Abstract
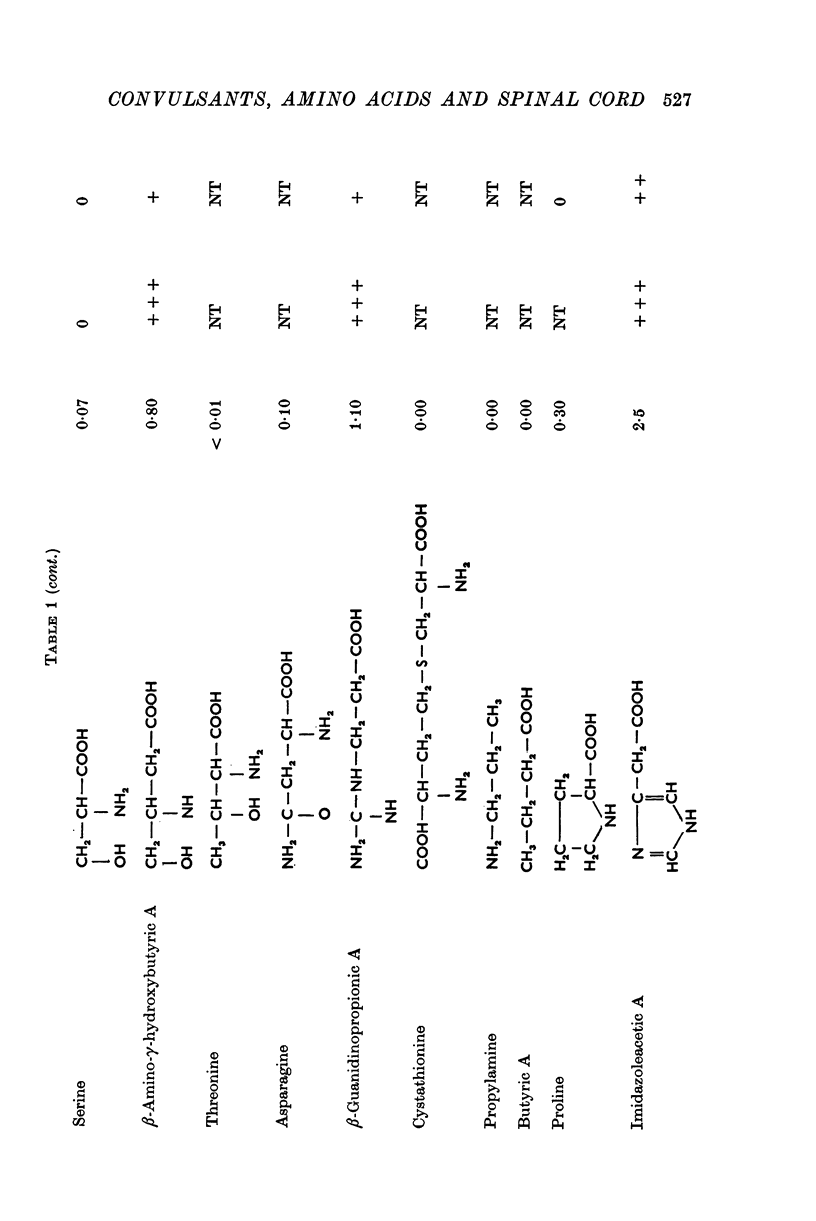
1. The isolated frog spinal cord was used to study the effects of picrotoxin, bicuculline, and strychnine on the responses of primary afferents to amino acids. Recording was by sucrose gap technique. 2. A series of neutral amino acids was found to depolarize primary afferents. Optimal activity was obtained by an amino acid whose carboxyl and amino groups were separated by a three-carbon chain length (i.e. GABA). Amino acids with shorter (i.e. beta-alanine, glycine) or longer (i.e. delta-aminovaleric acid, epsilon-aminocaproic acid) distances between the charged groups were less potent. Imidazoleacetic acid was the most potent depolarizing agent tested. 3. Picrotoxin and bicuculline antagonized the primary afferent depolarizations of a number of amino acids tested with equal specificity. Depolarizing responses to standard (10- minus 3 M) concentrations of beta-alanine and taurine were completely blocked by these convulsants, while depolarizations to 10- minus 3 gamma-aminobutyric acid (GABA) were only partially antagonized. Glycine responses were unaffected by these agentsk; Strychnine completely blocked beta-alanine and taurine depolarizations and incompletely antagonized several other neutral amino acids. GABA, glutamate, and glycine depolarizations were not affected. 5. These results suggest that there are at least three distinct populations of neutral amino acid receptors on primary afferent terminals: a GABA-like receptor, a taurine/beta-alanine receptor, and a glycine-like receptor. The strychnine resistance of the glycine responses indictaes that the primary afferent receptors for glycine differ from those on the somata of spinal neurones.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. L., Nicoll R. A. Gamma-aminobutyric acid: role in primary afferent depolarization. Science. 1972 Jun 2;176(4038):1043–1045. doi: 10.1126/science.176.4038.1043. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A., Padjen A. Studies on convulsants in the isolated frog spinal cord. II. Effects on root potentials. J Physiol. 1975 Mar;245(3):537–548. doi: 10.1113/jphysiol.1975.sp010860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A. The pharmacology and ionic dependency of amino acid responses in the frog spinal cord. J Physiol. 1973 Jan;228(2):259–277. doi: 10.1113/jphysiol.1973.sp010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Brown D. A. Depolarizing actions of gamma-aminobutyric acid and related compounds on rat superior cervical ganglia in vitro. Br J Pharmacol. 1974 Feb;50(2):205–218. doi: 10.1111/j.1476-5381.1974.tb08563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., WATKINS J. C. The excitation and depression of spinal neurones by structurally related amino acids. J Neurochem. 1960 Sep;6:117–141. doi: 10.1111/j.1471-4159.1960.tb13458.x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971 Sep 10;32(1):69–96. doi: 10.1016/0006-8993(71)90156-9. