Abstract
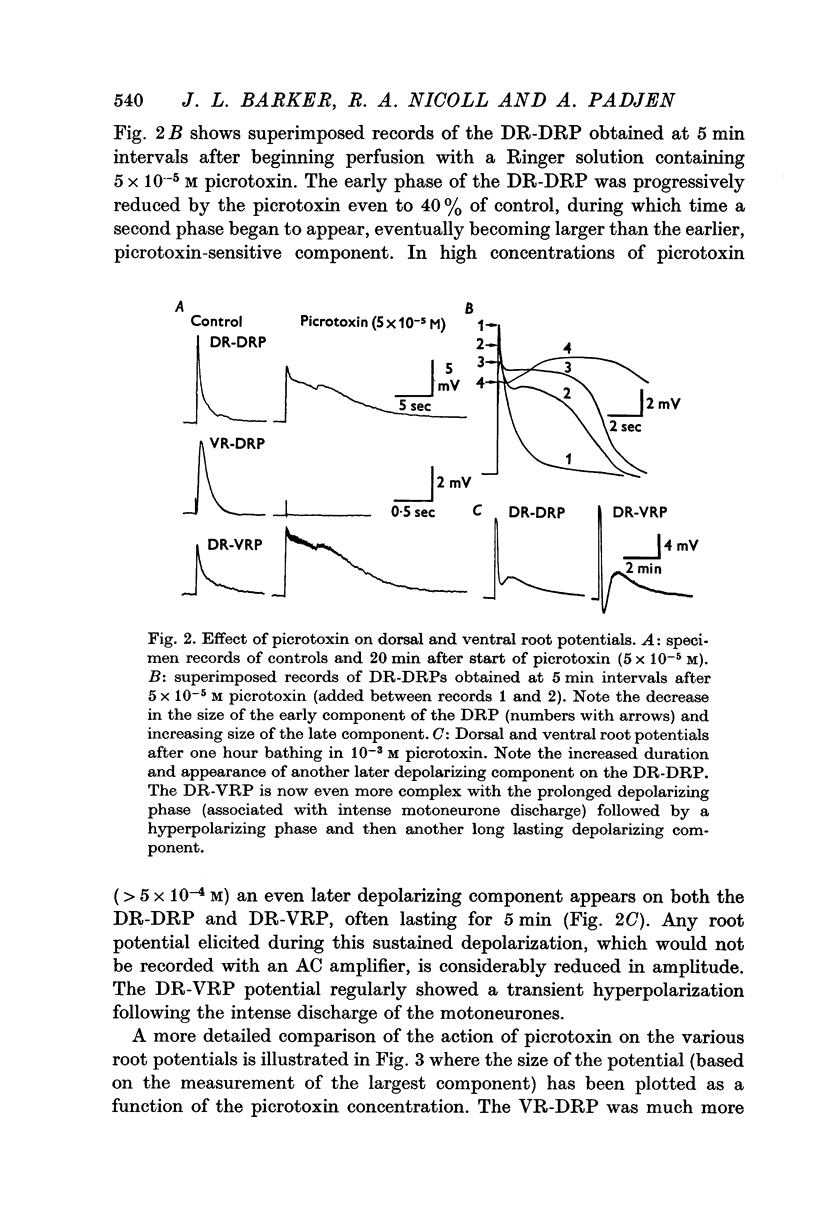
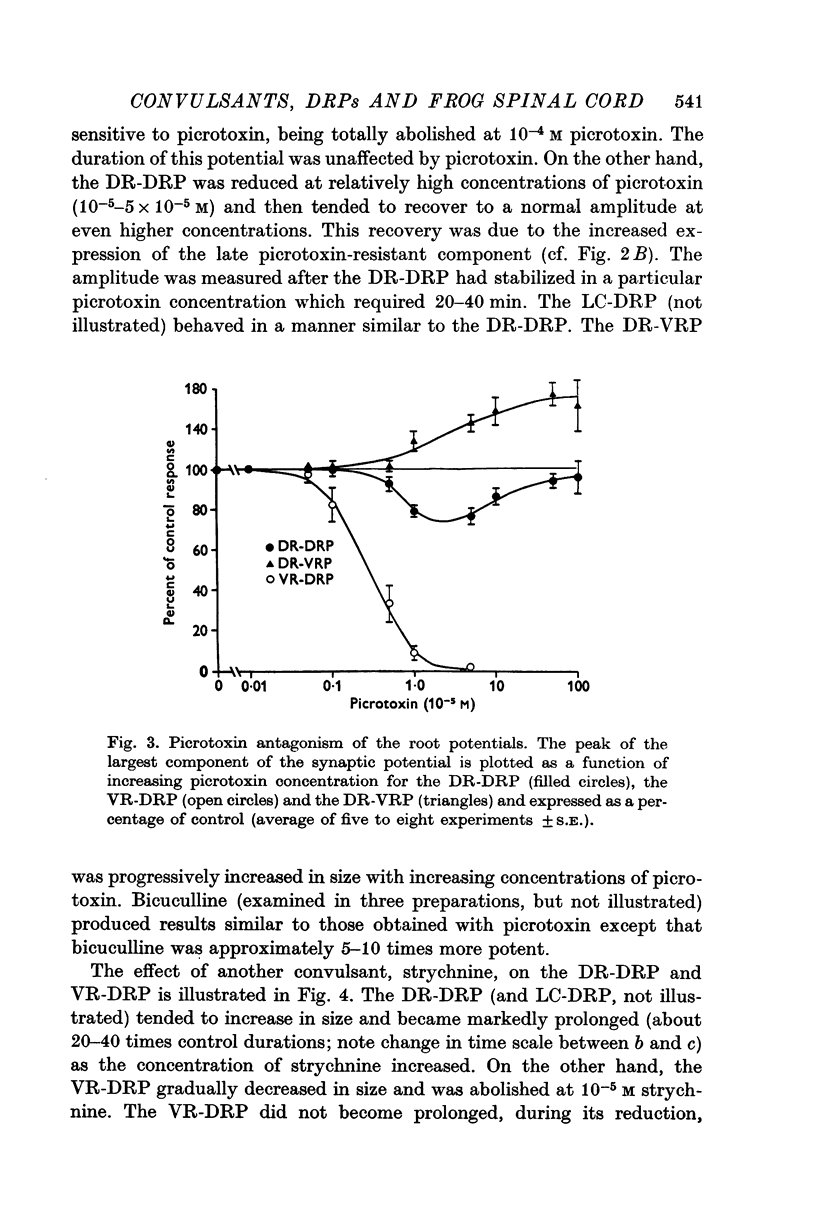
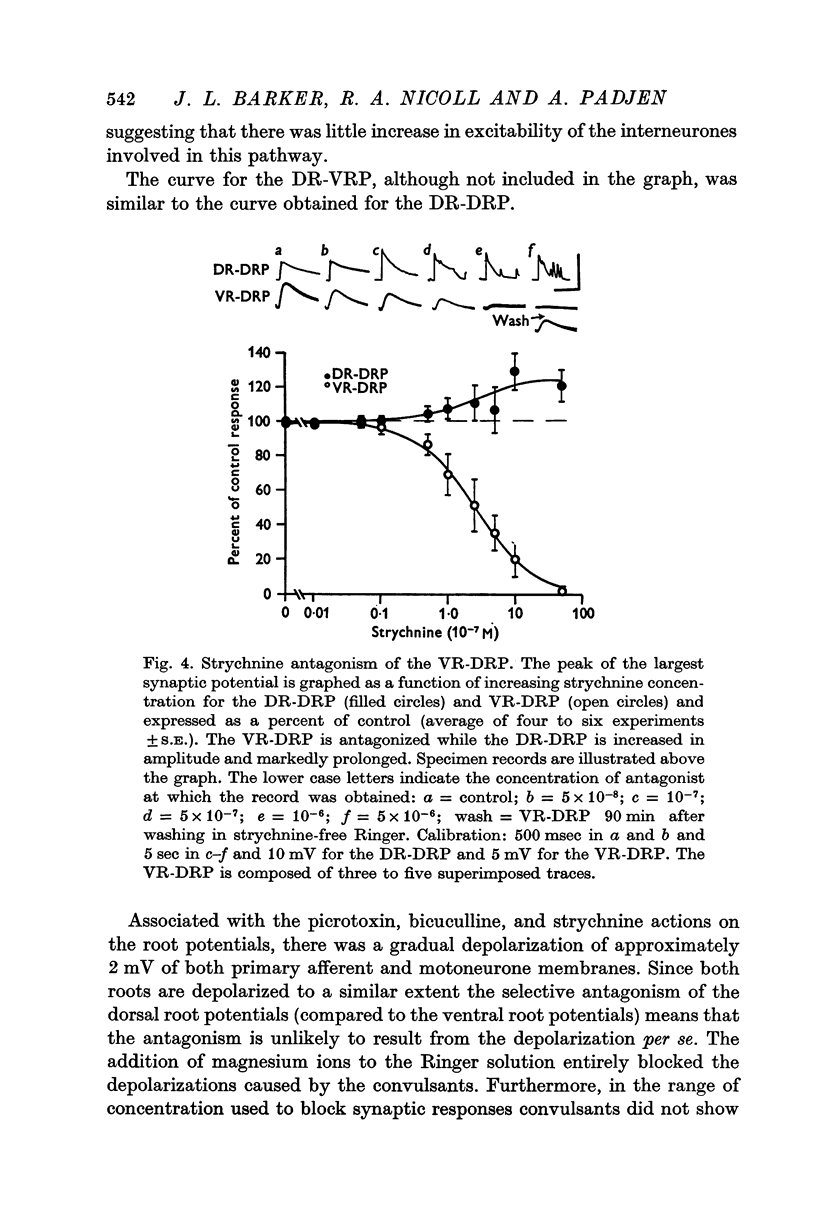
1. In the isolated frog spinal cord picrotoxin, bicuculline, and strychnine were evaluated for their effects on synaptically induced root potentials recorded by the sucrose gap technique. 2. Picrotoxin (greater than 10- minus 4 M) completely blocked the dorsal root potential (DRP) elicited by stimulating the ventral root of the same segment (VR-DRP). Although picrotoxin antagonized the DRP elicited by stimulation of either an adjacent dorsal root (DR-DRP) or the lateral column (LC-DRP), a slower component to these potentials appeared and increased in size as the concentration of picrotoxin was increased. Thus picrotoxin brings out a later, picrotoxin resistant component to the DR-DRP and LC-DRP. 3. Strychnine (10- minus 8-10- minus 5 M) reduced and abolished the VR-DRP without prolongation and progressively increased and prolonged the DR-DRP (and LC-DRP) and the DR-VRP. Strychnine in higher concentrations (greater than 10- minus 4 M) also reduced the amplitude and prolonged the duration of the compound action potential of afferent fibres. 4. These results combined with those presented in the preceding paper (Barker, Nicoll & Padjen, 1975) suggest that (1) a GABA-like transmitter mediates the final step in the DR-DRP and LC-DRP pathways and that (2) either taurine or beta-alanine may mediate the last step in the VR-DRP pathway.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVING B. O. The action of strychnine at cholinergic junctions. Arch Int Pharmacodyn Ther. 1961 Apr 1;131:123–150. [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A. Gamma-aminobutyric acid: role in primary afferent depolarization. Science. 1972 Jun 2;176(4038):1043–1045. doi: 10.1126/science.176.4038.1043. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A., Padjen A. Studies on convulsants in the isolated frog spinal cord. I. Antagonism of amino acid responses. J Physiol. 