Abstract
1. Intracellular recordings of membrane potential, input resistance and time constant have been made in vitro from the exocrine acinar cells of the mouse pancreas using glass micro-electrodes. The acinar cells were stimulated by acetylcholine (ACh). In some cases ACh was simply directly added to the tissue superfusion bath, in other experiments ACh was applied locally to pancreatic acini by micro-iontophoresis. 2. Current-voltage relations were investigated by injecting rectangular de- or hyperpolarizing current pulses through the recording micro-electrode. Within a relatively wide range (-20 to -70 mV) there was a linear relation between injected current and change in membrane potential. The slope of such linear curves corresponded to an input resistance of about 3-8 M omega. The membrane time constant was about 5-10 msec. 3. ACh depolarized the cell membrane and caused a marked reduction of input resistance and time constant. The minimum latency of the ACh-induced depolarization (microiontophoretic application) was 100-300 msec. Maximal depolarization was about 20 mV. The effect of this local ACh application was abolished by atropine (1-4 x 10-6 M). The blocking effect of atropine was fully reversible. 4. Stimulating with ACh during the passage of large depolarizing current pulses made it possible simultaneously to observe the effect of ACh at two different levels of resting potential (RP). At the spontaneous RP of about minus 40 mV ACh evoked a depolarization of usual magnitude (15-20 mV) while at the artificially displaced level of about -10 mV a small hyperpolarization (about 5 mV) was observed. It therefore appears that the reversal potential of the transmitter equilibrium potential is about -20 mV. 5. Replacement of the superfusion fluid C1 by sulphate or methylsulphate caused an initial short-lasting depolarization, thereafter the normal resting potential was reassumed...
Full text
PDF









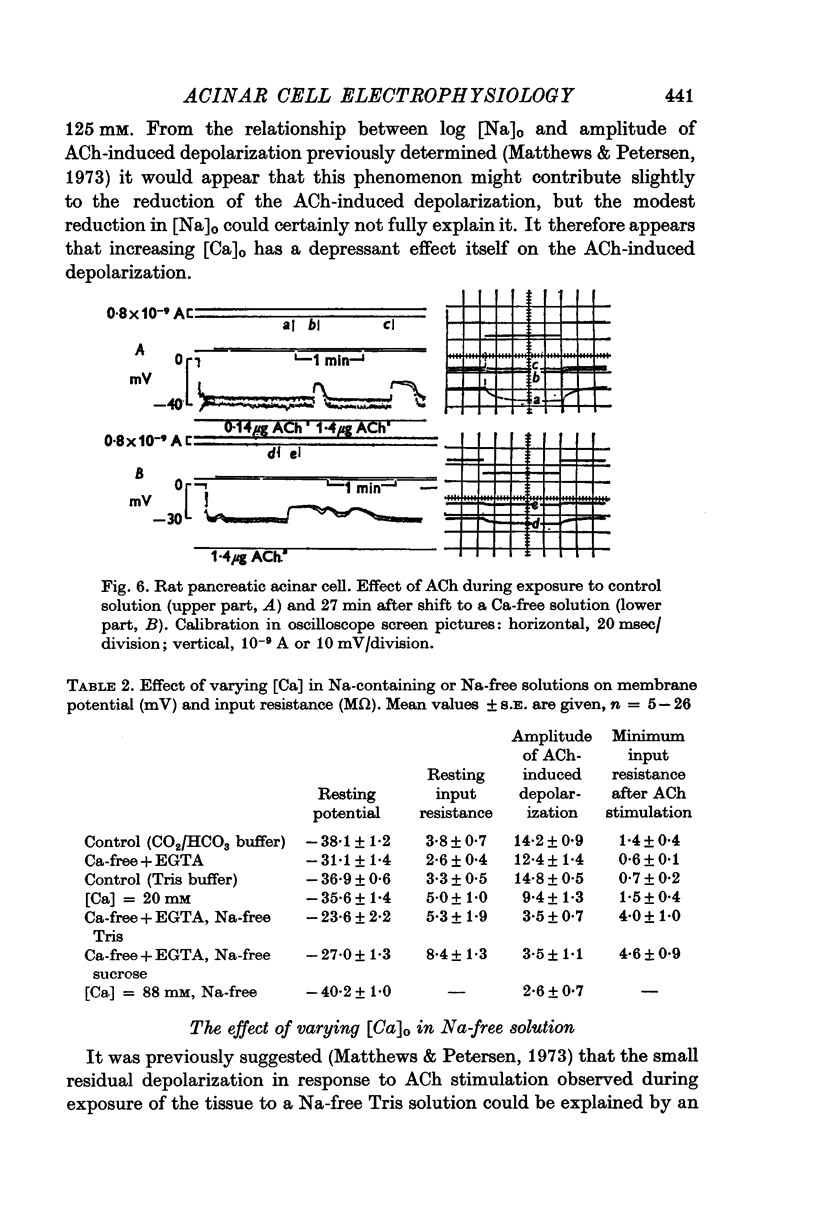






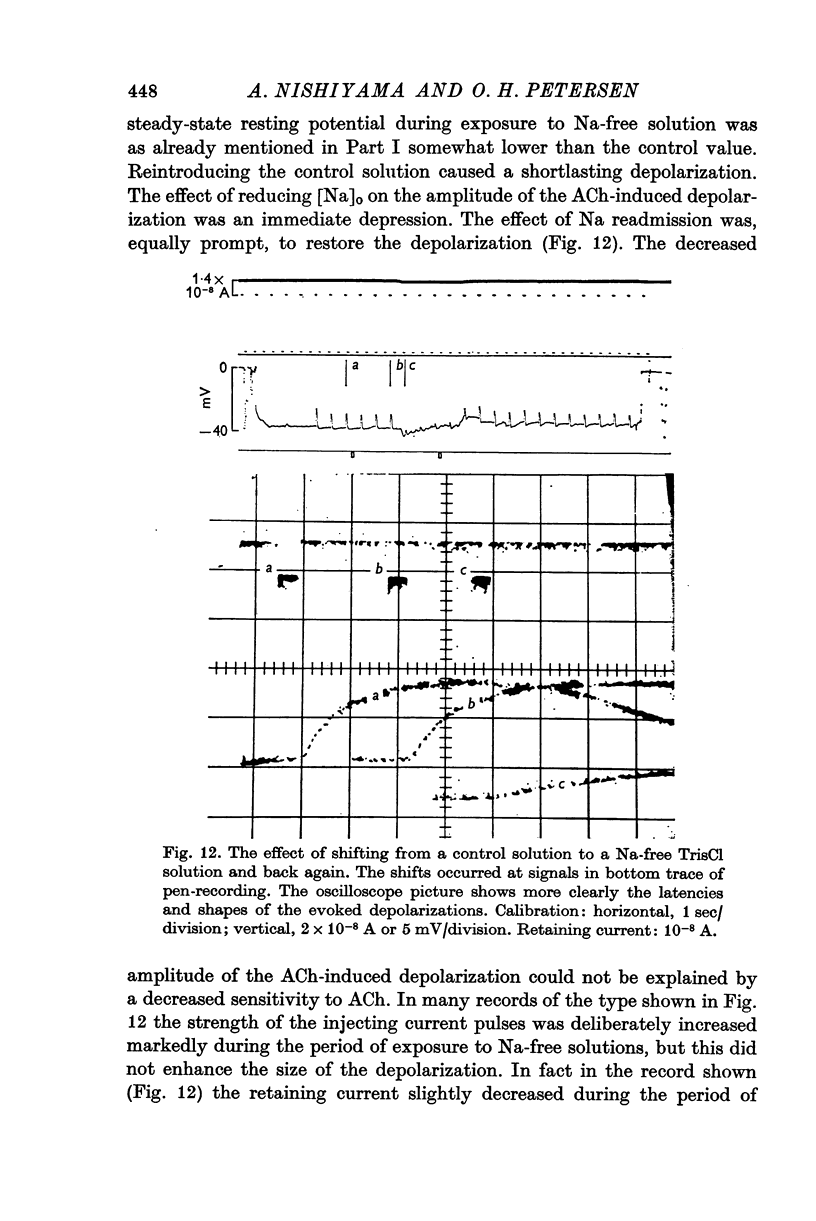






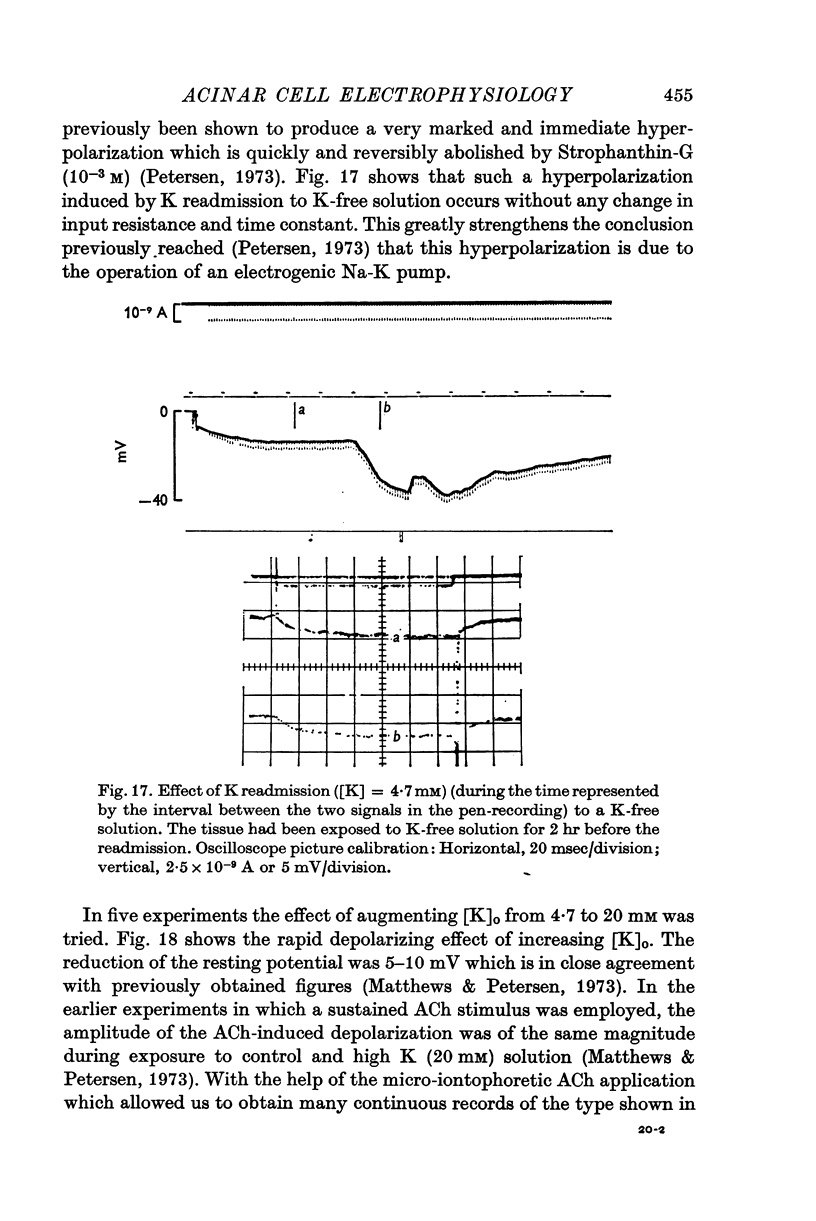
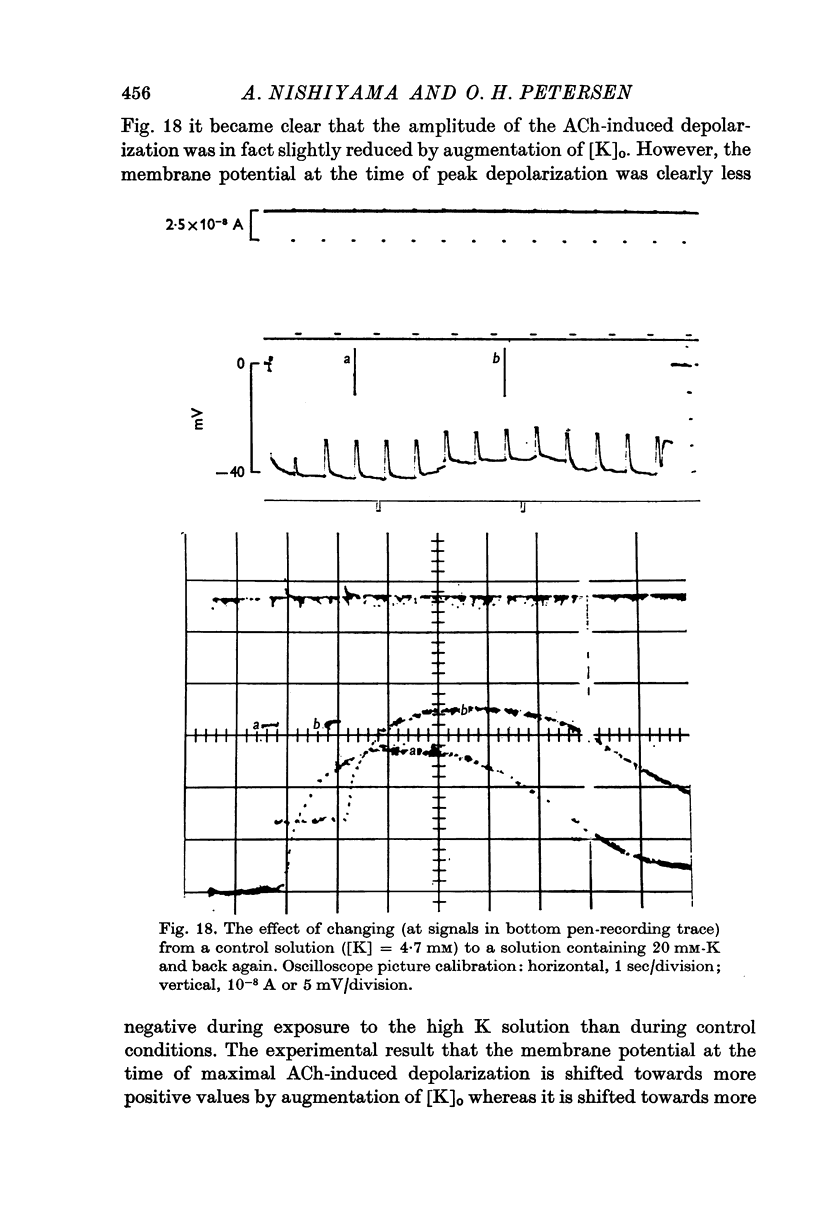








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz L., Eckstein B., Matthews E. K., Williams J. A. Control of pancreatic amylase release in vitro: effects of ions, cyclic AMP, and colchicine. Br J Pharmacol. 1972 Sep;46(1):66–67. doi: 10.1111/j.1476-5381.1972.tb06849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. The role of electrogenic sodium pumping in the response of smooth muscle to acetylcholine. J Physiol. 1973 Feb;228(3):713–731. doi: 10.1113/jphysiol.1973.sp010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Clausen T. The relationship between calcium exchange and enzyme secretion in the isolated rat pancreas. J Physiol. 1973 Nov;235(1):75–102. doi: 10.1113/jphysiol.1973.sp010379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Harper A. A., Scratcherd T. The secretion of electrolytes and enzymes by the pancreas of the anaesthetized cat. J Physiol. 1969 Apr;201(2):335–348. doi: 10.1113/jphysiol.1969.sp008759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. E., Williams J. A. Pancreatic acinar cells: effects of lanthanum ions on amylase release and calcium ion fluxes. J Physiol. 