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A., McLennan H. Antagonism between bicuculline and GABA in the cat brain. Brain Res. 1971 Oct 8;33(1):57–73. doi: 10.1016/0006-8993(71)90305-2. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Johnston G. A. The specificity of strychnine as a glycine antagonist in the mammalian spinal cord. Exp Brain Res. 1971 Jun 29;12(5):547–565. doi: 10.1007/BF00234248. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Felix D. The effect of bicuculline upon synaptic inhibition in the cerebral and cerebellar corticles of the cat. Brain Res. 1971 Nov;34(2):301–321. doi: 10.1016/0006-8993(71)90283-6. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Hösli L., Johnston G. A. A pharmacological study of the depression of spinal neurones by glycine and related amino acids. Exp Brain Res. 1968;6(1):1–18. doi: 10.1007/BF00235443. [DOI] [PubMed] [Google Scholar]
- DUDEL J. PRESYNAPTIC AND POSTSYNAPTIC EFFECTS OF INHIBITORY DRUGS ON THE CRAYFISH NEUROMUSCULAR JUNCTION. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965 Mar 18;283:104–118. doi: 10.1007/BF00363182. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A. Gamma-aminobutyric acid antagonism and presynaptic inhibition in the frog spinal cord. Science. 1972 Jan 21;175(4019):331–333. doi: 10.1126/science.175.4019.331. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A. The effects of bicuculline on the isolated spinal cord of the frog. Exp Neurol. 1972 Apr;35(1):179–193. doi: 10.1016/0014-4886(72)90068-4. [DOI] [PubMed] [Google Scholar]
- De Groat W. C., Lalley P. M., Saum W. R. Depolarization of dorsal root ganglia in the cat by GABA and related amino acids: antagonism by picrotoxin and bicuculline. Brain Res. 1972 Sep 15;44(1):273–277. doi: 10.1016/0006-8993(72)90383-6. [DOI] [PubMed] [Google Scholar]
- EDWARDS C., KUFFLER S. W. The blocking effect of gamma-aminobutyric acid (GABA) and the action of related compounds on single nerve cells. J Neurochem. 1959 Apr;4(1):19–30. doi: 10.1111/j.1471-4159.1959.tb13170.x. [DOI] [PubMed] [Google Scholar]
- Florey E., Woodcock B. Presynaptic excitatory action of glutamate applied to crab nerve-muscle preparations. Comp Biochem Physiol. 1968 Aug;26(2):651–661. doi: 10.1016/0010-406x(68)90657-9. [DOI] [PubMed] [Google Scholar]
- Galindo A. GABA-picrotoxin interaction in the mammalian central nervous system. Brain Res. 1969 Aug;14(3):763–767. doi: 10.1016/0006-8993(69)90220-0. [DOI] [PubMed] [Google Scholar]
- Godfraind J. M., Krnjević K., Maretić H., Pumain R. Inhibition of cortical neurones by imidazole and some derivatives. Can J Physiol Pharmacol. 1973 Nov;51(11):790–797. doi: 10.1139/y73-122. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Anderson E. G., Hösli L. Histamine and metabolites: their effects and interactions with convulsants on brain stem neurones. Brain Res. 1973 Mar 15;51:269–278. doi: 10.1016/0006-8993(73)90378-8. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Johnston G. A. GABA uptake in rat central nervous system: comparison of uptake in slices and homogenates and the effects of some inhibitors. J Neurochem. 1971 Oct;18(10):1939–1950. doi: 10.1111/j.1471-4159.1971.tb09600.x. [DOI] [PubMed] [Google Scholar]
