Abstract
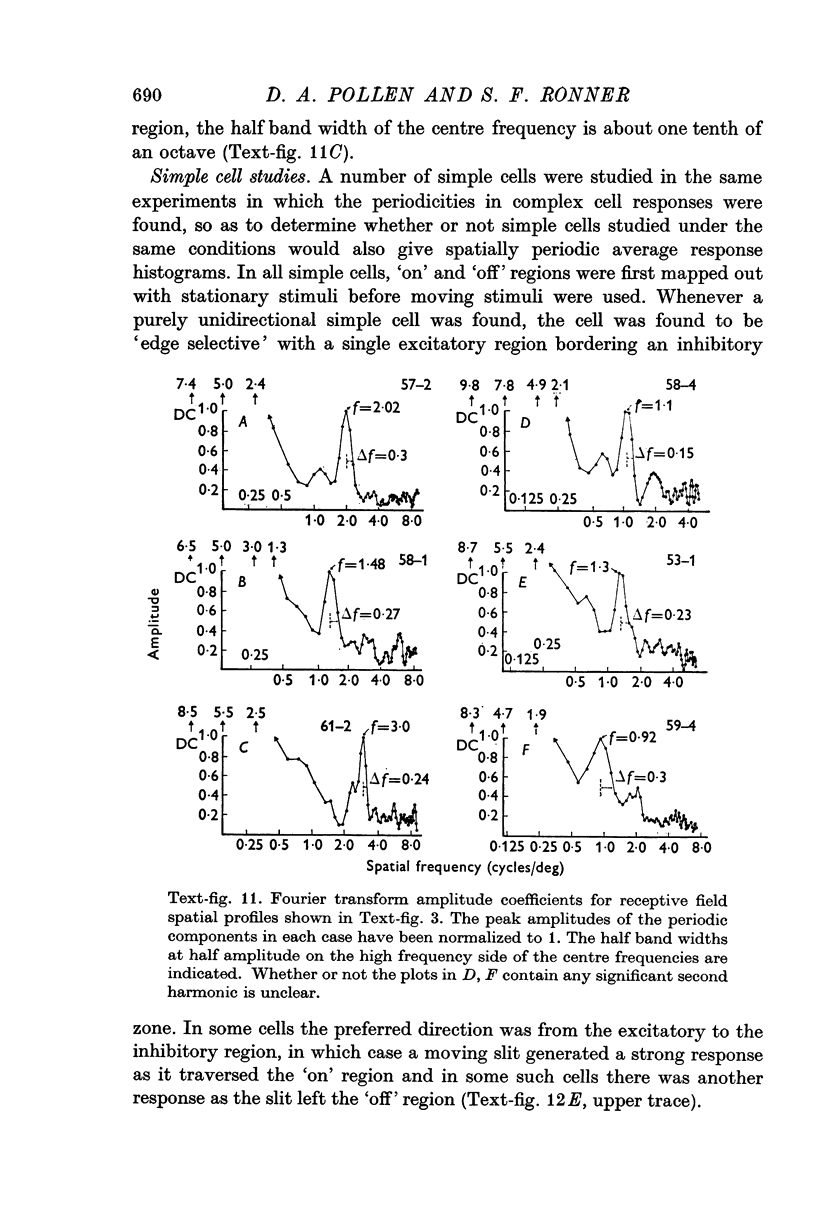
1. Complex cells in cortical areas 17 and 18 of the cat have been studied in response to narrow slits and edges moving across the receptive field in the preferred direction and also to stationary slits of different widths. 2. Average response histograms, recorded as a narrow slit was moved across the receptive field, displayed a periodic series of peaks above a base line level. The response histogram for most area 17 and 18 cells contained five principal peaks; sometimes one or two weaker peaks were present at receptive field borders. The histogram for one cell located at the area 17-18 border showed thirteen distinct peaks. Periodic response patterns were also generated as an extended edge was moved across the receptive field. Plots of cell responses versus slit width for stationary slits of different widths also indicated periodic response pattern. 3. The accuracy of determining the preferred slit orientation was the single most important requirement for demonstrating the periodic response pattern. Significant changes in the appearance of the periodic pattern occurred even upon 5 degrees rotations away from the preferred orientation. 4. Average response histograms were also studied over a wide range of moving slit velocities. The number of peaks across corresponding spacings within the recewptive field remained constant over a range of velocities. Response amplitudes, however, were velocity dependent. Thus the response peaks remain associated with fixed positions within visual space independent of stimulus velocity, even though temporal as well as spatial factors may be involved in response selectivity and the periodic modulation. The most striking periodic response histograms were generated at the velocities which produced the greatest cell firing rates. Area 17 complex cells responded well to velocities of less than 0-5 degrees to 6-0 degrees/sec, but cells in area 18 generally required higher velocities, sometimes as high as 20 degrees--30 degrees/sec, for a good response. 5. Spatial frequencies for the periodic component of the receptive field for area 17 cells in the central visual area covered a range of three octaves up to 5 cycles/degree, and area 18 cells included another octave on the low frequency side. The spatial frequency of a cell was found to be roughly inversely proportional to the receptive field width. Only a small sample of area 18 cells was studied, but these cells tended to represent low spatial frequencies and to respond selectively to high velocity stimuli...
Full text
PDF






























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. O., Coombs J. S., Henry G. H. Responses to visual contours: spatio-temporal aspects of excitation in the receptive fields of simple striate neurones. J Physiol. 1971 Dec;219(3):625–657. doi: 10.1113/jphysiol.1971.sp009681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Campbell F. W. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. J Physiol. 1969 Jul;203(1):237–260. doi: 10.1113/jphysiol.1969.sp008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Cooper G. F., Enroth-Cugell C. The spatial selectivity of the visual cells of the cat. J Physiol. 1969 Jul;203(1):223–235. doi: 10.1113/jphysiol.1969.sp008861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer V. D., Ivanoff V. A., Tscherbach T. A. Investigation of complex and hypercomplex receptive fields of visual cortex of the cat as spatial frequency filters. Vision Res. 1973 Oct;13(10):1875–1904. doi: 10.1016/0042-6989(73)90061-8. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959 Oct;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K. P., Stone J. Conduction velocity of afferents to cat visual cortex: a correlation with cortical receptive field properties. Brain Res. 1971 Sep 24;32(2):460–466. doi: 10.1016/0006-8993(71)90340-4. [DOI] [PubMed] [Google Scholar]
- Hubel D. H. Tungsten Microelectrode for Recording from Single Units. Science. 1957 Mar 22;125(3247):549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Cortical and callosal connections concerned with the vertical meridian of visual fields in the cat. J Neurophysiol. 1967 Nov;30(6):1561–1573. doi: 10.1152/jn.1967.30.6.1561. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Res. 1973 Jul;13(7):1255–1267. doi: 10.1016/0042-6989(73)90201-0. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- Pollen D. A., Lee J. R., Taylor J. H. How does the striate cortex begin the reconstruction of the visual world? Science. 1971 Jul 2;173(3991):74–77. doi: 10.1126/science.173.3991.74. [DOI] [PubMed] [Google Scholar]
- SCHADE O. H., Sr Optical and photoelectric analog of the eye. J Opt Soc Am. 1956 Sep;46(9):721–739. doi: 10.1364/josa.46.000721. [DOI] [PubMed] [Google Scholar]
- Venes J. L., Collins W. F., Taub A. Nitrous oxide: an anesthetic for experiments in cats. Am J Physiol. 1971 Jun;220(6):2028–2031. doi: 10.1152/ajplegacy.1971.220.6.2028. [DOI] [PubMed] [Google Scholar]




