Abstract
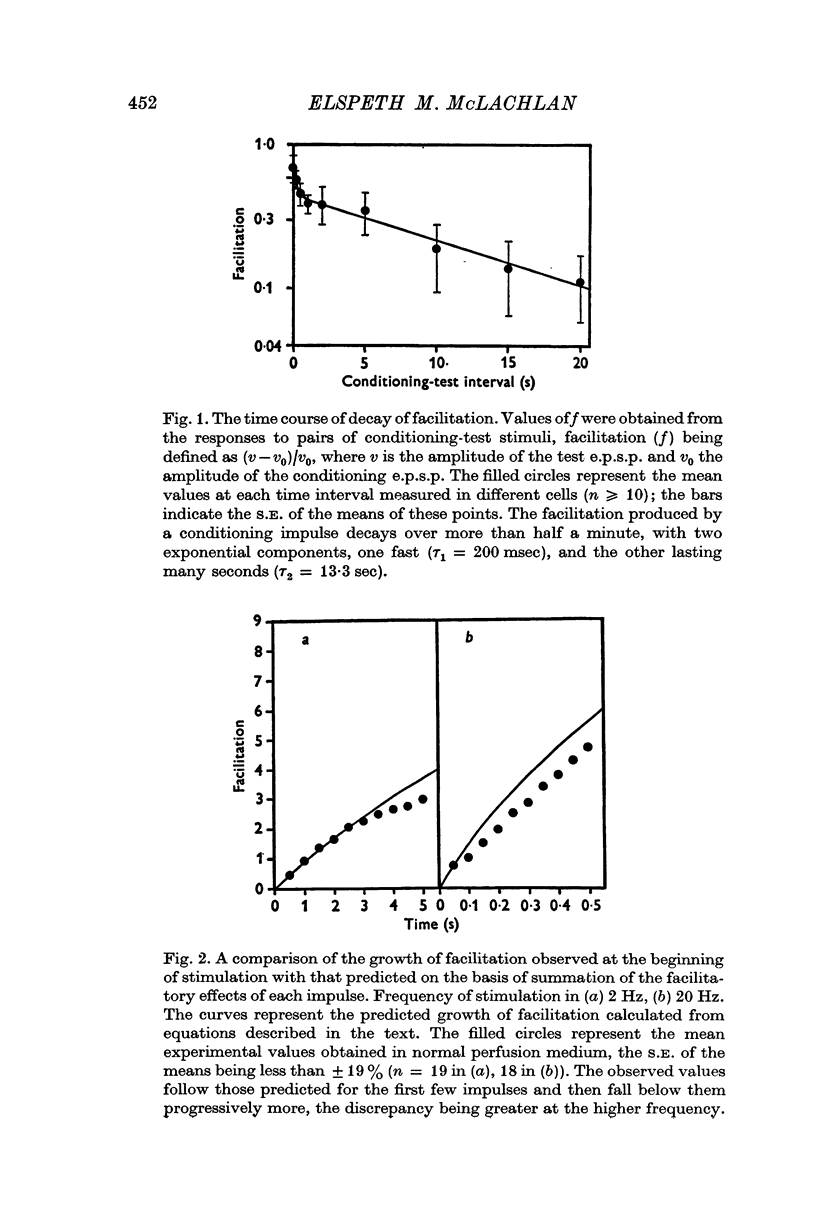
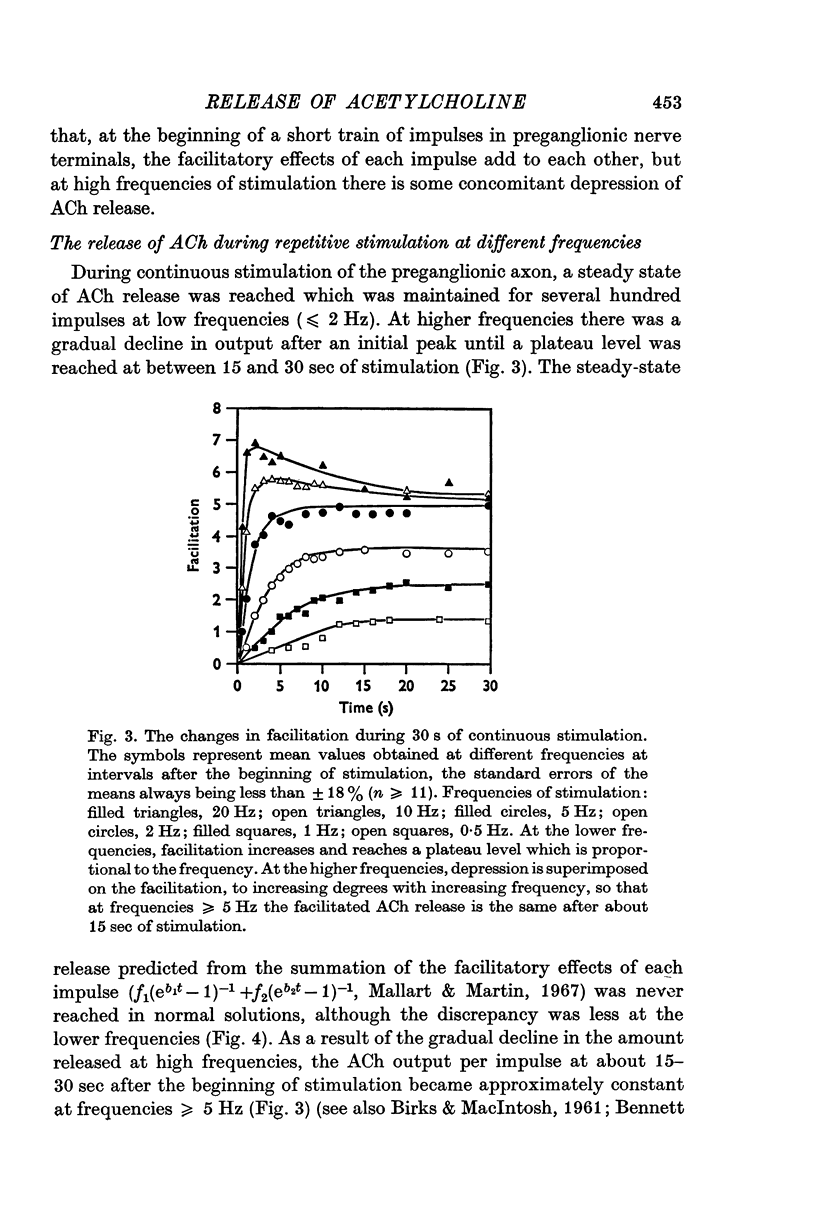
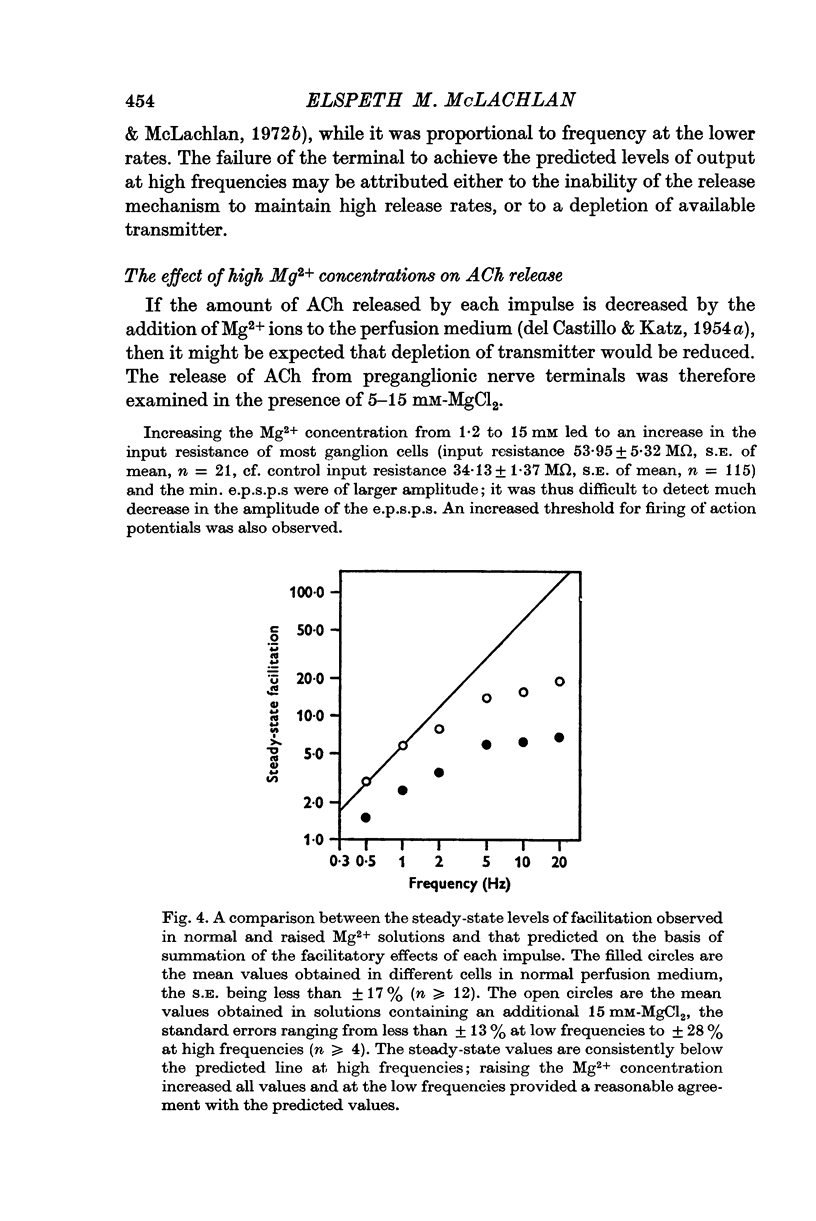
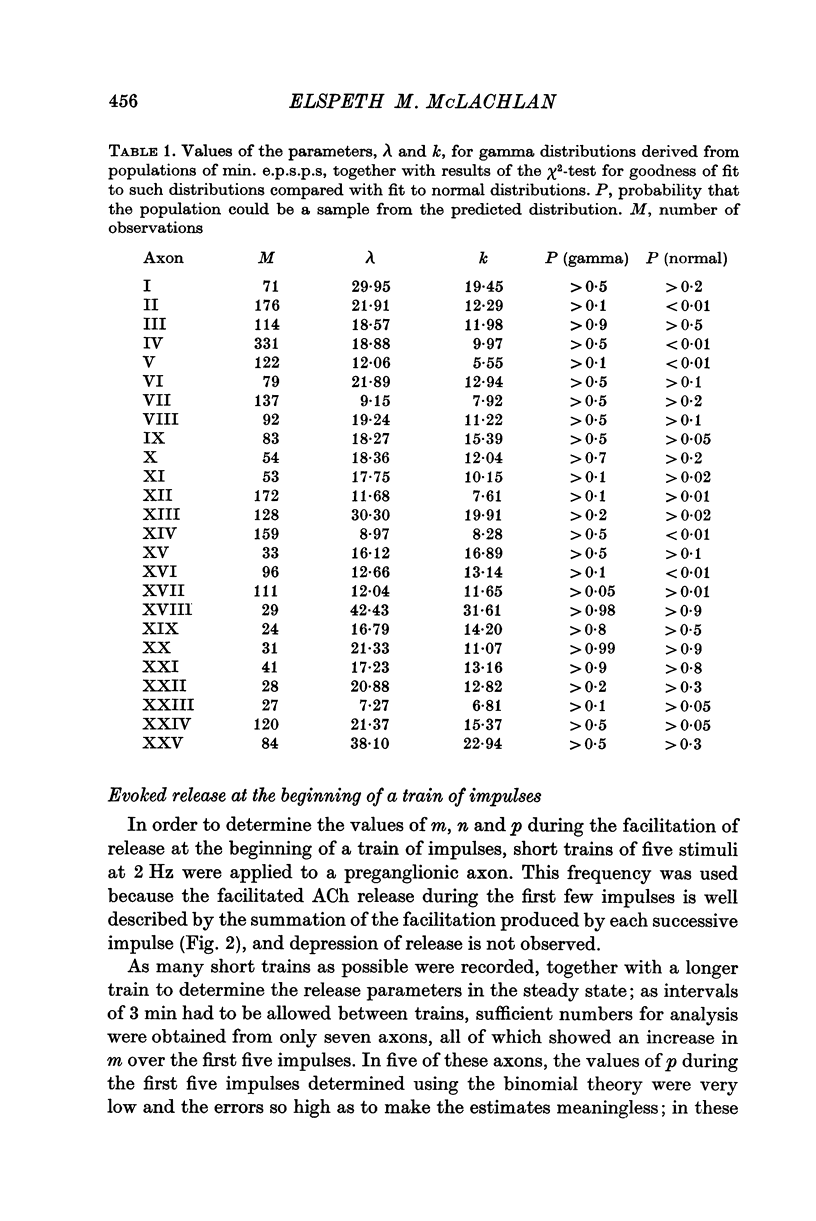
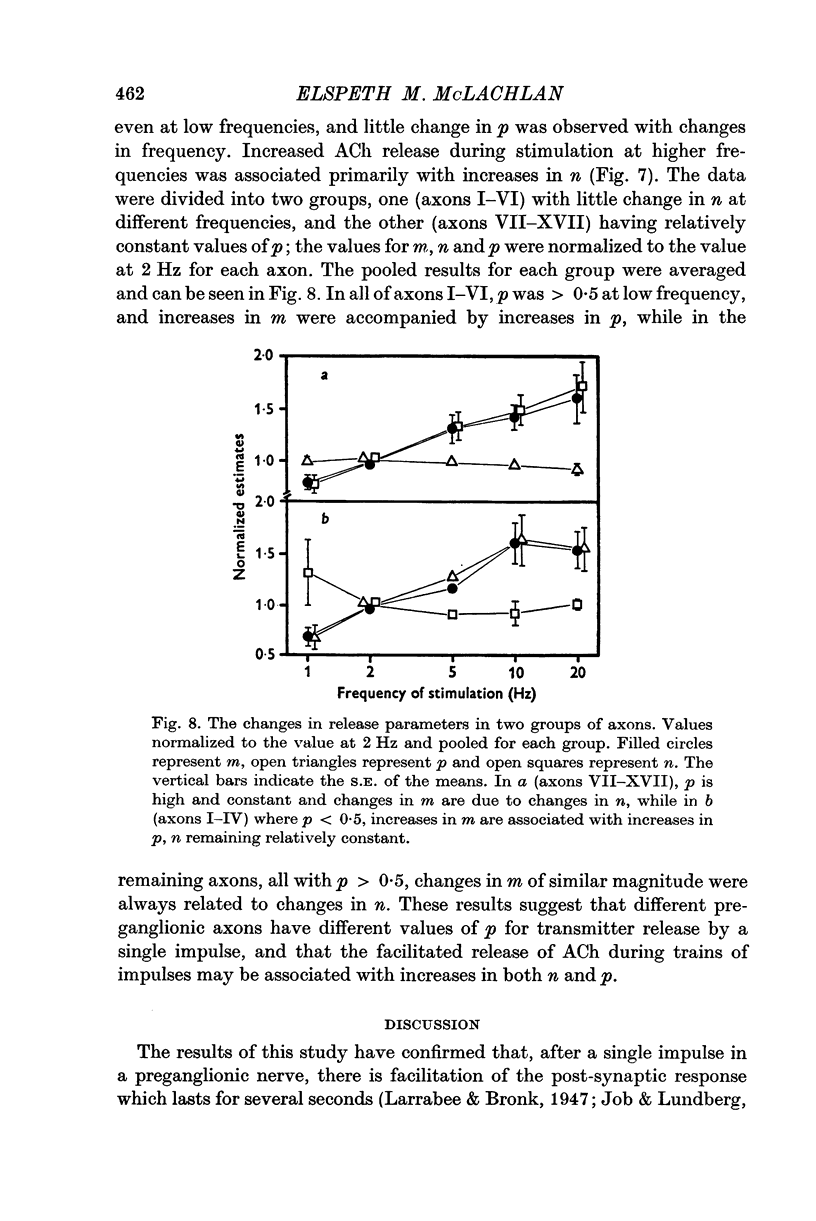
1. A study has been made of the release of acetylcholine (ACh) from the preganglionic nerve terminals of the isolated superior cervical ganglion of the guinea-pig during short trains of impulses, using the amplitude of the excitatory post-synaptic potential (e.p.s.p.) as a measure of the ACh released by each impulse. 2. The time course of decay of facilitation following a single impulse could be described by two exponential components, with T1 = 200 msec, and T2 = 13-3 sec. The increase in ACh output at the beginning of stimulation at frequencies smaller than or equal to 2Hz was reasonably predicted in terms of summation of the individual facilitatory effects of each impulse in the train, but fell short of the prediction at higher frequencies. 3. The steady-state output of ACh during repetitive stimulation at frequencies between 0-5 and 20 Hz was lower than that predicted by summation of the facilitatory effects of each impulse, but reached the predicted level at frequencies smaller than or equal to 2Hz in raised Mg2+ concentrations. 4. Statistical analysis of the quantal content (m) of e.p.s.p.s evoked by each of the first five impulses in a train showed that Poisson statistics described release of ACh at the beginning of a train in most cases; when binomial statistics could be applied (two of seven axons studied), the increase in m was accompanied by an increase in the statistical parameter, n. 5. Analyses were also made of release during continuous stimulation; at the time when the steady state of release was reached, the statistical parameter, p, had also increased. Increased release of ACh at increased frequencies of stimulation was associated with increases in p in axons with p smaller than 0-5; however, in most axons (eleven of seventeen), p was greater than 0-5 in the steady state, and increases in m with frequency were due to increases in n.