- Koketsu K., Karczmar A. G., Kitamura R. Acetylcholine depolarization of the dorsal root nerve terminals in the amphibian spinal cord. Int J Neuropharmacol. 1969 Jul;8(4):329–336. doi: 10.1016/0028-3908(69)90018-5. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Schwartz S. The action of gamma-aminobutyric acid on cortical neurones. Exp Brain Res. 1967;3(4):320–336. doi: 10.1007/BF00237558. [DOI] [PubMed] [Google Scholar]
- NISHI S., SOEDA H., KOKETSU K. EFFECT OF ALKALI-EARTH CATIONS ON FROG SPINAL GANGLION CELL. J Neurophysiol. 1965 May;28:457–472. doi: 10.1152/jn.1965.28.3.457. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Barker J. L. Effect of strychnine on dorsal root potentials and amino acid responses in frog spinal cord. Nat New Biol. 1973 Dec 19;246(155):224–225. doi: 10.1038/newbio246224a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Pharmacological evidence for GABA as the transmitter in granule cell inhibition in the olfactory bulb. Brain Res. 1971 Dec 10;35(1):137–149. doi: 10.1016/0006-8993(71)90600-7. [DOI] [PubMed] [Google Scholar]
- Obata K., Highstein S. M. Blocking by picrotoxin of both vestibular inhibition and GABA action on rabbit oculomotor neurones. Brain Res. 1970 Mar 17;18(3):538–541. doi: 10.1016/0006-8993(70)90136-8. [DOI] [PubMed] [Google Scholar]
- Obata K., Ito M., Ochi R., Sato N. Pharmacological properties of the postsynaptic inhibition by Purkinje cell axons and the action of gamma-aminobutyric acid on deiters NEURONES. Exp Brain Res. 1967;4(1):43–57. doi: 10.1007/BF00235216. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Tebecis A. K., York D. H. Histamine and some antihistamines: their actions on cerebral cortical neurones. Br J Pharmacol Chemother. 1968 Jul;33(3):426–440. doi: 10.1111/j.1476-5381.1968.tb00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swagel M. W., Ikeda K., Roberts E. Effects of GABA, imidazoleacetic acid, and related substances on conductance of crayfish abdominal stretch receptor. Nat New Biol. 1973 Nov 21;246(151):91–92. doi: 10.1038/newbio246091a0. [DOI] [PubMed] [Google Scholar]
- Tebecis A. K., Phillis J. W. The use of convulsants in studying possible functions of amino acids in the toad spinal cord. Comp Biochem Physiol. 1969 Mar;28(3):1303–1315. doi: 10.1016/0010-406x(69)90568-4. [DOI] [PubMed] [Google Scholar]
- Usherwood P. N., Machili P. Chemical transmission at the insect excitatory neuromuscular synapse. Nature. 1966 May 7;210(5036):634–636. doi: 10.1038/210634a0. [DOI] [PubMed] [Google Scholar]
- Werman R., Davidoff R. A., Aprison M. H. Inhibitory of glycine on spinal neurons in the cat. J Neurophysiol. 1968 Jan;31(1):81–95. doi: 10.1152/jn.1968.31.1.81. [DOI] [PubMed] [Google Scholar]


