Abstract
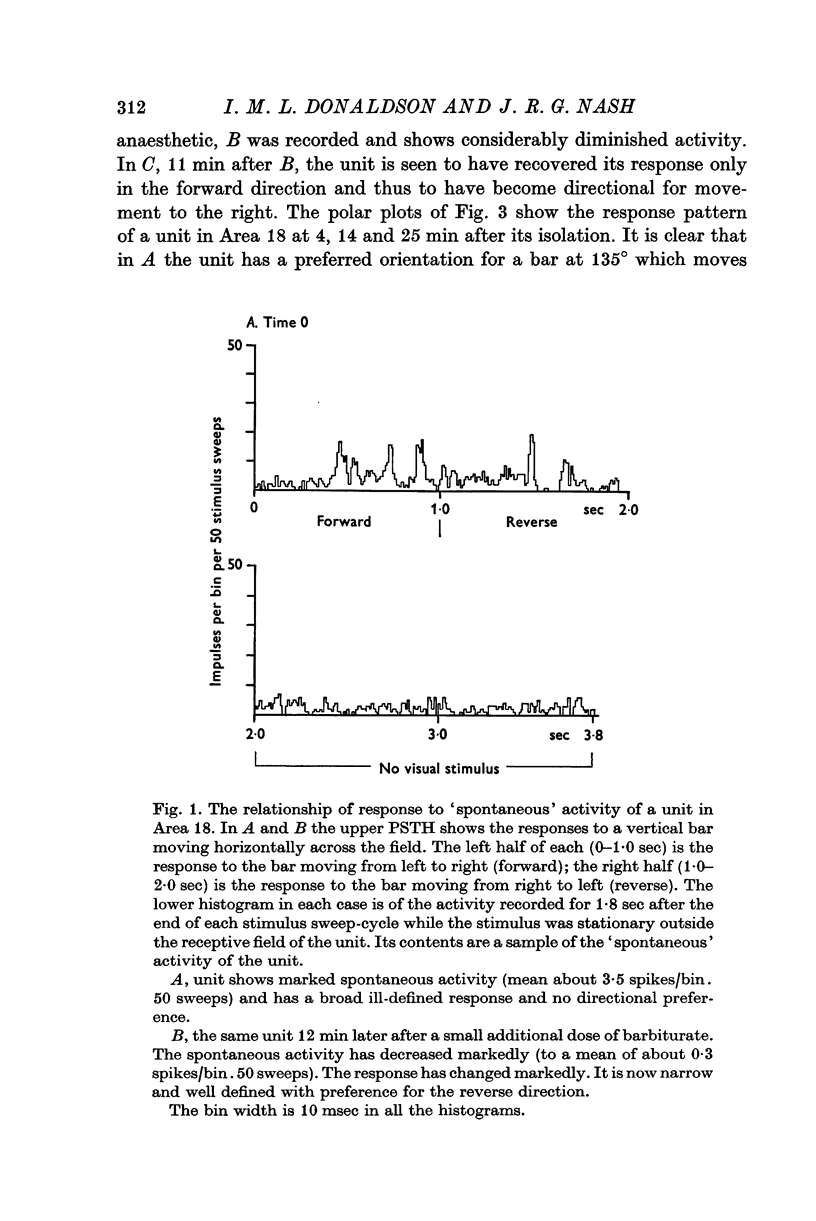
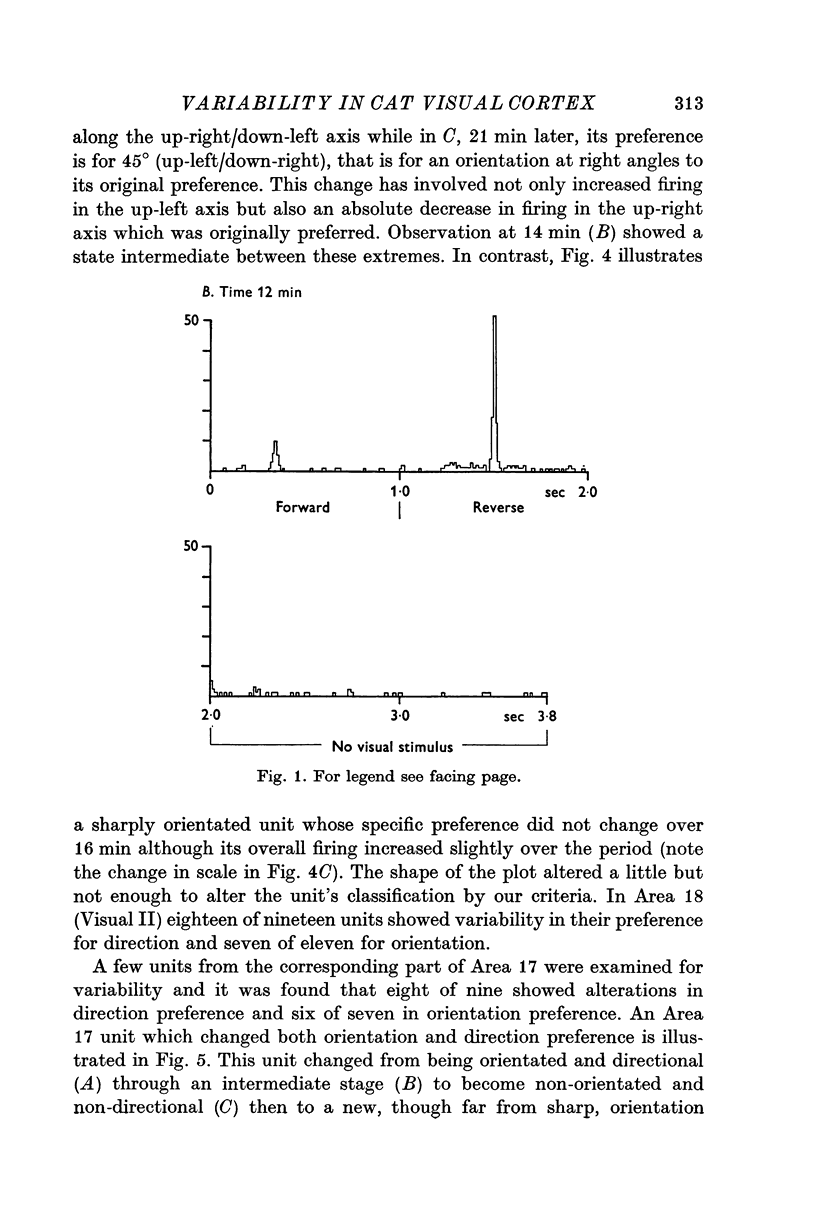
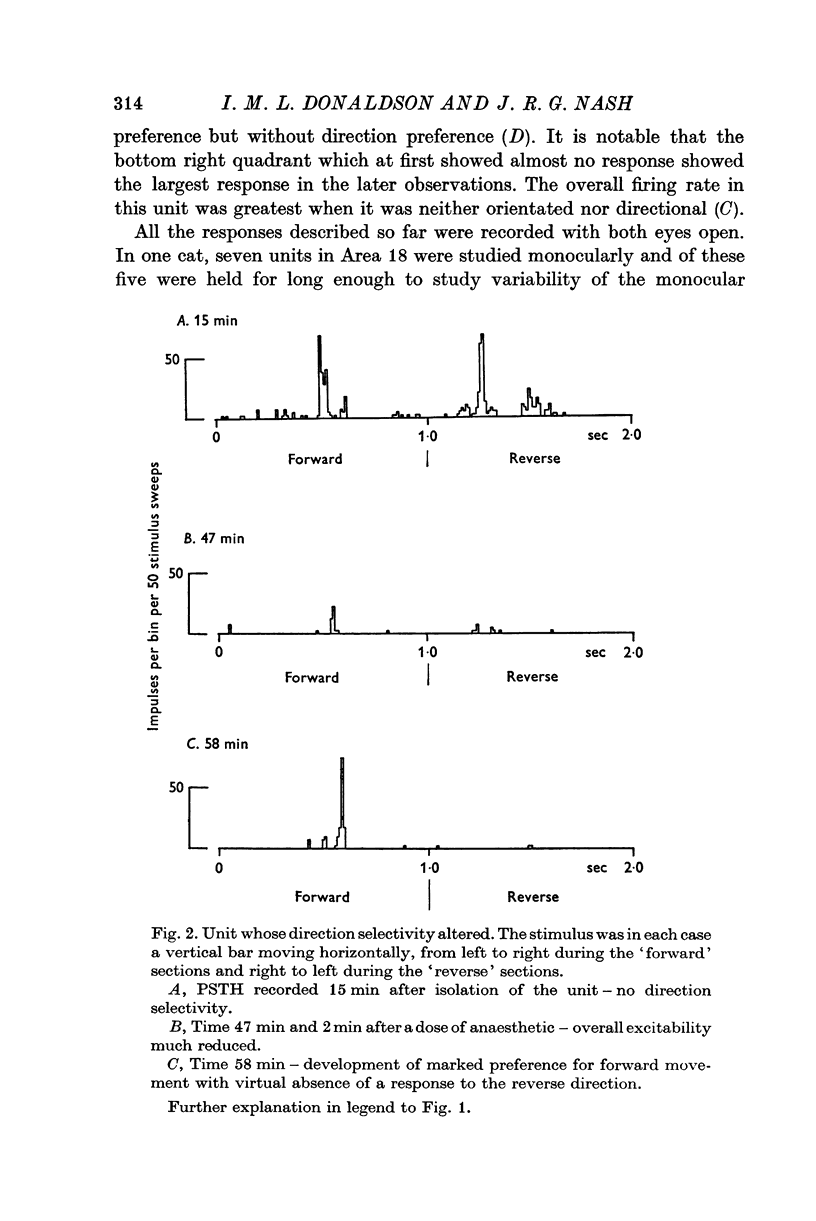
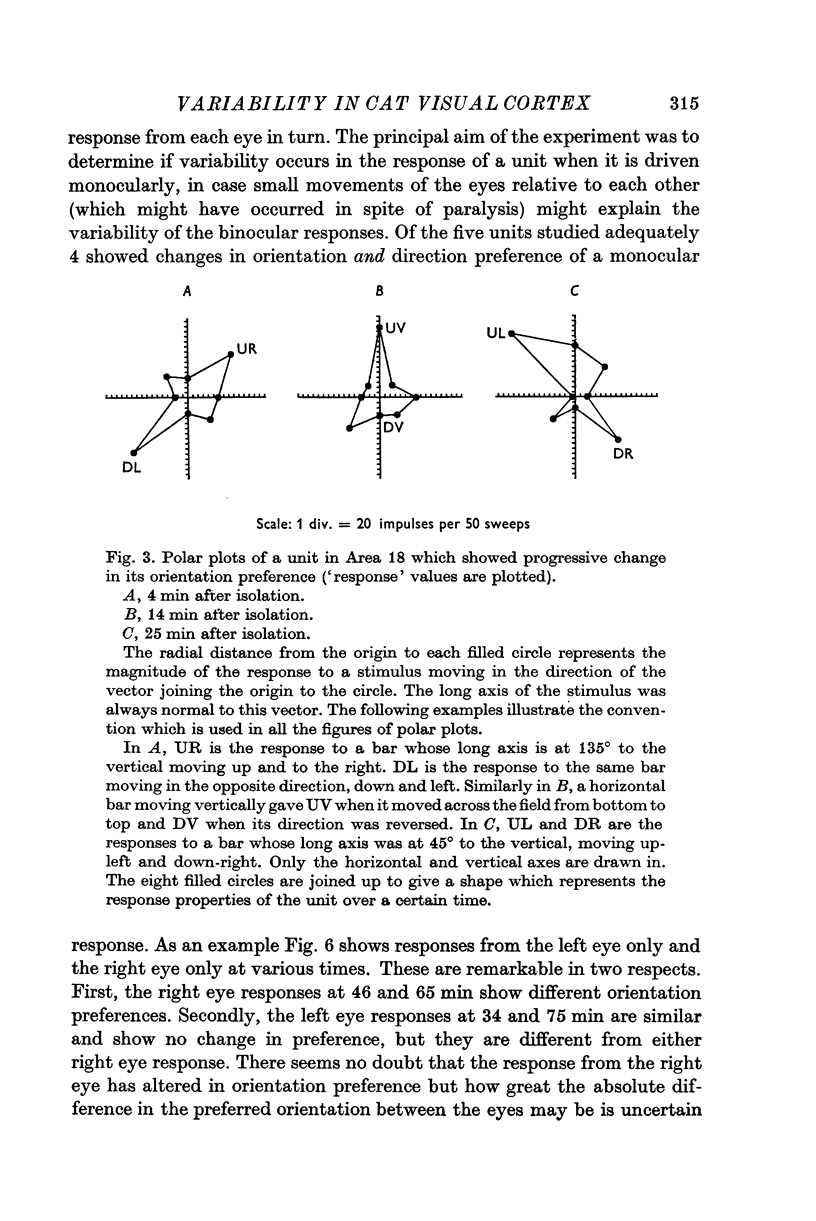
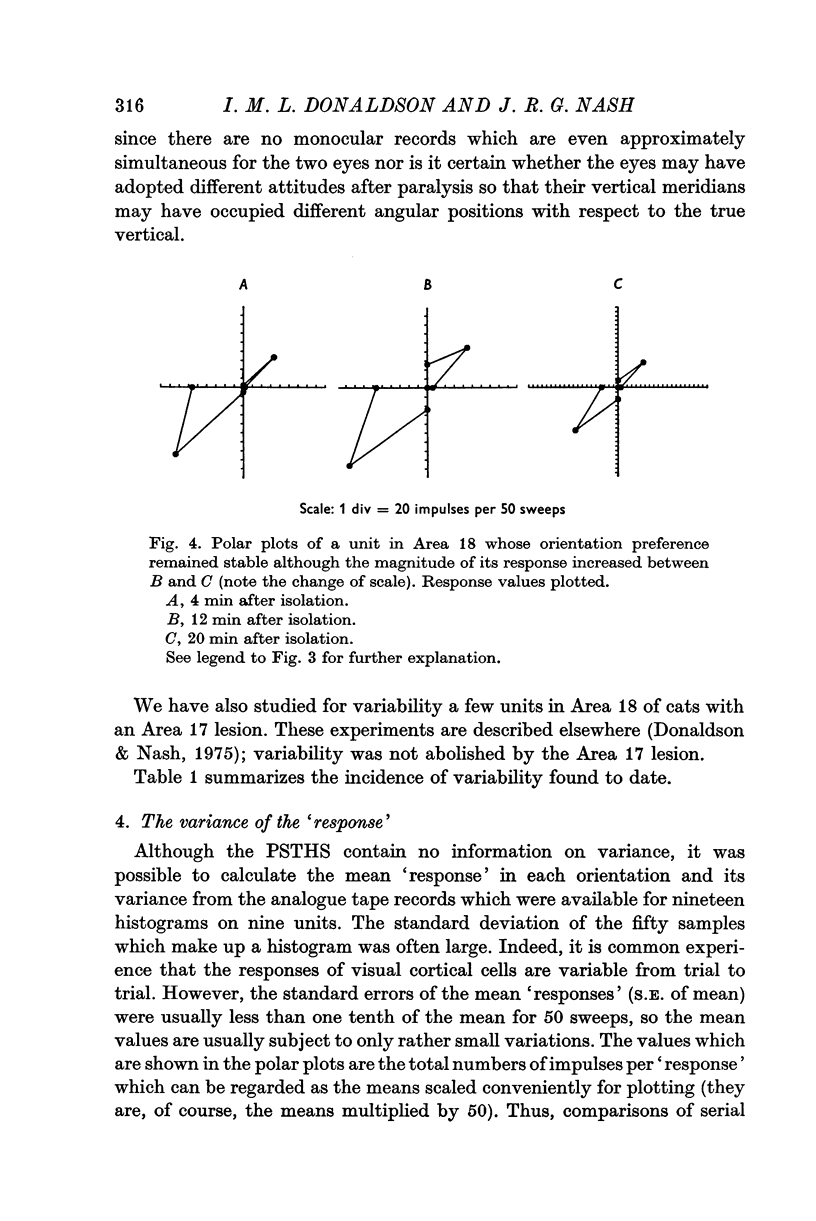
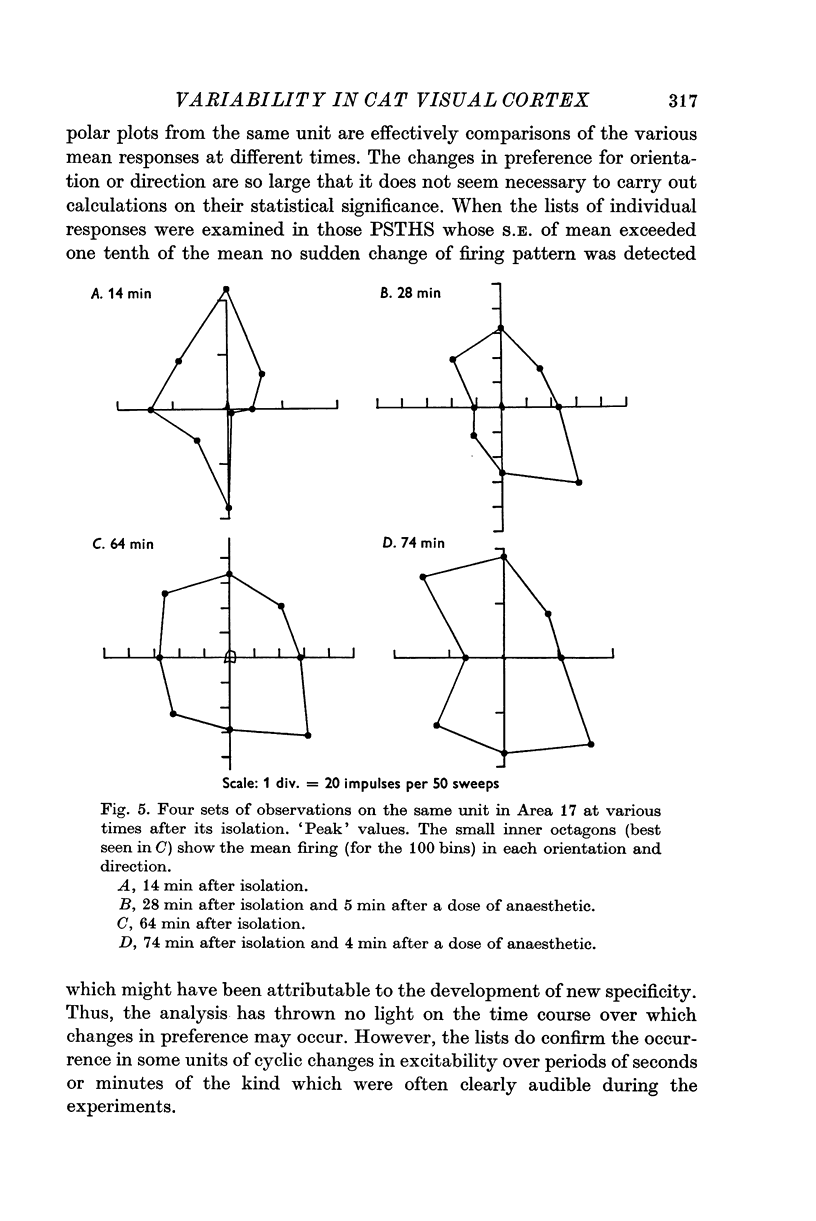
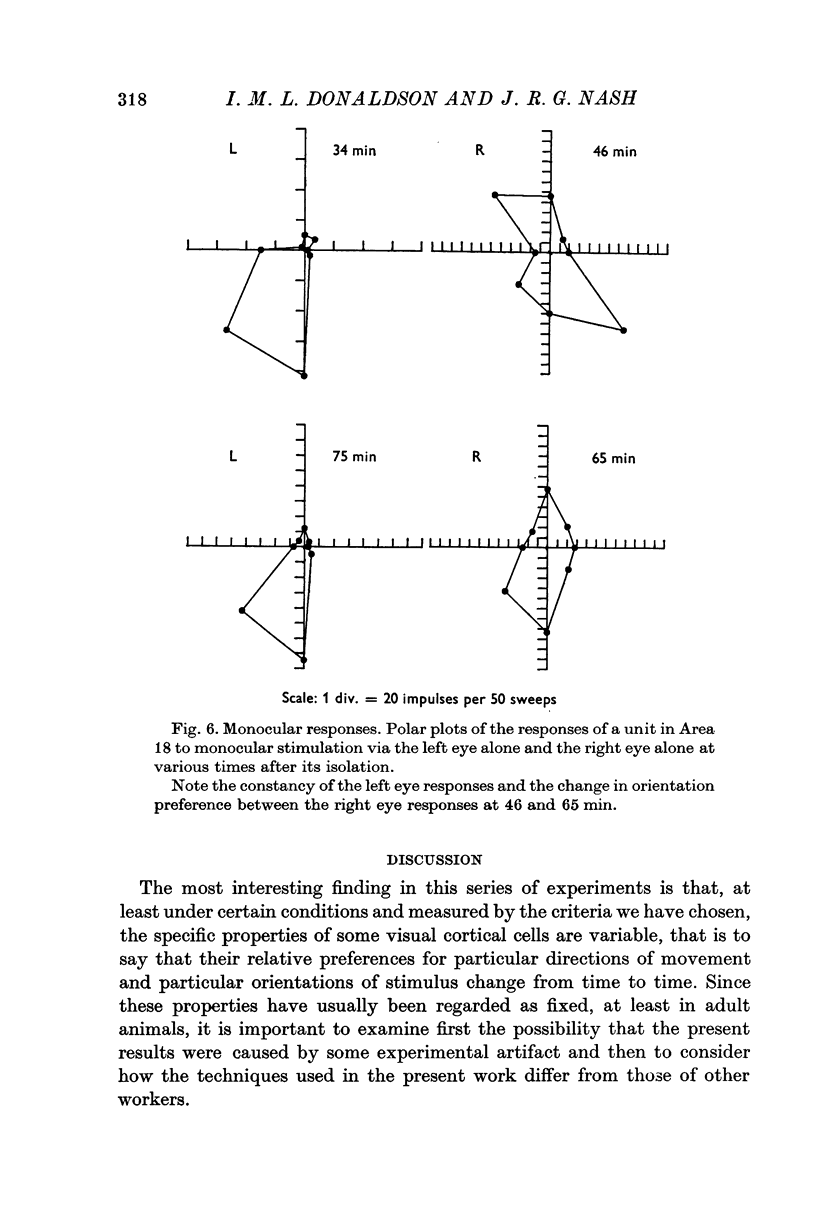
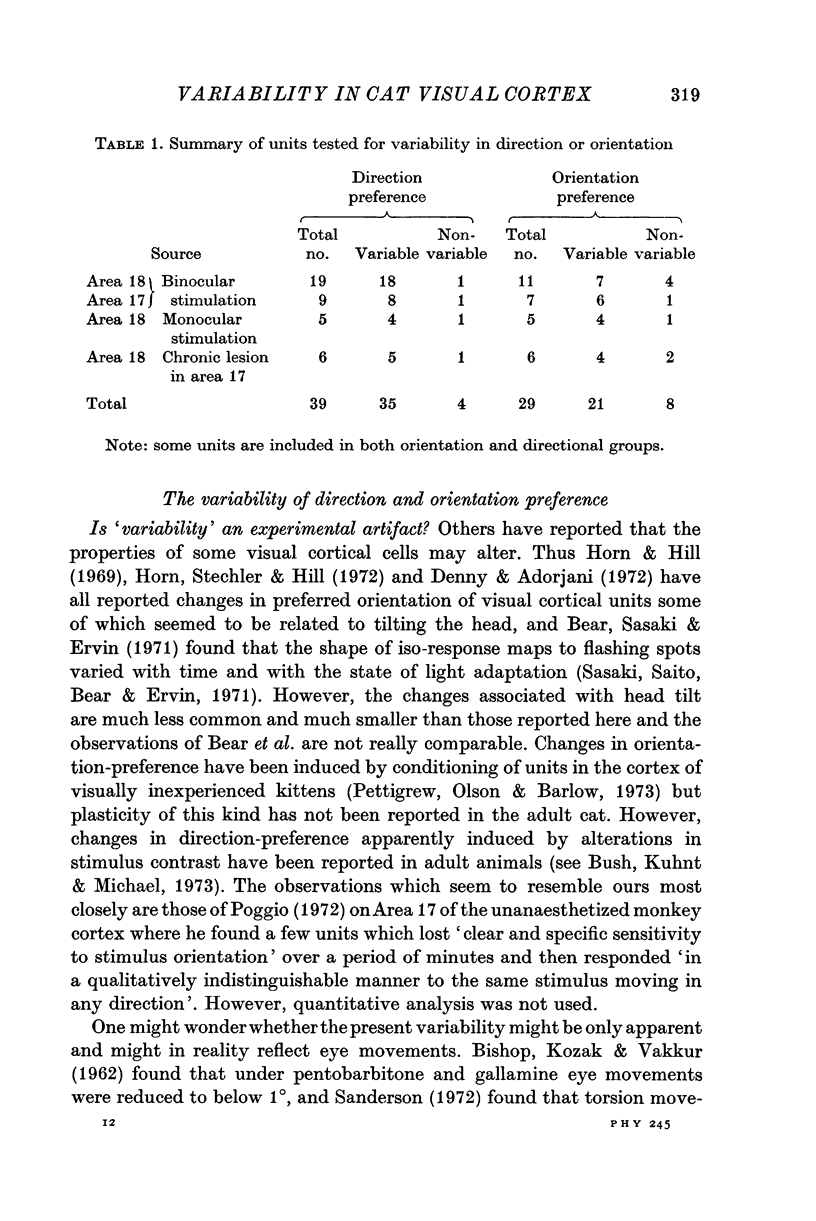
1. The responses to visual stimuli of single units in the cortex of cats anaesthetized with pentobarbitone were recorded extracellularly with glass micropipettes. All had receptive field centres more than 5 degrees and most lay between 5 and 20 degrees from the area centralis. Most units were in Area 18 but some were in the corresponding field representation in Area 17. 