Full text
PDF






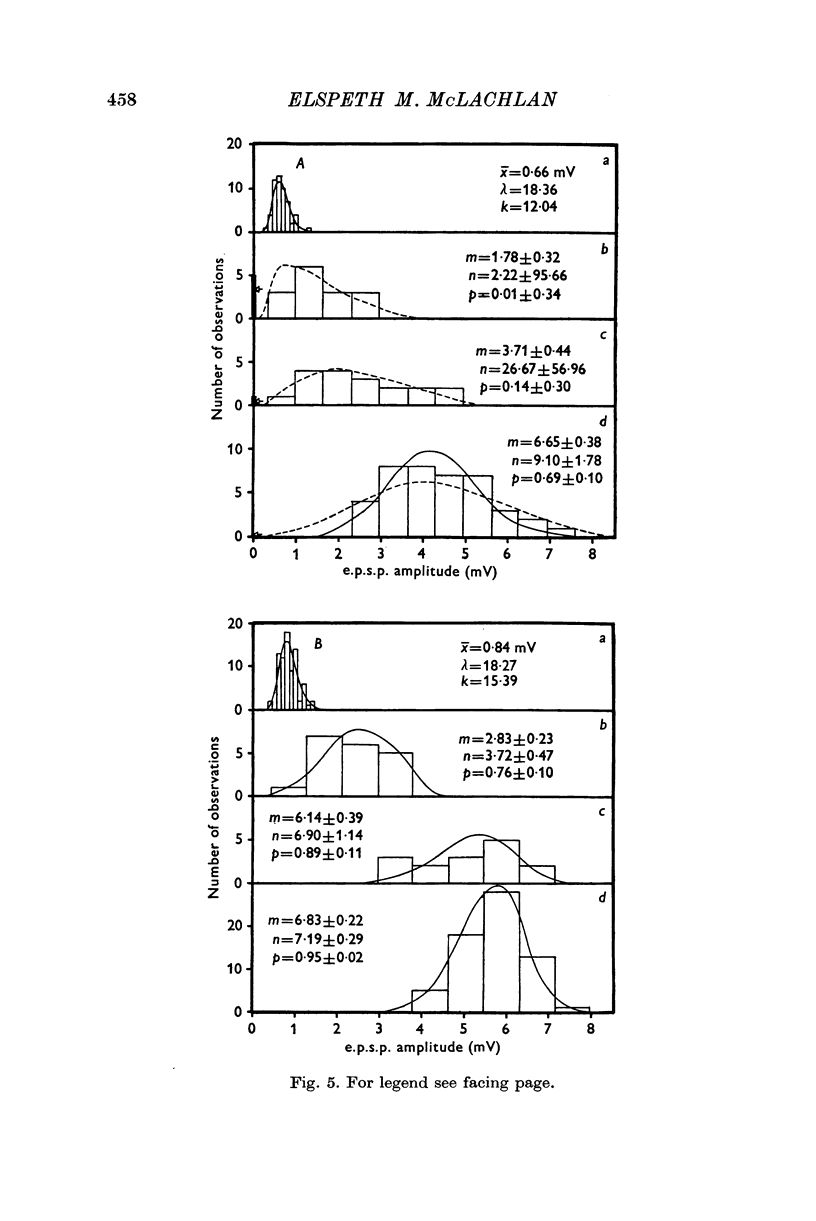
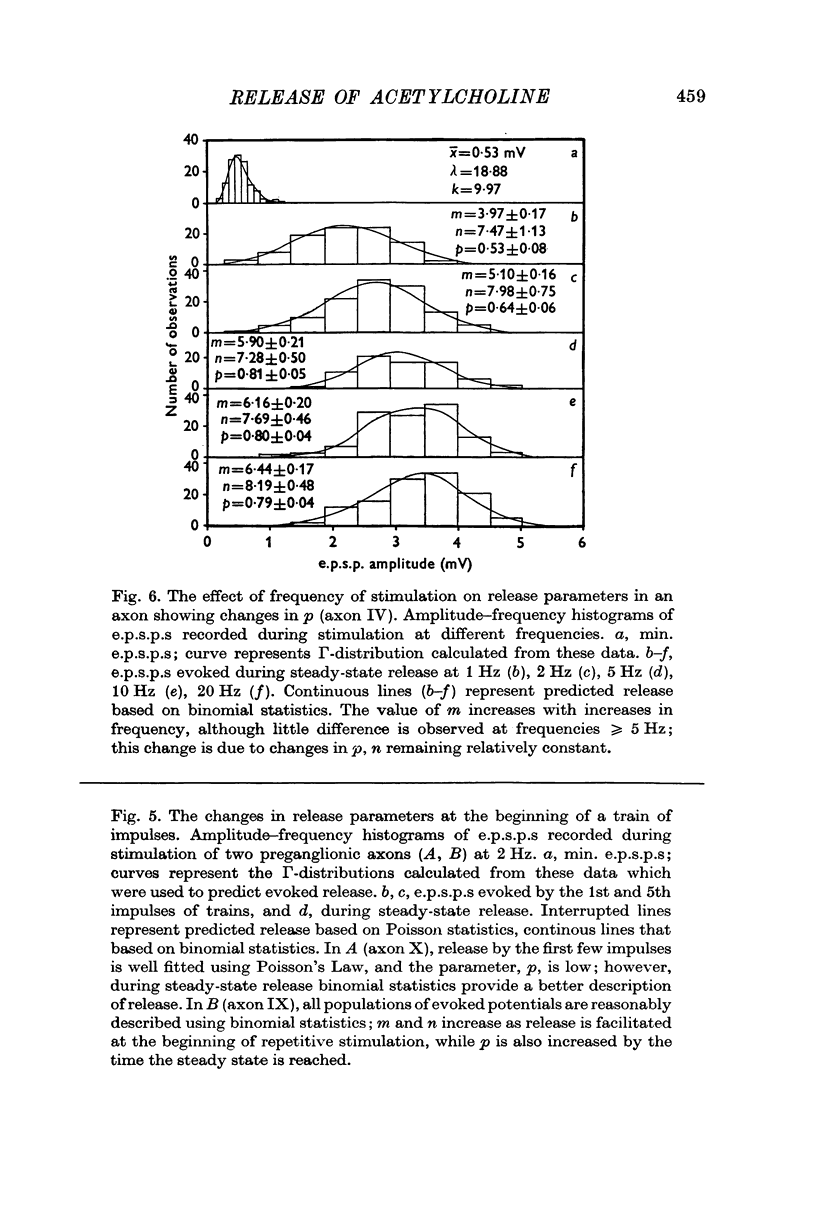
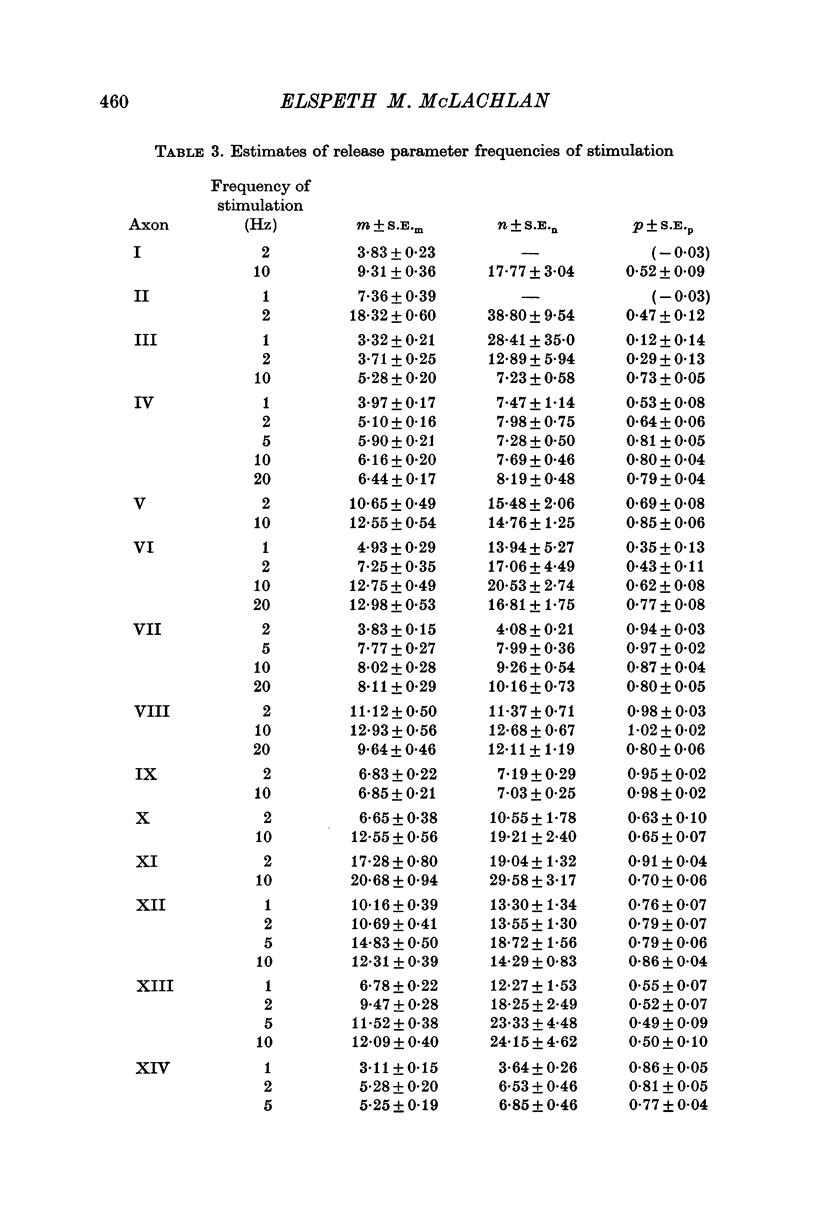
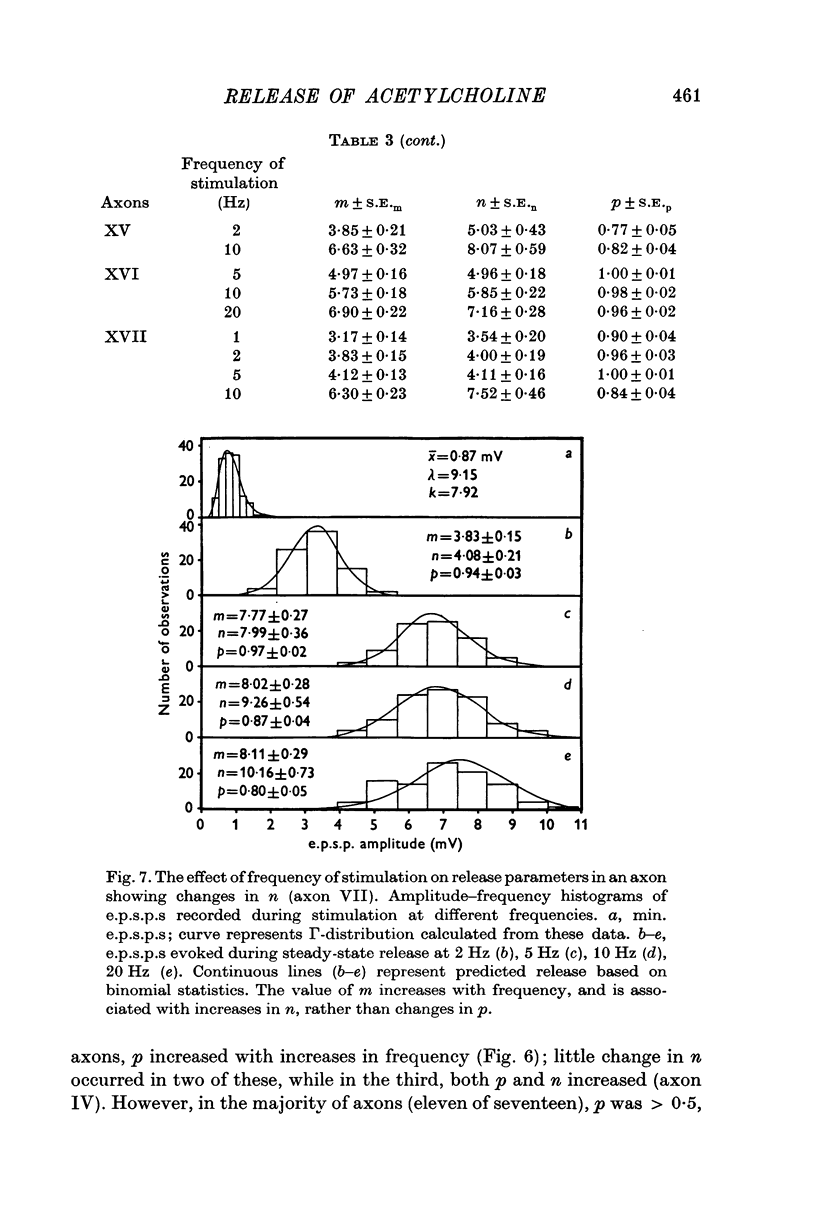




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACKMAN J. G., GINSBORG B. L., RAY C. On the quantal release of the transmitter at a sympathetic synapse. J Physiol. 1963 Jul;167:402–415. doi: 10.1113/jphysiol.1963.sp007158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKMAN J. G., GINSBORG B. L., RAY C. Spontaneous synaptic activity in sympathetic ganglion cells of the frog. J Physiol. 1963 Jul;167:389–401. doi: 10.1113/jphysiol.1963.sp007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. The end-plate potential in mammalian muscle. J Physiol. 1956 Apr 27;132(1):74–91. doi: 10.1113/jphysiol.1956.sp005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. The transmission of excitation from autonomic nerve to smooth muscle. J Physiol. 1961 Jan;155:115–133. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. An electrophysiological analysis of the storage and release of noradrenaline at sympathetic nerve terminals. J Physiol. 1973 Mar;229(2):515–531. doi: 10.1113/jphysiol.1973.sp010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T. A statistical analysis of the release of acetylcholine at newly formed synapses in striated muscle. J Physiol. 1974 Apr;238(1):93–107. doi: 10.1113/jphysiol.1974.sp010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M. An electrophysiological analysis of the storage of acetylcholine in preganglionic nerve terminals. J Physiol. 1972 Mar;221(3):657–668. doi: 10.1113/jphysiol.1972.sp009774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M. An electrophysiological analysis of the synthesis of acetylcholine in preganglionic nerve terminals. J Physiol. 