1974 Dec;243(3):831–846. doi: 10.1113/jphysiol.1974.sp010779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The membrane change produced by the neuromuscular transmitter. J Physiol. 1954 Sep 28;125(3):546–565. doi: 10.1113/jphysiol.1954.sp005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Pancreatic acinar cells: measurement of membrane potential and miniature depolarization potentials. J Physiol. 1972 Aug;225(1):1–13. doi: 10.1113/jphysiol.1972.sp009926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G. J. The action of scretin, cholecystokinin-pancreozymin and caerulein on pancreatic secretion in the rat. J Physiol. 1972 Sep;225(3):679–692. doi: 10.1113/jphysiol.1972.sp009963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. The effect of sodium ions on neuromuscular transmission. J Physiol. 1952 Sep;118(1):73–87. doi: 10.1113/jphysiol.1952.sp004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. D., Dale M. M., Haylett D. G. Effect of adrenergic amines on the membrane potential of guinea-pig liver parenchymal cells in short term tissue culture. Experientia. 1972 Sep 15;28(9):1073–1074. doi: 10.1007/BF01918681. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Effects of calcium omission on acetylcholine-stimulated amylase secretion and phospholipid synthesis in pigeon pancreas slices. Biochim Biophys Acta. 1966 Jan 25;115(1):219–221. doi: 10.1016/0304-4165(66)90066-3. [DOI] [PubMed] [Google Scholar]