2. A quantitative method is described in which the visual stimuli (slits or light bars) were presented repetitively by mechanical means in each of four orientations of the stimulus and in two directions of movement for each orientation. The responses were analysed quantitatively and criteria are described for classification of units according to their preference for particular orientations or directions of movement of the stimulus. 3. Some units were studied continuously for up to 2 hr using the quantitative technique. In Area 18, of nineteen units, eighteen showed changes in their preference for direction of stimulus movement and, in seven of eleven units, the orientation preference changed. In Area 17 direction preference changed in eight of nine units and orientation preference in six of seven. In some cases both orientation and direction preference altered. 4. The relationship of these changes to alterations in the 'spontaneous activity' of the cortical units and to variations in the depth of anaesthesia are considered. Neither would appear to be the sole cause of the phenomenon.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP P. O., KOZAK W., VAKKUR G. J. Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol. 1962 Oct;163:466–502. doi: 10.1113/jphysiol.1962.sp006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear D. M., Sasaki H., Ervin F. R. Sequential change in receptive fields of striate neurons in dark adapted cats. Exp Brain Res. 1971;113(3):256–272. doi: 10.1007/BF00234949. [DOI] [PubMed] [Google Scholar]
- Denney D., Adorjani C. Orientation specificity of visual cortical neurons after head tilt. Exp Brain Res. 1972;14(3):312–317. doi: 10.1007/BF00816165. [DOI] [PubMed] [Google Scholar]
- Donaldson I. M., Nash J. R. The effect of a chronic lesion in cortical area 17 on the visual responses of units in area 18 of the cat. J Physiol. 1975 Feb;245(2):325–332. doi: 10.1113/jphysiol.1975.sp010848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H. Single unit activity in striate cortex of unrestrained cats. J Physiol. 