Abstract
1. The smooth muscle layer of the bovine trachea was studied in vitro with the micro-electrode and sucrose-gap techniques. The membrane potential was stable at--47-6 plus or minus 0-98 (S.E. of mean) mV, and there was no spontaneous electrical or mechanical activity. 2. The cell membrane had strong rectifying properties, making it impossible to elicit action potentials by electrical stimulation in normal Krebs Solution. The rectification was abolished by TEA (30 mmol/l), which depolarized the membrane and produced plateau-type action potentials. 3. The spontaneous repetitive action potentials produced by TEA were associated with rhythmic oscillatory contractions of the muscle strips. 4. Histamine caused an increased tone, with superimposed rhythmic fluctuations in tension. The electrical response consisted of depolarization, with rhythmic slow oscillations in potential (slow waves) which were synchronous with the fluctuations in tension. 5. Acetylcholine produced smooth, tonic contractures of tracheal muscle strips, and caused simple depolarization of the membrane. No action potentials were recorded. 6. In calcium-free solutions containing EGTA, the mechanical response to TEA was completely abolished; the response to histamine was greatly reduced; the response to acetylcholine was reduced to a lesser extent. All responses reverted to normal when normal concentrations of extracellular calcium were restored. 7. Lanthanum added to the bathing solution abolished the contraction due to TEA even though the solution contained calcium. It reduced the histamine-induced contraction to 26% of control, and reduced the acetylcholine-induced contraction to 58% of control; extracellular calcium was present throughout. 8. It is suggested that TEA produces contraction by promoting influx of calcium ions into the cytoplasm. Acetylcholine, and to a smaller extent histamine, are less dependent upon the presence of extracellular calcium, and may be capable of releasing calcium sequestered within the cell; acetylcholine appears to be more effective in releasing sequestered calcium.
Full text
PDF



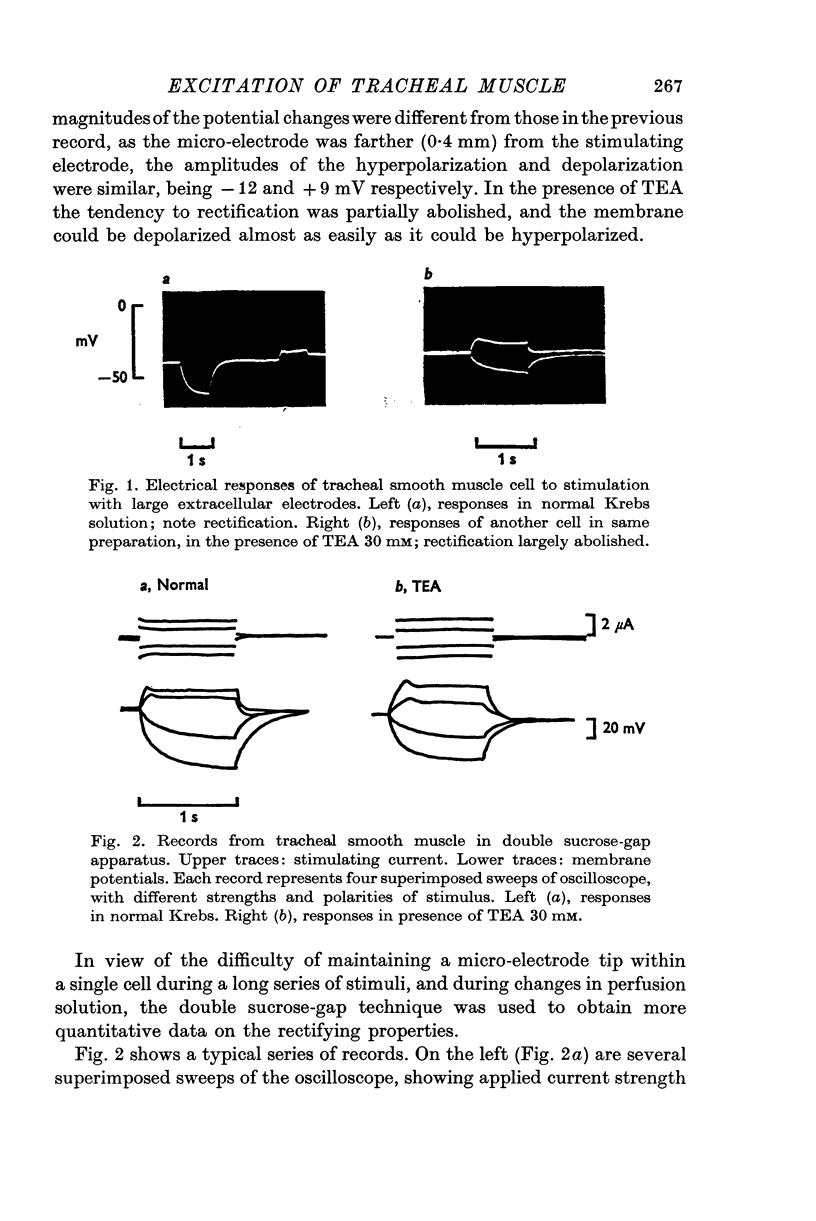
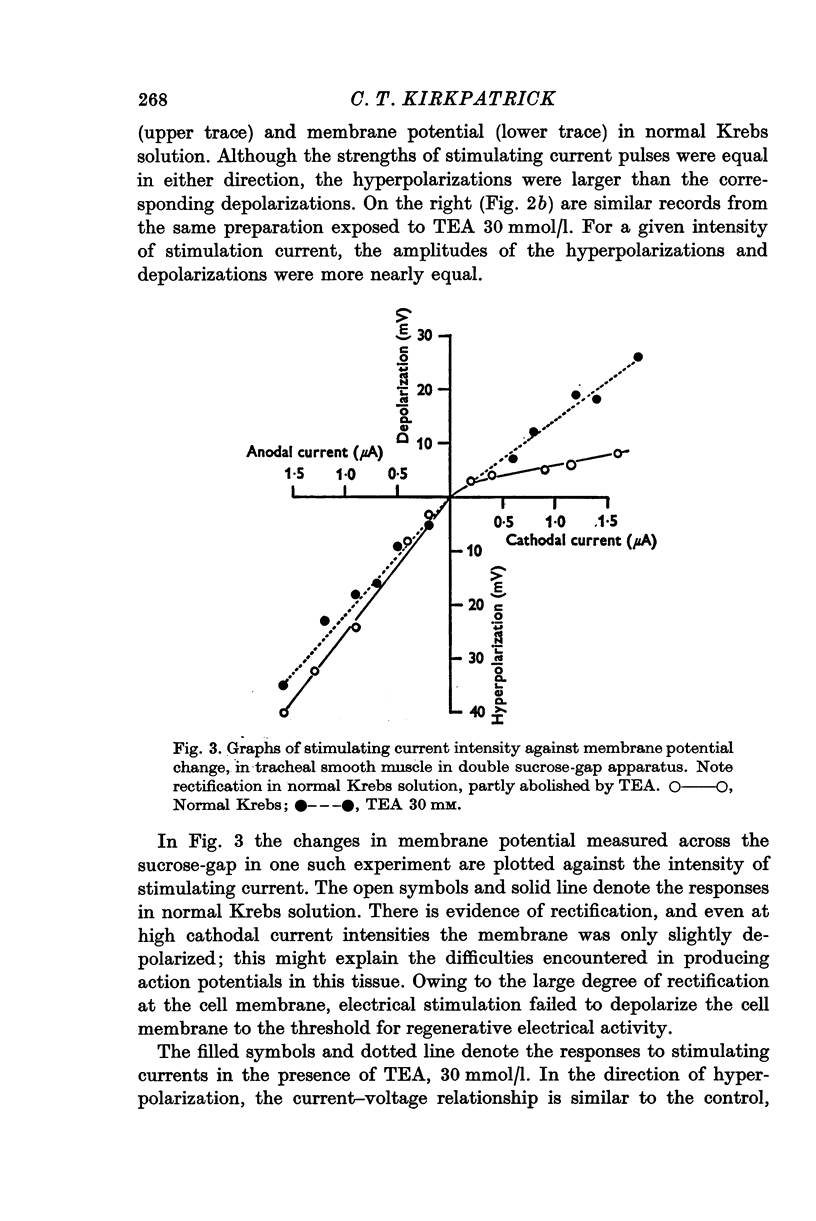
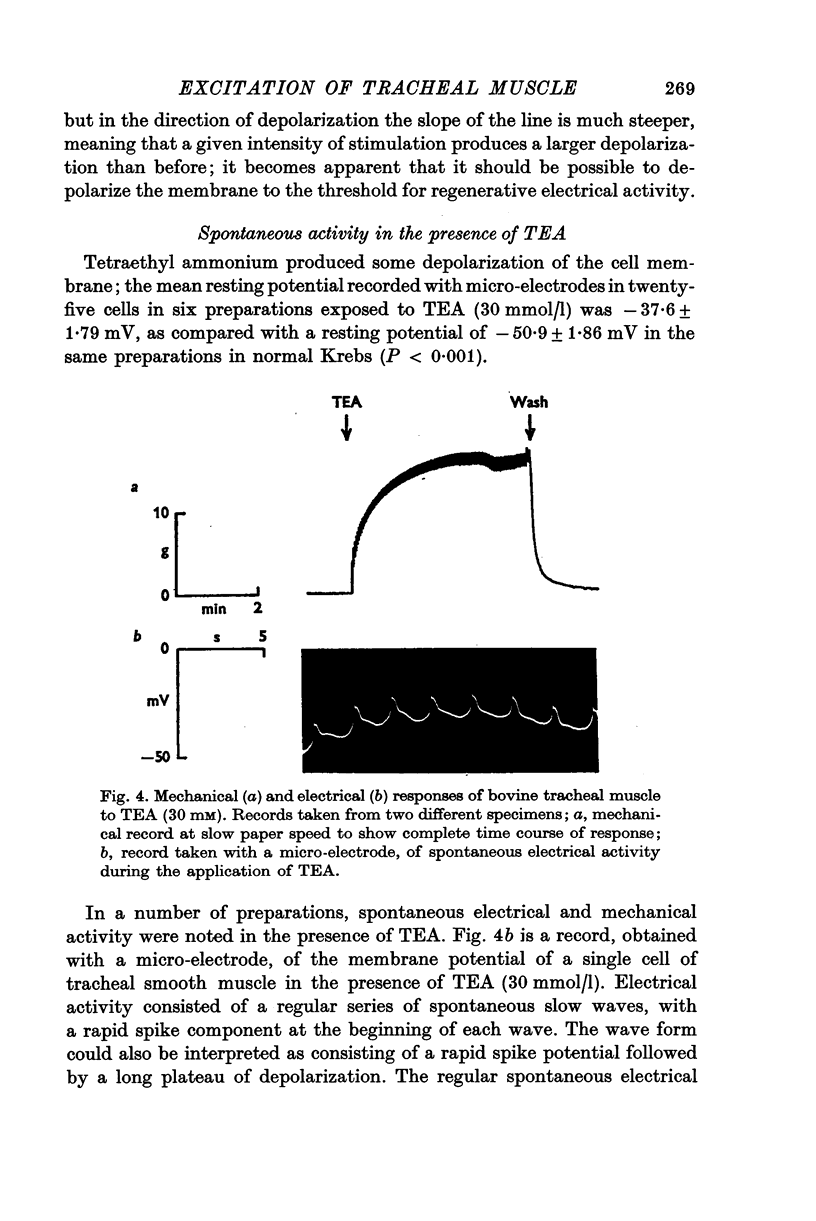
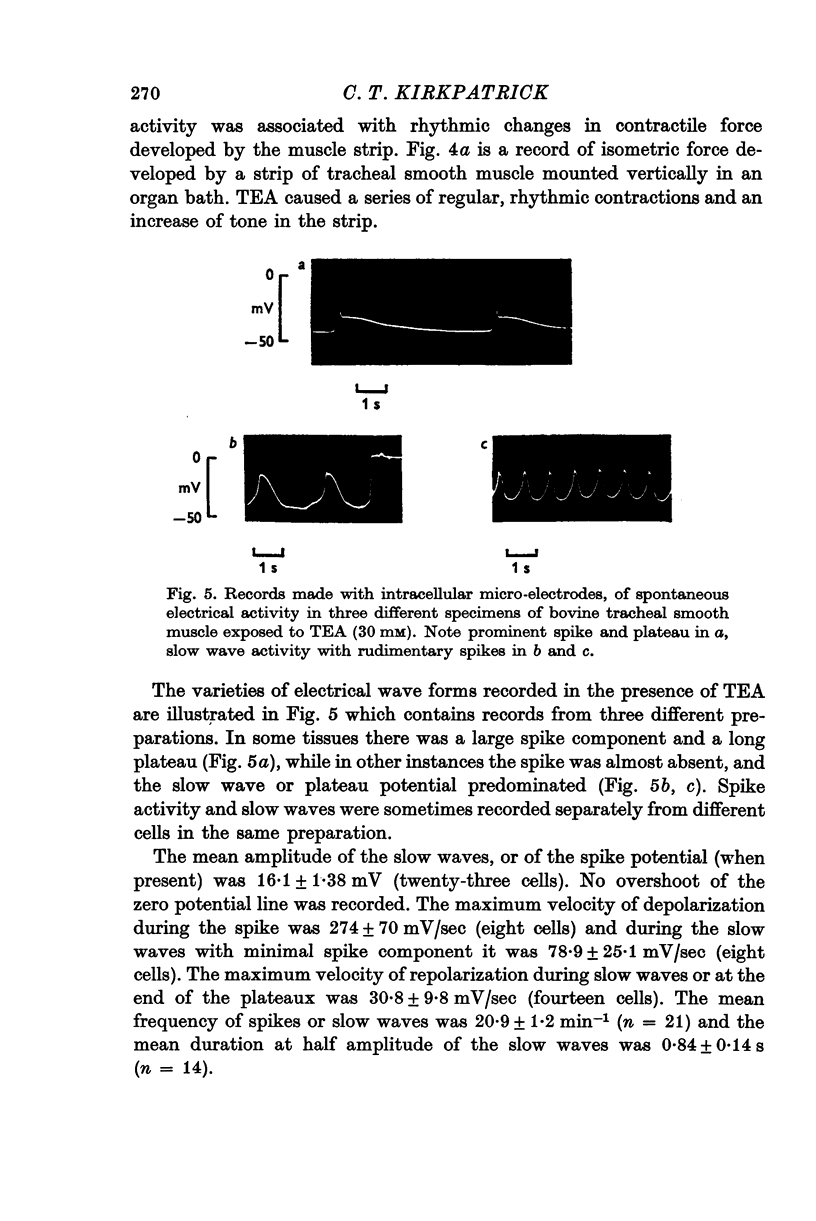
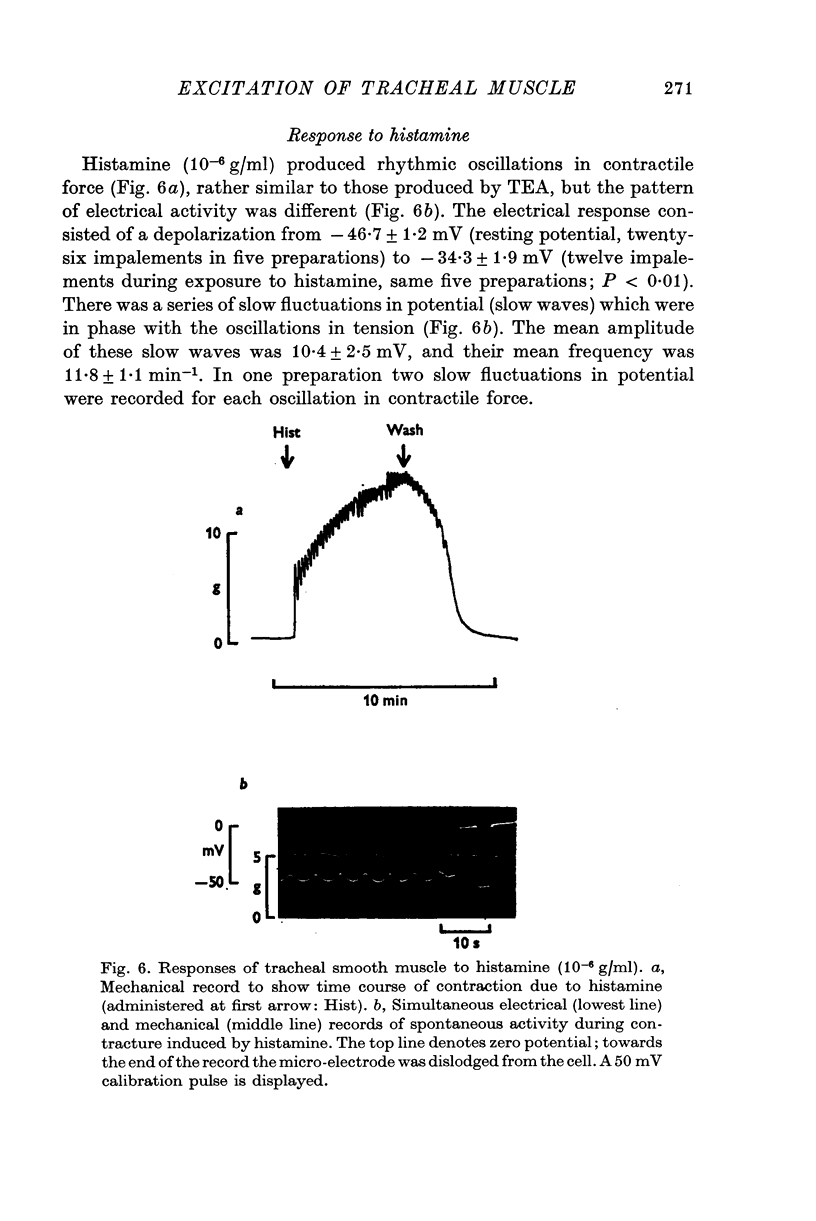