- Kagayama M., Nishiyama A. Membrane potential and input resistance in acinar cells from cat and rabbit submaxillary glands in vivo: effects of autonomic nerve stimulation. J Physiol. 1974 Oct;242(1):157–172. doi: 10.1113/jphysiol.1974.sp010699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T. Calcium-dependent amylase release and electrophysiological measurements in cells of the pancreas. J Physiol. 1972 Oct;226(2):353–371. doi: 10.1113/jphysiol.1972.sp009988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Selective inhibition of the transmembrane Ca conductivity of mammalian myocardial fibres by Ni, Co and Mn ions. Pflugers Arch. 1973 Jan 22;338(2):115–123. doi: 10.1007/BF00592747. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. The electrophysiology of the submaxillary gland of the cat. Acta Physiol Scand. 1955 Dec 22;35(1):1–25. doi: 10.1111/j.1748-1716.1955.tb01258.x. [DOI] [PubMed] [Google Scholar]
- MORRILL G. A., KABACK H. R., ROBBINS E. EFFECT OF CALCIUM ON INTRACELLULAR SODIUM AND POTASSIUM CONCENTRATIONS IN PLANT AND ANIMAL CELLS. Nature. 1964 Nov 14;204:641–642. doi: 10.1038/204641a0. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H. Pancreatic acinar cells: ionic dependence of the membrane potential and acetycholine-induced depolarization. J Physiol. 1973 Jun;231(2):283–295. doi: 10.1113/jphysiol.1973.sp010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H., Williams J. A. Analysis of tissue amylase output by an automated method. Anal Biochem. 1974 Mar;58(1):155–160. doi: 10.1016/0003-2697(74)90452-7. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H., Williams J. A. Pancreatic acinar cells: acetylcholine-induced membrane depolarization, calcium efflux and amylase release. J Physiol. 