1959 Sep 2;147:226–238. doi: 10.1113/jphysiol.1959.sp006238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O., Tupper R. M., Dreher B. Orientation specificity and response variability of cells in the striate cortex. Vision Res. 1973 Sep;13(9):1771–1779. doi: 10.1016/0042-6989(73)90094-1. [DOI] [PubMed] [Google Scholar]
- Horn G., Hill R. M. Modifications of receptive fields of cells in the visual cortex occurring spontaneously and associated with bodily tilt. Nature. 1969 Jan 11;221(5176):186–188. doi: 10.1038/221186a0. [DOI] [PubMed] [Google Scholar]
- Horn G., Stechler G., Hill R. M. Receptive fields of units in the visual cortex of the cat in the presence and absence of bodily tilt. Exp Brain Res. 1972;15(2):113–132. doi: 10.1007/BF00235577. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. A re-examination of stereoscopic mechanisms in area 17 of the cat. J Physiol. 1973 Jul;232(1):29P–30P. [PubMed] [Google Scholar]
- Nikara T., Bishop P. O., Pettigrew J. D. Analysis of retinal correspondence by studying receptive fields of binocular single units in cat striate cortex. Exp Brain Res. 1968;6(4):353–372. doi: 10.1007/BF00233184. [DOI] [PubMed] [Google Scholar]
- Noda H., Freeman R. B., Jr, Gies B., Creutzfeldt O. D. Neuronal responses in the visual cortex of awake cats to stationary and moving targets. Exp Brain Res. 1971 May 26;112(4):389–405. doi: 10.1007/BF00234494. [DOI] [PubMed] [Google Scholar]
