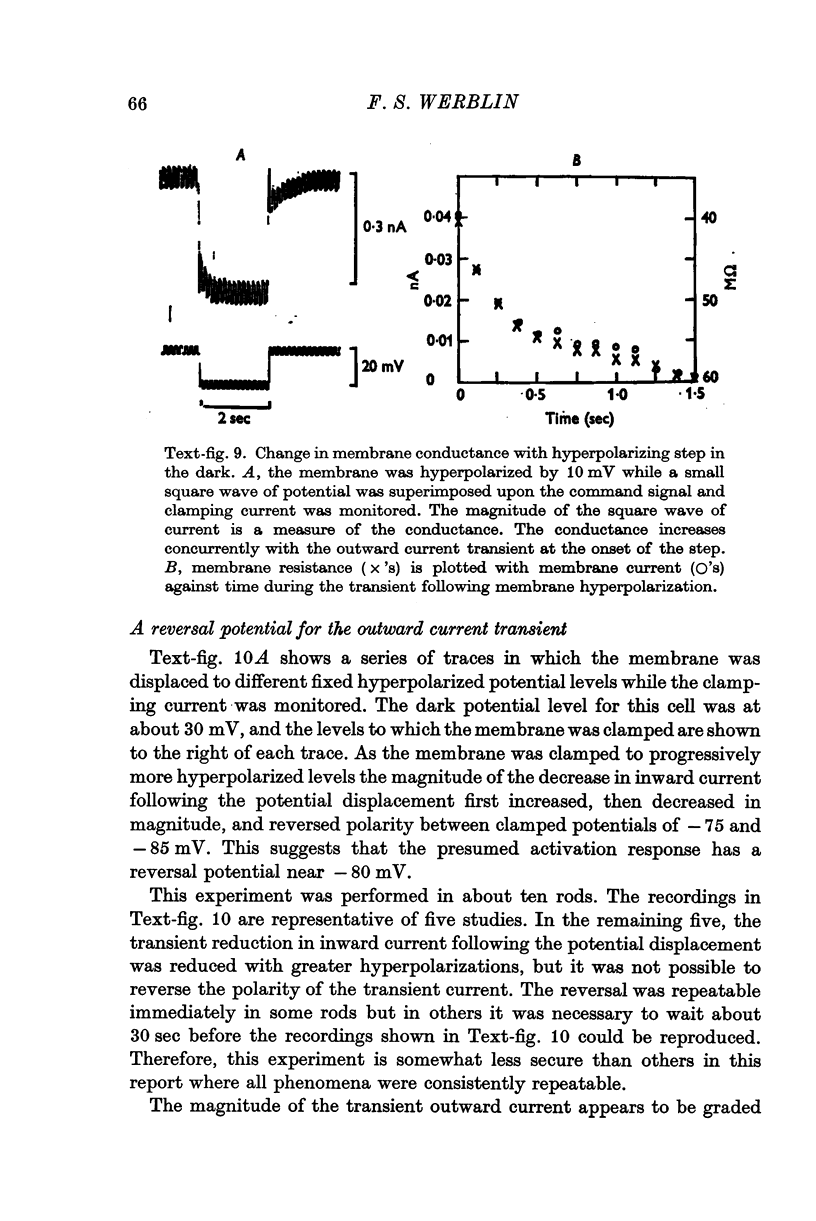
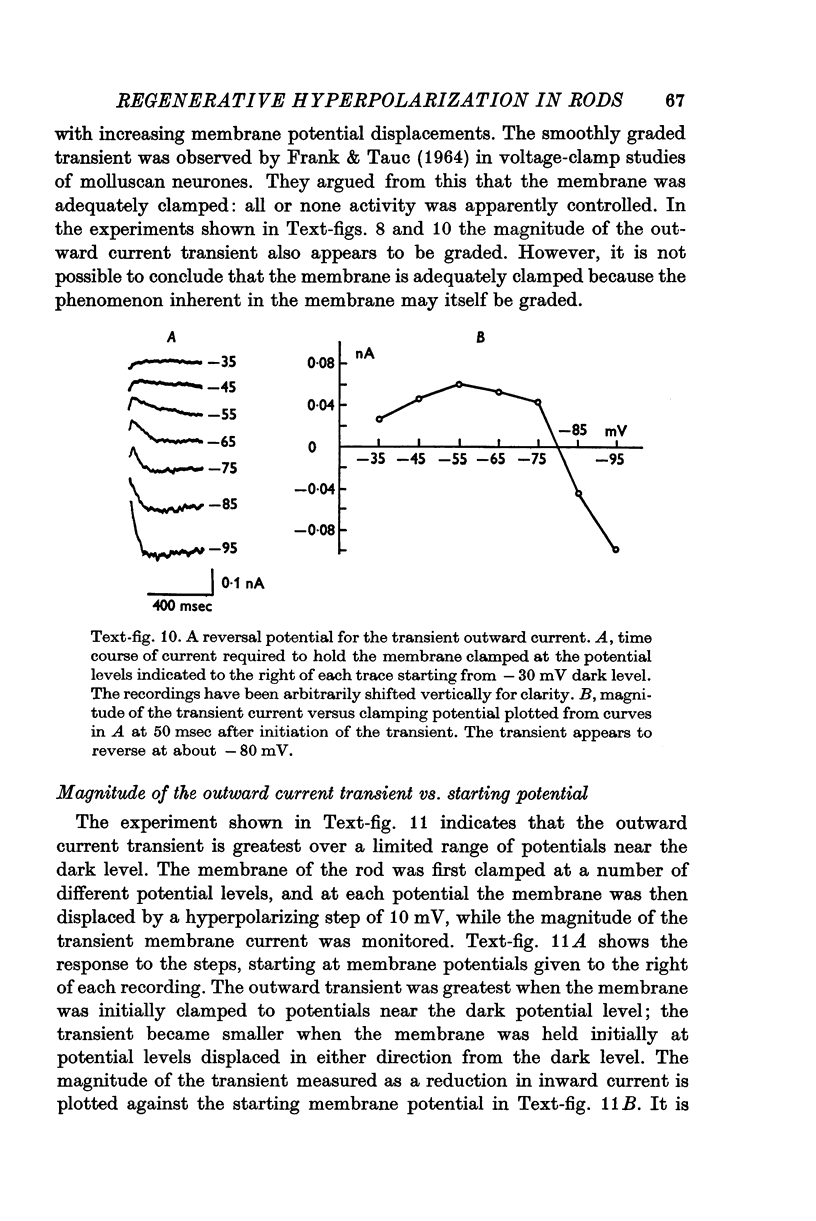
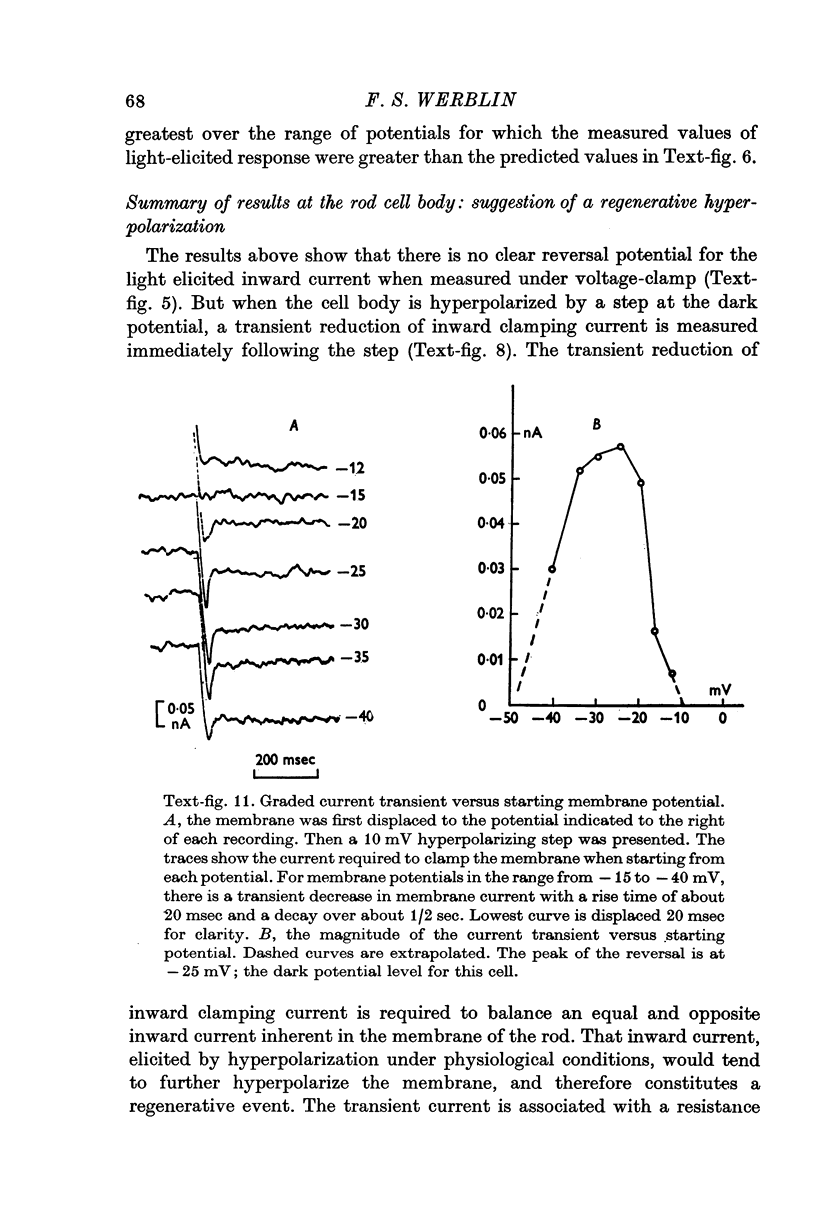
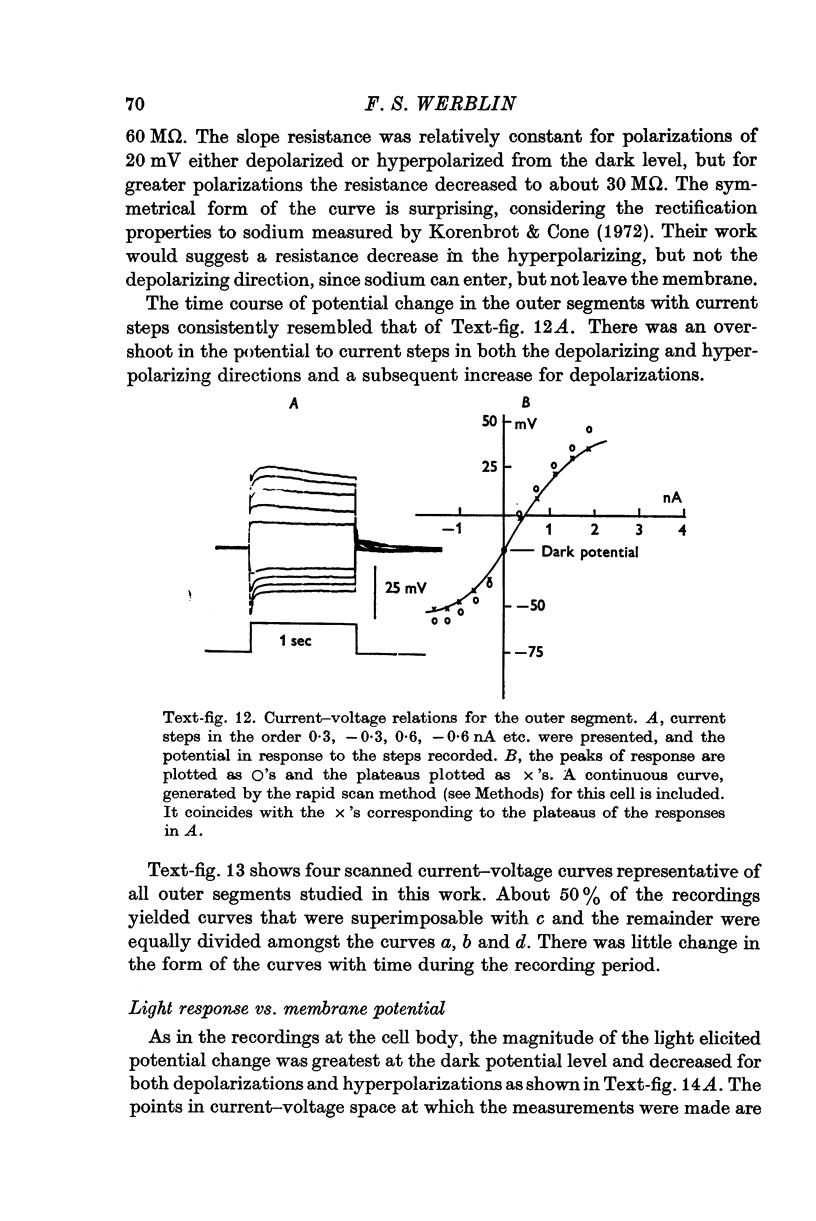
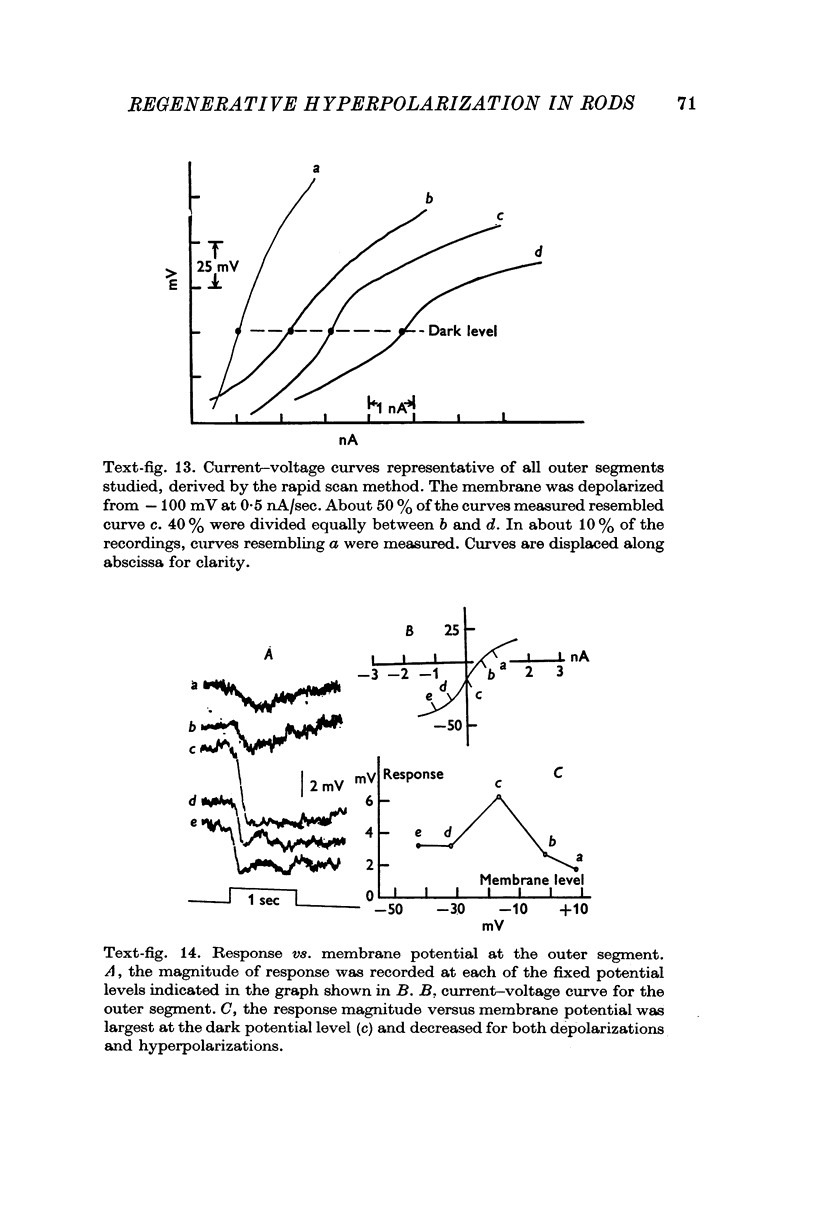
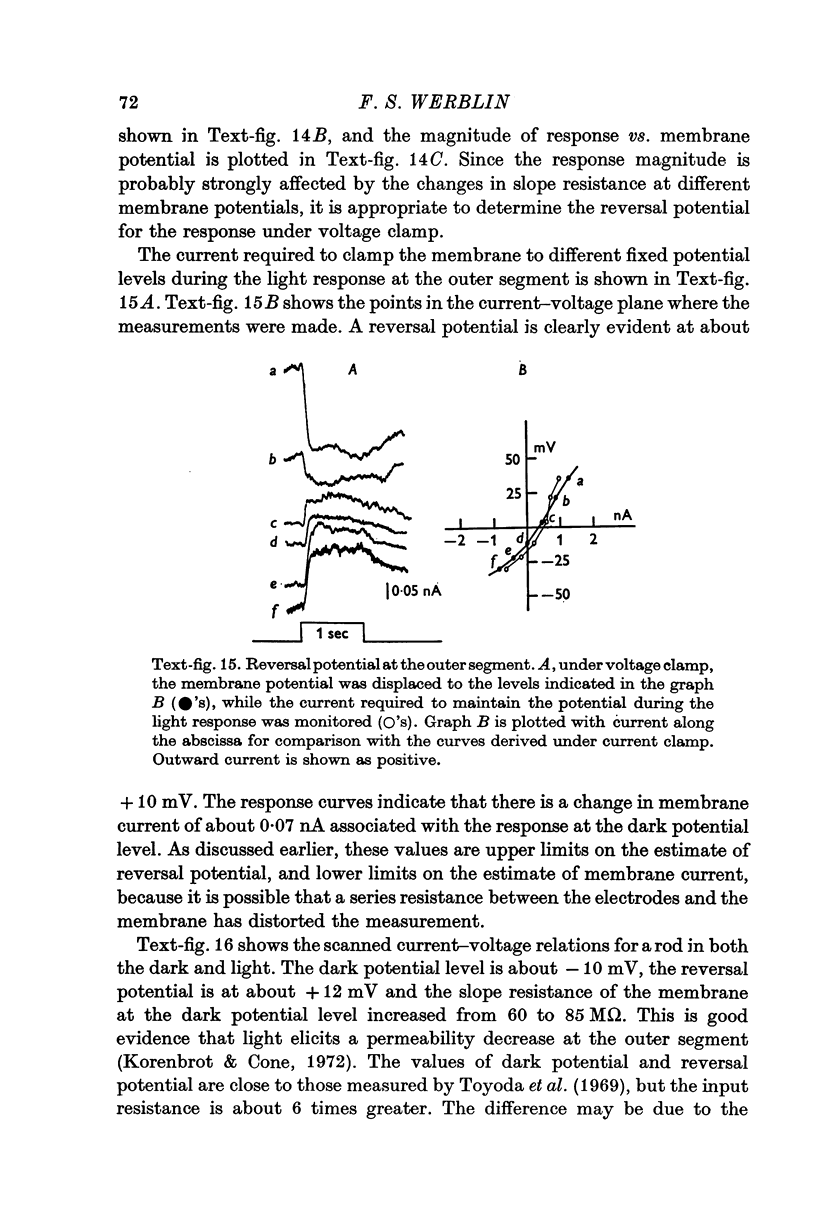
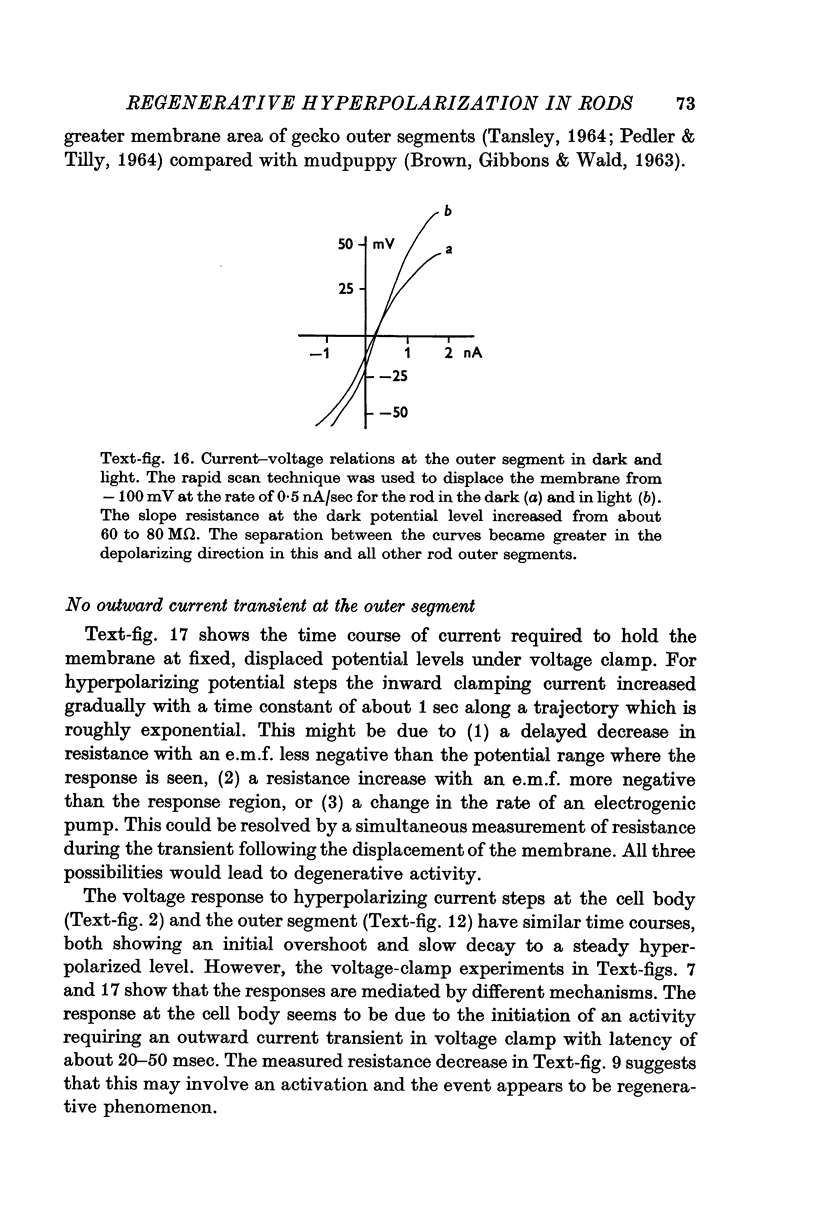
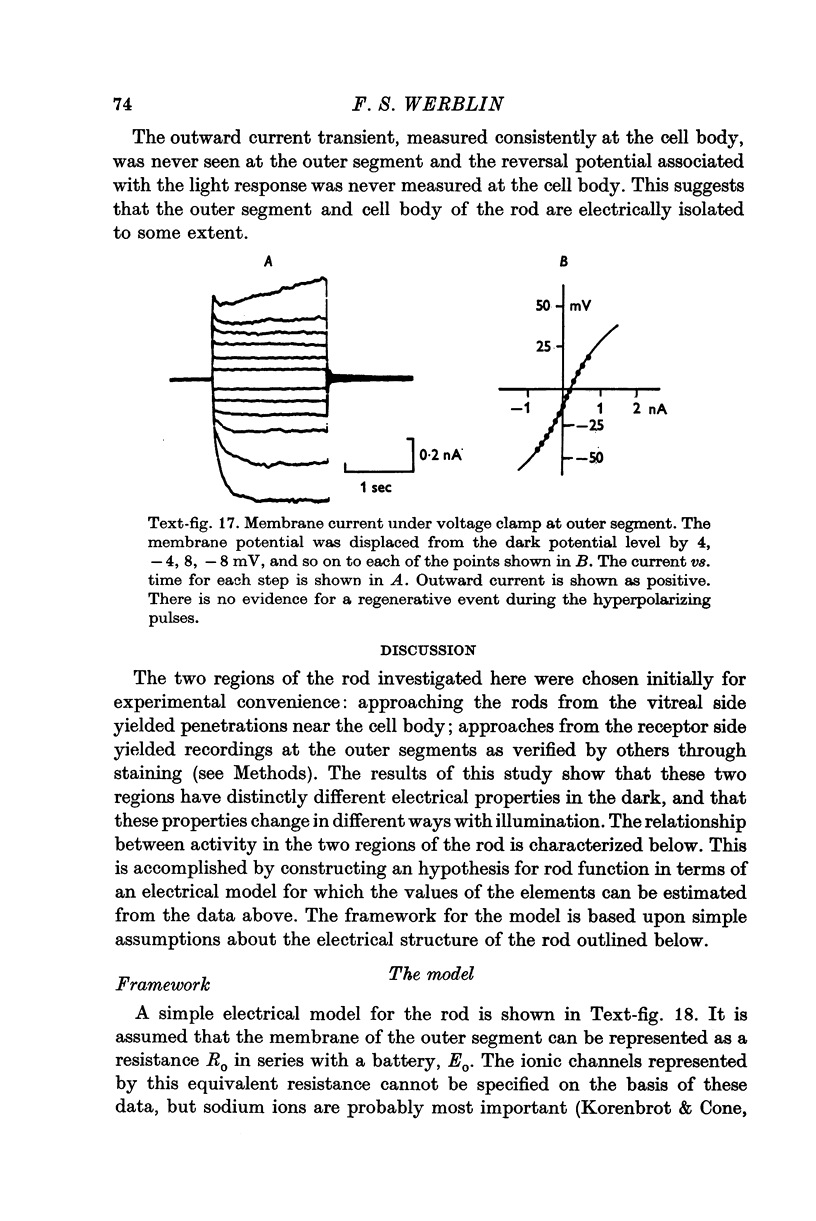
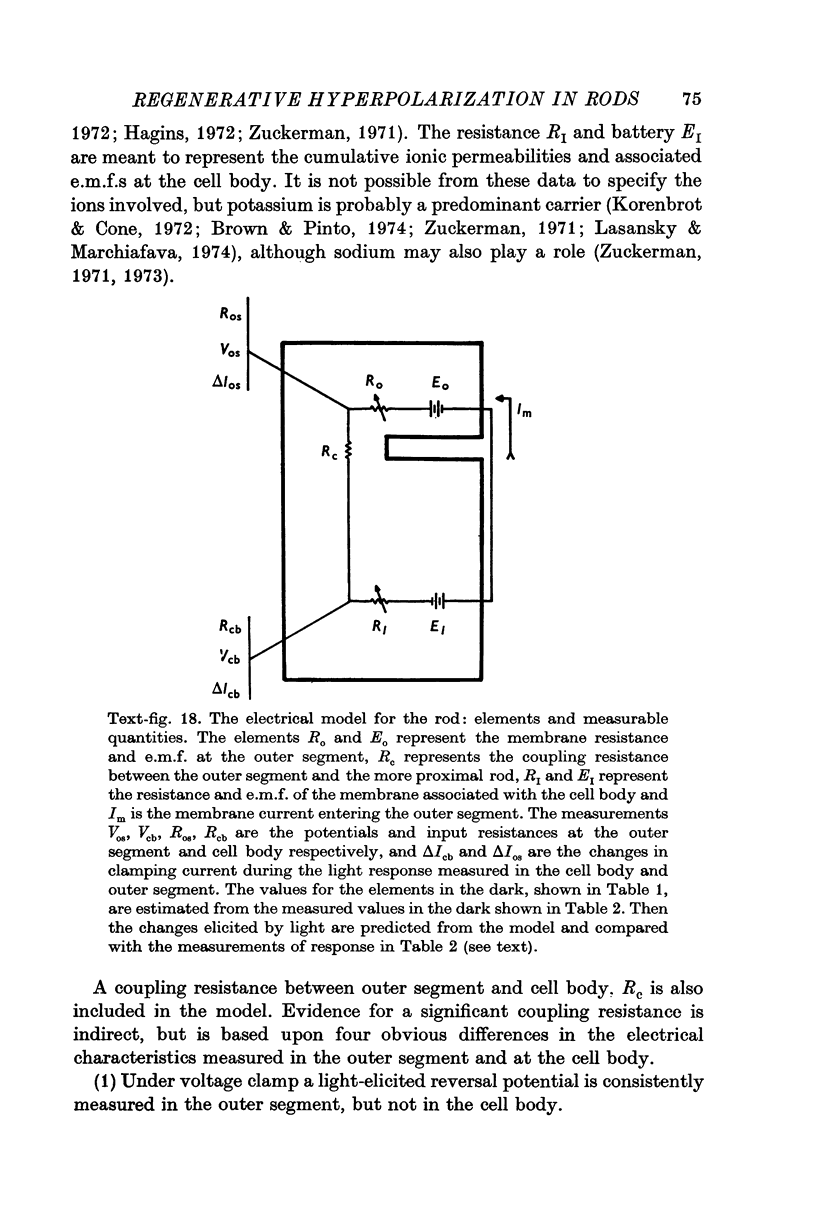
1. The electrical properties of the rods in Necturus maculosus were studied at the cell body and the outer segments in dark and light under current and voltage clamp with a pair of intracellular electrodes separated by about 1 mum. 2. The membrane resistance in the dark was voltage- and time-dependent both for the cell body and the outer segment. Slight depolarizations in the cell body reduced the slope resistance from 60 to 10 M omega with a time constant of about 1 sec. Polarization in either direction, at the outer segment, when greater than about 20 mV, reduced the slope resistance from 60 to 30 M omega. The dark potential in the cell body was typically -30 to -35 m V; at the outer segment it was typically only -10 to -15 mV. 3. The light-elicited voltage response in both the cell body and the outer segment was largest with the membrane near the dark potential level. In both regions, the response was reduced when the membrane was polarized in either direction. 4. Under voltage-clamp conditions, a reversal potential for the light response near + 10 mV was measured at the outer segment. At the cell body no reversal potential for the light response was measured; there the clamping current required during the light response was almost of the same magnitude at all potential levels. 5. When the membrane at the cell body was hyperpolarized in the dark under voltage clamp, a transient outward current, typically about one-half the magnitude of the initial inward clamping current was required to maintain the membrane at the clamped potential level. This outward current transient was associated with a decrease in membrane resistance with similar time course. The transient outward current reversed and became inward when the membrane was clamped to potentials more negative than -80 mV. Thus, the transient outward current appears to involve a transient activation initiated by hyperpolarization. I is regenerative in that it is initiated by hyperpolarization and tends to further hyperpolarize the membrane. 6. The reversal potential for the light response was measured at the outer segment but not at the cell body. The regenerative hyperpolarization was measured at the cell body but not at the outer segment. Thus, the outer segment and cell body appear to have different electrical properties: a light-elicited resistance increase at the outer segment causes a potential-dependent transient decrease at the inner rod. 7. An electrical model of the rod, based upon estimates of the membrane resistances and membrane e.m.f.s. in the dark, was derived from the data. This model predicts the appropriate response potentials at outer segment and cell body when perturbed by the measured light-elicited resistance increase at the outer segment. An estimate of membrane current in dark, of 0-2 mA, is also derived from the model.