1975 Mar;245(3):521–536. doi: 10.1113/jphysiol.1975.sp010859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A. The pharmacology and ionic dependency of amino acid responses in the frog spinal cord. J Physiol. 1973 Jan;228(2):259–277. doi: 10.1113/jphysiol.1973.sp010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. A., Anderson E. G. The influence of semicarbazide-induced depletion of -aminobutyric acid on presynaptic inhibition. Brain Res. 1972 Aug 11;43(1):161–169. doi: 10.1016/0006-8993(72)90281-8. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Ryall R. W. Pharmacological studies upon spinal presynaptic fibres. Exp Brain Res. 1966;1(2):195–204. doi: 10.1007/BF00236871. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A. Gamma-aminobutyric acid antagonism and presynaptic inhibition in the frog spinal cord. Science. 1972 Jan 21;175(4019):331–333. doi: 10.1126/science.175.4019.331. [DOI] [PubMed] [Google Scholar]
- Diamond J., Roper S., Yasargil G. M. The membrane effects, and sensitivity to strychnine, of neural inhibition of the Mauthner cell, and its inhibition by glycine and GABA. J Physiol. 1973 Jul;232(1):87–111. doi: 10.1113/jphysiol.1973.sp010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P. Biophysics of junctional transmission. Physiol Rev. 1954 Oct;34(4):674–710. doi: 10.1152/physrev.1954.34.4.674. [DOI] [PubMed] [Google Scholar]
- Freeman A. R. Electrophysiological analysis of the actions of strychnine, bicuculline and picrotoxin on the axonal membrane. J Neurobiol. 1973;4(6):567–582. doi: 10.1002/neu.480040609. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D. A study of the interaction between motoneurones in the frog spinal cord. J Physiol. 1966 Feb;182(3):612–648. doi: 10.1113/jphysiol.1966.sp007841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell A. D. Electrical interaction between antidromically stimulated frog motoneurones and dorsal root afferents: enhancement by gallamine and TEA. J Physiol. 