1972 Mar;221(3):669–682. doi: 10.1113/jphysiol.1972.sp009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Purves R. D. Intracellular recordings from ganglia of the thoracic sympathetic chain of the guinea-pig. J Physiol. 1969 Jul;203(1):173–198. doi: 10.1113/jphysiol.1969.sp008858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein J. C. Multiquantal release of acetylcholine in mammalian ganglia. Nature. 1974 Apr 5;248(448):529–531. doi: 10.1038/248529a0. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B. N., Martin A. R. Estimates of probability of transmitter release at the mammalian neuromuscular junction. J Physiol. 1970 Nov;210(4):933–945. doi: 10.1113/jphysiol.1970.sp009250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Mechanism of facilitation at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:530–542. doi: 10.1113/jphysiol.1961.sp006645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- HUBBARD J. I. REPETITIVE STIMULATION AT THE MAMMALIAN NEUROMUSCULAR JUNCTION, AND THE MOBILIZATION OF TRANSMITTER. J Physiol. 1963 Dec;169:641–662. doi: 10.1113/jphysiol.1963.sp007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOB C., LUNDBERG A. On the significance of post- and pre-synaptic events for facilitation and inhibition in the sympathetic ganglion of the cat. Acta Physiol Scand. 1953 Mar 31;28(1):14–28. doi: 10.1111/j.1748-1716.1953.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Johnson E. W., Wernig A. The binomial nature of transmitter release at the crayfish neuromuscular junction. J Physiol. 1971 Nov;218(3):757–767. doi: 10.1113/jphysiol.1971.sp009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Weakly J. N. Facilitation of monosynaptic excitatory synaptic potentials in spinal motoneurones evoked by internuncial impulses. J Physiol. 