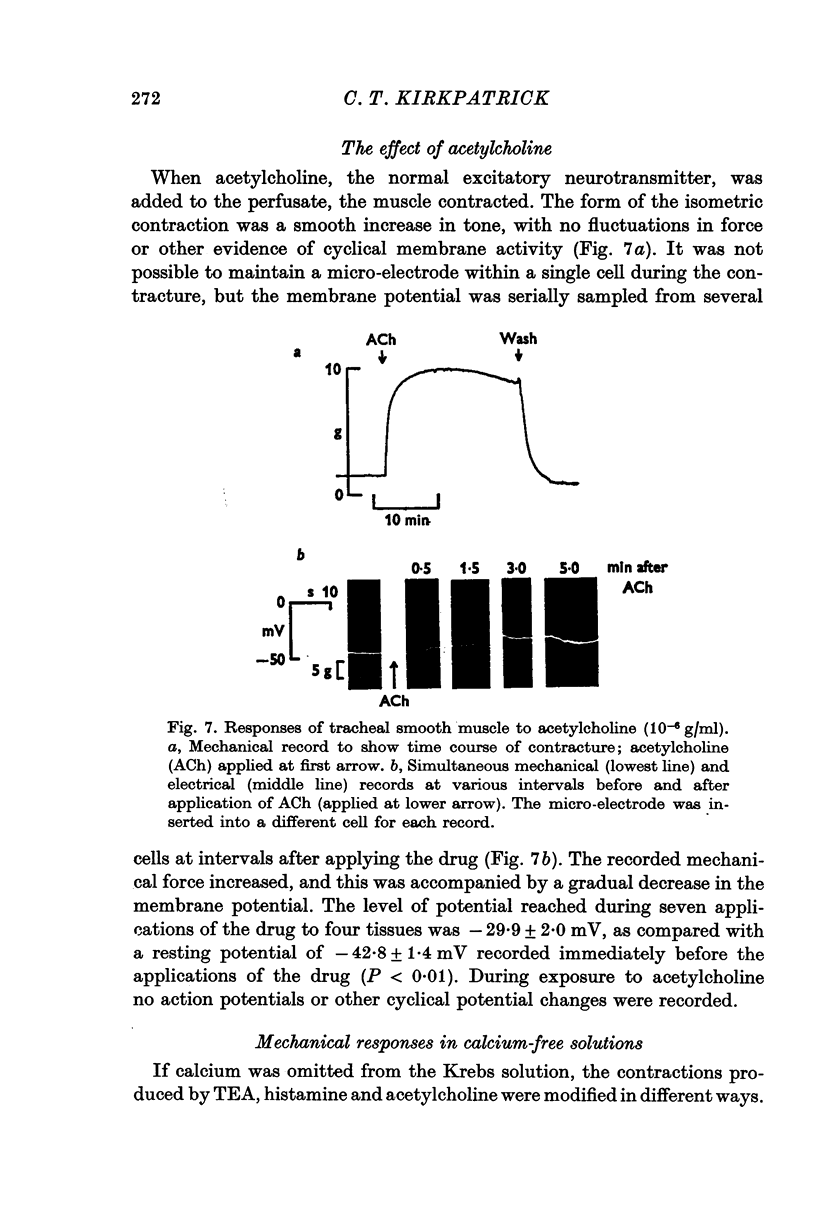
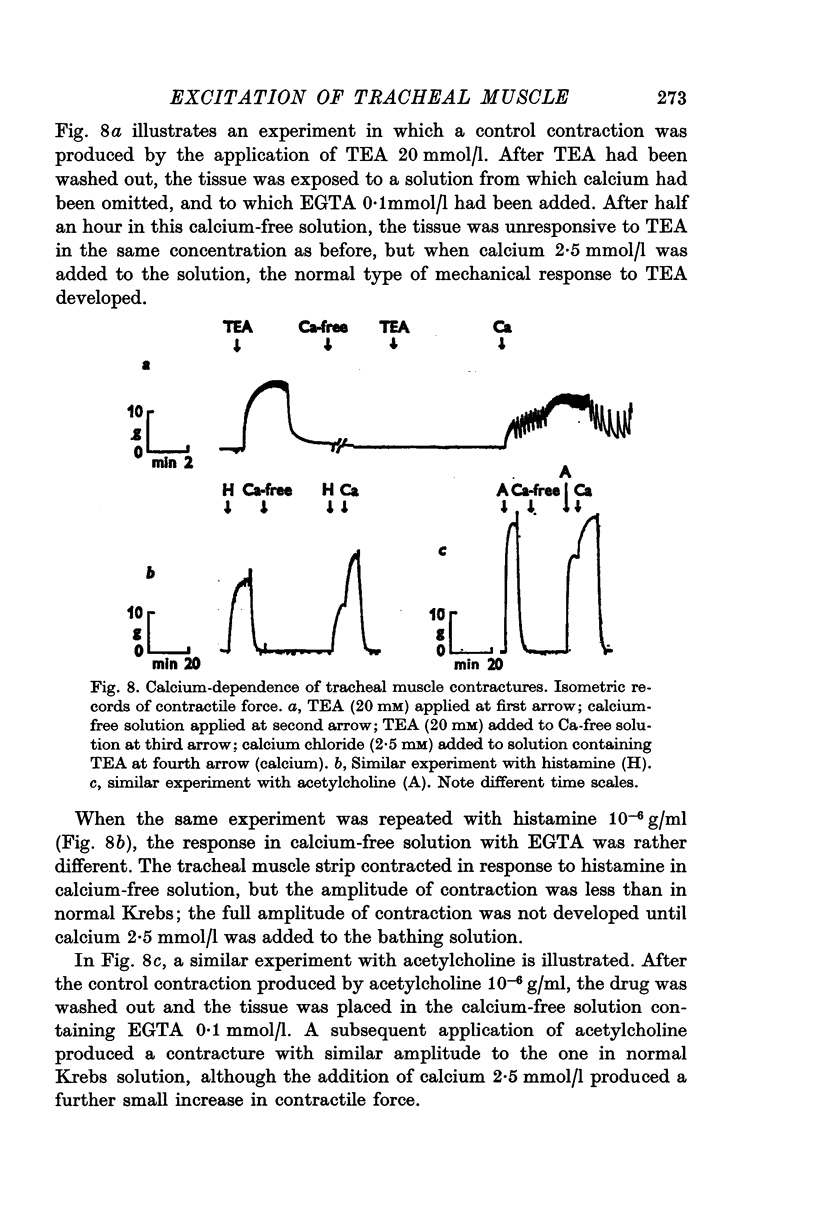
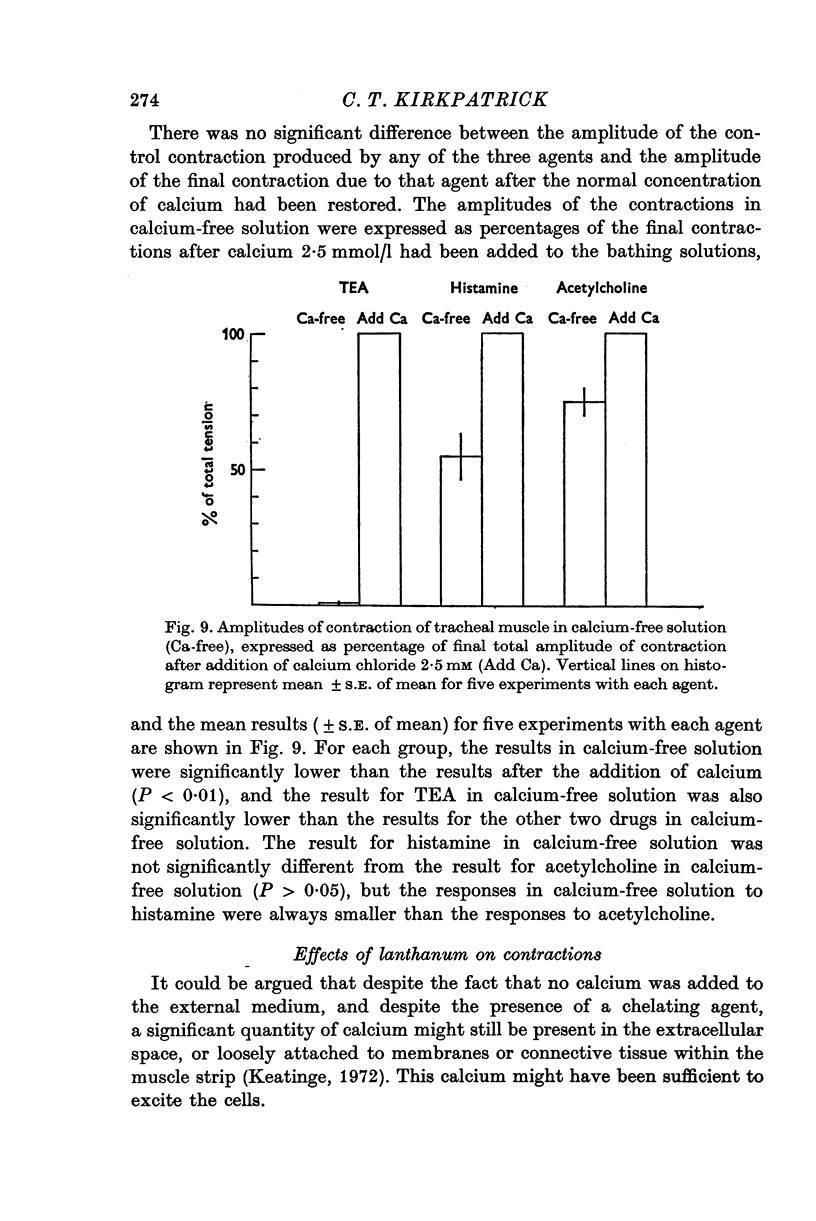






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
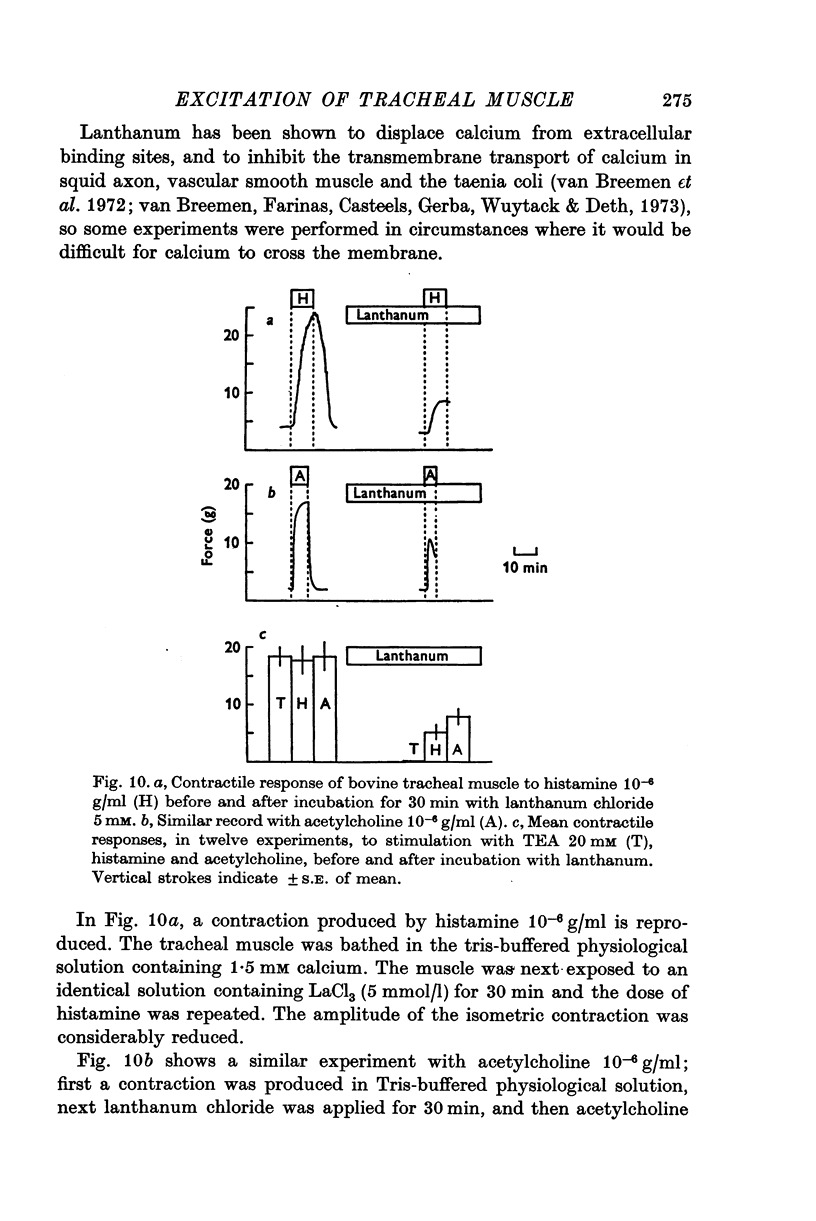
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGER W. DIE DOPPELSACCHAROSETRENNWANDTECHNIK: EINE METHODE ZUR UNTERSUCHUNG DES MEMBRANPOTENTIALS UND DER MEMBRANEIGENSCHAFTEN GLATTER MUSKELZELLEN. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963 Sep 9;277:570–576. [PubMed] [Google Scholar]
- BULBRING E. Changes in configuration of spontaneously discharged spike potentials from smooth muscle of the guinea-pig's taenia coli; the effect of electrotonic currents and of adrenaline, acetylcholine and histamine. J Physiol. 1957 Feb 15;135(2):412–425. doi: 10.1113/jphysiol.1957.sp005720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E., PROSSER C. L. Electrophysiology of smooth muscle. Physiol Rev. 1963 Jul;43:482–527. doi: 10.1152/physrev.1963.43.3.482. [DOI] [PubMed] [Google Scholar]