1973 Nov;234(3):689–701. doi: 10.1113/jphysiol.1973.sp010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASTUK W. L. Some ionic factors that influence the action of acetylcholine at the muscle end-plate membrane. Ann N Y Acad Sci. 1959 Aug 28;81:317–327. doi: 10.1111/j.1749-6632.1959.tb49316.x. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: membrane potential and resistance change evoked by acetylcholine. J Physiol. 1974 Apr;238(1):145–158. doi: 10.1113/jphysiol.1974.sp010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Proceedings: Effect of calcium on the potential and resistance change of the pancreatic acinar cell membrane during the action of acetylcholine. J Physiol. 1974 Apr;238(1):55P–56P. [PubMed] [Google Scholar]
- Petersen O. H. Electrogenic sodium pump in pancreatic acinar cells. Proc R Soc Lond B Biol Sci. 1973 Aug 31;184(1074):115–119. doi: 10.1098/rspb.1973.0037. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Electrophysiological studies on gland cells. Experientia. 1974 Feb 15;30(2):130–134. doi: 10.1007/BF01927689. [DOI] [PubMed] [Google Scholar]
- Romero P. J., Whittam R. The control by internal calcium of membrane permeability to sodium and potassium. J Physiol. 1971 May;214(3):481–507. doi: 10.1113/jphysiol.1971.sp009445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI N. Effects of calcium on the conductance change of the end-plate membrane during the action of transmitter. J Physiol. 1963 Jun;167:141–155. doi: 10.1113/jphysiol.1963.sp007137. [DOI] [PMC free article] [PubMed] [Google Scholar]