1970 Sep;210(1):17–43. doi: 10.1113/jphysiol.1970.sp009194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRALY J. K., PHILLIS J. W. Action of some drugs on the dorsal root potentials of the isolated toad spinal cord. Br J Pharmacol Chemother. 1961 Oct;17:224–231. doi: 10.1111/j.1476-5381.1961.tb01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee M. R., Faber D. S., Heiss W. D. Strychnine- and pentylenetetrazol-induced changes of excitability in aplysia neurons. Science. 1973 Mar 16;179(4078):1133–1136. doi: 10.1126/science.179.4078.1133. [DOI] [PubMed] [Google Scholar]
- Koketsu K., Karczmar A. G., Kitamura R. Acetylcholine depolarization of the dorsal root nerve terminals in the amphibian spinal cord. Int J Neuropharmacol. 1969 Jul;8(4):329–336. doi: 10.1016/0028-3908(69)90018-5. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Morris M. E. Extracellular K + activity and slow potential changes in spinal cord and medulla. Can J Physiol Pharmacol. 1972 Dec;50(12):1214–1217. doi: 10.1139/y72-177. [DOI] [PubMed] [Google Scholar]
- Kríz N., Syková E., Ujec E., Vyklický L. Changes of extracellular potassium concentration induced by neuronal activity in the sinal cord of the cat. J Physiol. 1974 Apr;238(1):1–15. doi: 10.1113/jphysiol.1974.sp010507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau E. M. The effect of strychnine on the neuro-muscular junction of the rat. Life Sci. 1967 Dec 1;6(23):2515–2517. doi: 10.1016/0024-3205(67)90315-3. [DOI] [PubMed] [Google Scholar]
- MARUHASHI J., OTANI T., TAKAHASHI H., YAMADA M. On the effects of strychnine upon the myelinated nerve fibres of toads. Jpn J Physiol. 1956 Jun 15;6(2):175–189. doi: 10.2170/jjphysiol.6.175. [DOI] [PubMed] [Google Scholar]
- Meij H. S., Holemans K. C. Inhibitory interaction between motoneurons of adjacent segments in the frog spinal cord. Exp Neurol. 1969 Feb;23(2):174–186. doi: 10.1016/0014-4886(69)90054-5. [DOI] [PubMed] [Google Scholar]