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., TERZUOLO C. A. Membrane currents in spinal motoneurons associated with the action potential and synaptic activity. J Neurophysiol. 1962 Nov;25:772–789. doi: 10.1152/jn.1962.25.6.772. [DOI] [PubMed] [Google Scholar]
- BROWN P. K., GIBBONS I. R., WALD G. THE VISUAL CELLS AND VISUAL PIGMENT OF THE MUDPUPPY, NECTURUS. J Cell Biol. 1963 Oct;19:79–106. doi: 10.1083/jcb.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Piccolino M. Synaptic transmission between photoreceptors and horizontal cells in the turtle retina. Science. 1974 Feb 1;183(4123):417–419. doi: 10.1126/science.183.4123.417. [DOI] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G., NELSON P. G. Voltage clamp of motoneuron soma. Science. 1959 Jul 3;130(3366):38–39. doi: 10.1126/science.130.3366.38. [DOI] [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk G., Fatt P. An analysis of light-induced admittance changes in rod outer segments. J Physiol. 1973 Feb;229(1):185–220. doi: 10.1113/jphysiol.1973.sp010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski S. R., Pinto L. H., Pak W. L. Adaptation in retinal rods of axolotl: intracellular recordings. Science. 1972 Jun 16;176(4040):1240–1243. doi: 10.1126/science.176.4040.1240. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Anomalous rectification in the metacerebral giant cells and its consequences for synaptic transmission. J Physiol. 1966 Mar;183(2):287–304. doi: 10.1113/jphysiol.1966.sp007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot J. I., Cone R. A. Dark ionic flux and the effects of light in isolated rod outer segments. J Gen Physiol. 1972 Jul;60(1):20–45. doi: 10.1085/jgp.60.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A., Marchiafava P. L. Light-induced resistance changes in retinal rods and cones of the tiger salamander. J Physiol. 1974 Jan;236(1):171–191. doi: 10.1113/jphysiol.1974.sp010429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. 3. A voltage-clamp study. J Gen Physiol. 1969 Sep;54(3):331–351. doi: 10.1085/jgp.54.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Pak W. L. Intracellularly recorded early receptor potential of the vertebrate photoreceptors. Vision Res. 1970 Oct;10(10):965–975. doi: 10.1016/0042-6989(70)90074-x. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Frank K. Anomalous rectification in cat spinal motoneurons and effect of polarizing currents on excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1097–1113. doi: 10.1152/jn.1967.30.5.1097. [DOI] [PubMed] [Google Scholar]
- Normann R. A., Werblin F. S. Control of retinal sensitivity. I. Light and dark adaptation of vertebrate rods and cones. J Gen Physiol. 1974 Jan;63(1):37–61. doi: 10.1085/jgp.63.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryan P. M. Properties of the depolarizing synaptic potential evoked by peripheral illumination in cones of the turtle retina. J Physiol. 1973 Nov;235(1):207–223. doi: 10.1113/jphysiol.1973.sp010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedler C., Tilly R. The nature of the Gecko visual cell. A light and electron microscopic study. Vision Res. 1964 Nov;4(10):499–510. doi: 10.1016/0042-6989(64)90056-2. [DOI] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969 Jul 12;223(5202):201–204. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- Richardson T. M. Cytoplasmic and ciliary connections between the inner and outer segments of mammalian visual receptors. Vision Res. 1969 Jul;9(7):727–731. doi: 10.1016/0042-6989(69)90010-8. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of single rods in the retina of the turtle. J Physiol. 1973 Aug;232(3):503–514. doi: 10.1113/jphysiol.1973.sp010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillman A. J., Ito H., Tomita T. Studies on the mass receptor potential of the isolated frog retina. II. On the basis of the ionic mechanism. Vision Res. 1969 Dec;9(12):1443–1451. doi: 10.1016/0042-6989(69)90060-1. [DOI] [PubMed] [Google Scholar]
- TAYLOR R. E., MOORE J. W., COLE K. S. Analysis of certain errors in squid axon voltage clamp measurements. Biophys J. 1960 Nov;1:161–202. doi: 10.1016/s0006-3495(60)86882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansley K. The gecko retina. Vision Res. 1964 May;4(1):33–37. doi: 10.1016/0042-6989(64)90029-x. [DOI] [PubMed] [Google Scholar]
- Tomita T., Kaneko A. An intracellular coaxial microelectrode--its construction and application. Med Electron Biol Eng. 1965 Oct;3(4):367–376. doi: 10.1007/BF02476131. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Hashimoto H., Anno H., Tomita T. The rod response in the frog and studies by intracellular recording. Vision Res. 1970 Nov;10(11):1093–1100. doi: 10.1016/0042-6989(70)90026-x. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Nosaki H., Tomita T. Light-induced resistance changes in single photoreceptors of Necturus and Gekko. Vision Res. 1969 Apr;9(4):453–463. doi: 10.1016/0042-6989(69)90134-5. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Control of retinal sensitivity. II. Lateral interactions at the outer plexi form layer. J Gen Physiol. 1974 Jan;63(1):62–87. doi: 10.1085/jgp.63.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Zuckerman R. Ionic analysis of photoreceptor membrane currents. J Physiol. 1973 Dec;235(2):333–354. doi: 10.1113/jphysiol.1973.sp010390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman R. Mechanisms of photoreceptor current generation in light and darkness. Nat New Biol. 1971 Nov 3;234(44):29–31. doi: 10.1038/newbio234029a0. [DOI] [PubMed] [Google Scholar]