- OTSUKA R., HASSLER R. [On the structure and segmentation of the cortical center of vision in the cat]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1962;203:212–234. doi: 10.1007/BF00352744. [DOI] [PubMed] [Google Scholar]
- Pettigrew J., Olson C., Barlow H. B. Kitten visual cortex: short-term, stimulus-induced changes in connectivity. Science. 1973 Jun 15;180(4091):1202–1203. doi: 10.1126/science.180.4091.1202. [DOI] [PubMed] [Google Scholar]
- Poggio G. F. Spatial properties of neurons in striate cortex of unanesthetized macaque monkey. Invest Ophthalmol. 1972 May;11(5):368–377. [PubMed] [Google Scholar]
- Sanderson K. J. Does rolling of the eye occur in the anaesthetized paralysed cat? Vision Res. 1972 May;12(5):1045–1050. doi: 10.1016/0042-6989(72)90024-7. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Saito Y., Bear D. M., Ervin F. R. Quantitative variation in striate receptive fields of cats as a function of light and dark adaptation. Exp Brain Res. 1971;113(3):273–293. doi: 10.1007/BF00234950. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. Proceedings: Effects of the iontophoretic application of bicuculline on the receptive field properties of simple cells in the visual cortex of the cat. J Physiol. 1974 Oct;242(2):127P–128P. [PubMed] [Google Scholar]