- Beaulieu G., Frank G. B., Inoue F. Tetraethylammonium-induced contractions of frog's skeletal muscle. II. Effects on intramuscular nerve endings. Can J Physiol Pharmacol. 1967 Sep;45(5):833–844. doi: 10.1139/y67-098. [DOI] [PubMed] [Google Scholar]
- Beaulieu G., Frank G. B. Tetraethylammonium-induced contractions of frog's skeletal muscle. 3. Mechanism of action by calcium release. Can J Physiol Pharmacol. 1967 Sep;45(5):845–855. doi: 10.1139/y67-099. [DOI] [PubMed] [Google Scholar]
- Bergmann C., Nonner W., Stämpfli R. Sustained spontaneous activity of Ranvier nodes induced by the combined actions of TEA and lack of calcium. Pflugers Arch. 1968;302(1):24–37. doi: 10.1007/BF00586780. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Action of acetylcholine on the longitudinal muscle of guinea-pig ileum: the role of an electrogenic sodium pump. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):107–114. doi: 10.1098/rstb.1973.0013. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. On the nature of the oscillations of the membrane potential (slow waves) produced by acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1971 Jul;216(2):403–418. doi: 10.1113/jphysiol.1971.sp009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. The effects of varying the concentrations of ions in the external solution on the oscillations of the membrane potential (slow waves) produced by carbachol in longitudinal ileal miscle. Pflugers Arch. 1972;335(2):85–96. doi: 10.1007/BF00592036. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. The role of electrogenic sodium pumping in the response of smooth muscle to acetylcholine. J Physiol. 1973 Feb;228(3):713–731. doi: 10.1113/jphysiol.1973.sp010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A., Bülbring E., Tomita T. The effect of sodium and calcium on the action potential of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1969 Feb;200(3):637–654. doi: 10.1113/jphysiol.1969.sp008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Kuriyama H. The action of catecholamines on guinea-pig taenia coli. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):115–121. doi: 10.1098/rstb.1973.0014. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Increase of membrane conductance by adrenaline in the smooth muscle of guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):89–102. doi: 10.1098/rspb.1969.0013. [DOI] [PubMed] [Google Scholar]