1972 Jul;224(2):271–286. doi: 10.1113/jphysiol.1972.sp009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Weakly J. N. Quantal components of the inhibitory synaptic potential in spinal mononeurones of the cat. J Physiol. 1972 Jul;224(2):287–303. doi: 10.1113/jphysiol.1972.sp009895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The quantal components of the mammalian end-plate potential. J Physiol. 1956 Sep 27;133(3):571–587. doi: 10.1113/jphysiol.1956.sp005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG A., QUILISCH H. On the effect of calcium on presynaptic potentiation and depression at the neuro-muscular junction. Acta Physiol Scand Suppl. 1953;111:121–129. [PubMed] [Google Scholar]
- LUNDBERG A., QUILISCH H. Presynaptic potentiation and depression of neuromuscular transmission in frog and rat. Acta Physiol Scand Suppl. 1953;111:111–120. [PubMed] [Google Scholar]
- Linder T. M. Calcium and facilitation at two classes of crustacean neuromuscular synapses. J Gen Physiol. 1973 Jan;61(1):56–73. doi: 10.1085/jgp.61.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. PRESYNAPTIC AND POST-SYNAPTIC EVENTS DURING POST-TETANIC POTENTIATION AND FACILITATION IN THE AVIAN CILIARY GANGLION. J Physiol. 1964 Dec;175:17–30. doi: 10.1113/jphysiol.1964.sp007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. QUANTAL COMPONENTS OF THE SYNAPTIC POTENTIAL IN THE CILIARY GANGLION OF THE CHICK. J Physiol. 1964 Dec;175:1–16. doi: 10.1113/jphysiol.1964.sp007499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno T., Edwards C. Neuromuscular facilitation with low-frequency stimulation and effects of some drugs. J Neurophysiol. 1969 Sep;32(5):785–792. doi: 10.1152/jn.1969.32.5.785. [DOI] [PubMed] [Google Scholar]