- Miyata Y., Otsuka M. Distribution of -aminobutyric acid in cat spinal cord and the alteration produced by local ischaemia. J Neurochem. 1972 Jul;19(7):1833–1834. doi: 10.1111/j.1471-4159.1972.tb06233.x. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Barker J. L. Effect of strychnine on dorsal root potentials and amino acid responses in frog spinal cord. Nat New Biol. 1973 Dec 19;246(155):224–225. doi: 10.1038/newbio246224a0. [DOI] [PubMed] [Google Scholar]
- Roberts P. J., Mitchell J. F. The release of amino acids from the hemisected spinal cord during stimulation. J Neurochem. 1972 Nov;19(11):2473–2481. doi: 10.1111/j.1471-4159.1972.tb01307.x. [DOI] [PubMed] [Google Scholar]
- SCHMIDT R. F. PHARMACOLOGICAL STUDIES ON THE PRIMARY AFFERENT DEPOLARIZATION OF THE TOAD SPINAL CORD. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963 Jul 2;277:325–346. doi: 10.1007/BF00362515. [DOI] [PubMed] [Google Scholar]
- Somjen G. G., Lothman E. W. Potassium, sustained focal potential shifts, and dorsal root potentials of the mammalian spinal cord. Brain Res. 1974 Mar 29;69(1):153–157. doi: 10.1016/0006-8993(74)90382-5. [DOI] [PubMed] [Google Scholar]
- Tebecis A. K., Phillis J. W. The use of convulsants in studying possible functions of amino acids in the toad spinal cord. Comp Biochem Physiol. 1969 Mar;28(3):1303–1315. doi: 10.1016/0010-406x(69)90568-4. [DOI] [PubMed] [Google Scholar]
- Vyklicky L., Sykova E., Kriz N., Ujec E. Post-stimulation changes of extracellular potassium concentration in the spinal cord of the rat. Brain Res. 1972 Oct 27;45(2):608–611. doi: 10.1016/0006-8993(72)90492-1. [DOI] [PubMed] [Google Scholar]
- WALL P. D., MCCULLOCH W. S., LETTVIN J. Y., PITTS W. H. Effects of strychnine with special reference to spinal afferent fibres. Epilepsia. 1955 Nov;4:29–40. doi: 10.1111/j.1528-1157.1955.tb03171.x. [DOI] [PubMed] [Google Scholar]