- EVANS D. H., SCHILD H. O., THESLEFF S. Effects of drugs on depolarized plain muscle. J Physiol. 1958 Oct 31;143(3):474–485. doi: 10.1113/jphysiol.1958.sp006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Creed K. E., Muir T. C. The mechanisms of action of neurotransmitters. Electrical changes underlying excitation and inhibition in intestinal and related smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):95–106. doi: 10.1098/rstb.1973.0012. [DOI] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H., Sakamoto Y. Effects of tetraethylammonium chloride on the membrane activity of guinea-pig stomach smooth muscle. J Physiol. 1970 Dec;211(2):445–460. doi: 10.1113/jphysiol.1970.sp009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOKETSU K. Action of tetraethylammonium chloride on neuromuscular transmission in frogs. Am J Physiol. 1958 Apr;193(1):213–218. doi: 10.1152/ajplegacy.1958.193.1.213. [DOI] [PubMed] [Google Scholar]
- Keatinge W. R. Ca concentration and flux in Ca-deprived arteries. J Physiol. 1972 Jul;224(1):35–59. doi: 10.1113/jphysiol.1972.sp009880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. T. Proceedings: Various mechanisms of excitation-contraction coupling in bovine tracheal smooth muscle. J Physiol. 1974 Jan;236(1):28P–29P. [PubMed] [Google Scholar]