- Magleby K. L. The effect of repetitive stimulation on facilitation of transmitter release at the frog neuromuscular junction. J Physiol. 1973 Oct;234(2):327–352. doi: 10.1113/jphysiol.1973.sp010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L. The effect of tetanic and post-tetanic potentiation on facilitation of transmitter release at the frog neuromuscular junction. J Physiol. 1973 Oct;234(2):353–371. doi: 10.1113/jphysiol.1973.sp010349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. The relation between quantum content and facilitation at the neuromuscular junction of the frog. J Physiol. 1968 Jun;196(3):593–604. doi: 10.1113/jphysiol.1968.sp008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. The formation of synapses in mammalian sympathetic ganglia reinnervated with preganglionic or somatic nerves. J Physiol. 1974 Feb;237(1):217–242. doi: 10.1113/jphysiol.1974.sp010479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. Early facilitation at corticomotoneuronal synapses. J Physiol. 1970 May;207(3):733–745. doi: 10.1113/jphysiol.1970.sp009091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi O., Perri V. Quantal release of acetylcholine from the nerve endings of the guinea-pig superior cervical ganglion. Pflugers Arch. 1971;329(3):207–219. doi: 10.1007/BF00586615. [DOI] [PubMed] [Google Scholar]
- Wernig A. Changes in statistical parameters during facilitation at the crayfish neuromuscular junction. J Physiol. 1972 Nov;226(3):751–759. doi: 10.1113/jphysiol.1972.sp010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A. The effects of calcium and magnesium on statistical release parameters at the crayfish neuromuscular junction. J Physiol. 1972 Nov;226(3):761–768. doi: 10.1113/jphysiol.1972.sp010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Changes in the statistics of transmitter release during facilitation. J Physiol. 1973 Mar;229(3):787–810. doi: 10.1113/jphysiol.1973.sp010167. [DOI] [PMC free article] [PubMed] [Google Scholar]