- Mayer C. J., van Breemen C., Casteels T. The action of lanthanum and D600 on the calcium exchange in the smooth muscle cells of the guinea-pig Taenia coli. Pflugers Arch. 1972;337(4):333–350. doi: 10.1007/BF00586650. [DOI] [PubMed] [Google Scholar]
- Mekata F. Electrophysiological studies of the smooth muscle cell membrane of the rabbit common carotid artery. J Gen Physiol. 1971 Jun;57(6):738–751. doi: 10.1085/jgp.57.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F., Niu H. Biophysical effects of adrenaline on the smooth muscle of the rabbit common carotid artery. J Gen Physiol. 1972 Jan;59(1):92–102. doi: 10.1085/jgp.59.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SU C., BEVAN J. A., URSILLO R. C. ELECTRICAL QUIESCENCE OF PULMONARY ARTERY SMOOTH MUSCLE DURING SYMPATHOMIMETIC STIMULATION. Circ Res. 1964 Jul;15:26–27. doi: 10.1161/01.res.15.1.20. [DOI] [PubMed] [Google Scholar]
- Stephens N. L., Kroeger E. Effect of hypoxia on airway smooth muscle mechanics and electrophysiology. J Appl Physiol. 1970 May;28(5):630–635. doi: 10.1152/jappl.1970.28.5.630. [DOI] [PubMed] [Google Scholar]
- Szurszewski J. H., Bülbring E. The stimulant action of acetylcholine and catecholamines on the uterus. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):149–156. doi: 10.1098/rstb.1973.0017. [DOI] [PubMed] [Google Scholar]
- TASAKI I., HAGIWAR A. S. Demonstration of two stable potential states in the squid giant axon under tetraethylammonium chloride. J Gen Physiol. 1957 Jul 20;40(6):859–885. doi: 10.1085/jgp.40.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen C., Farinas B. R., Gerba P., McNaughton E. D. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res. 1972 Jan;30(1):44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]
- WIDDICOMBE J. G. Regulation of tracheobronchial smooth muscle. Physiol Rev. 1963 Jan;43:1–37. doi: 10.1152/physrev.1963.43.1.1. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Farinas B. R., Casteels R., Gerba P., Wuytack F., Deth R. Factors controlling cytoplasmic Ca 2+ concentration. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):57–71. doi: 10.1098/rstb.1973.0009. [DOI] [PubMed] [Google Scholar]


