Abstract
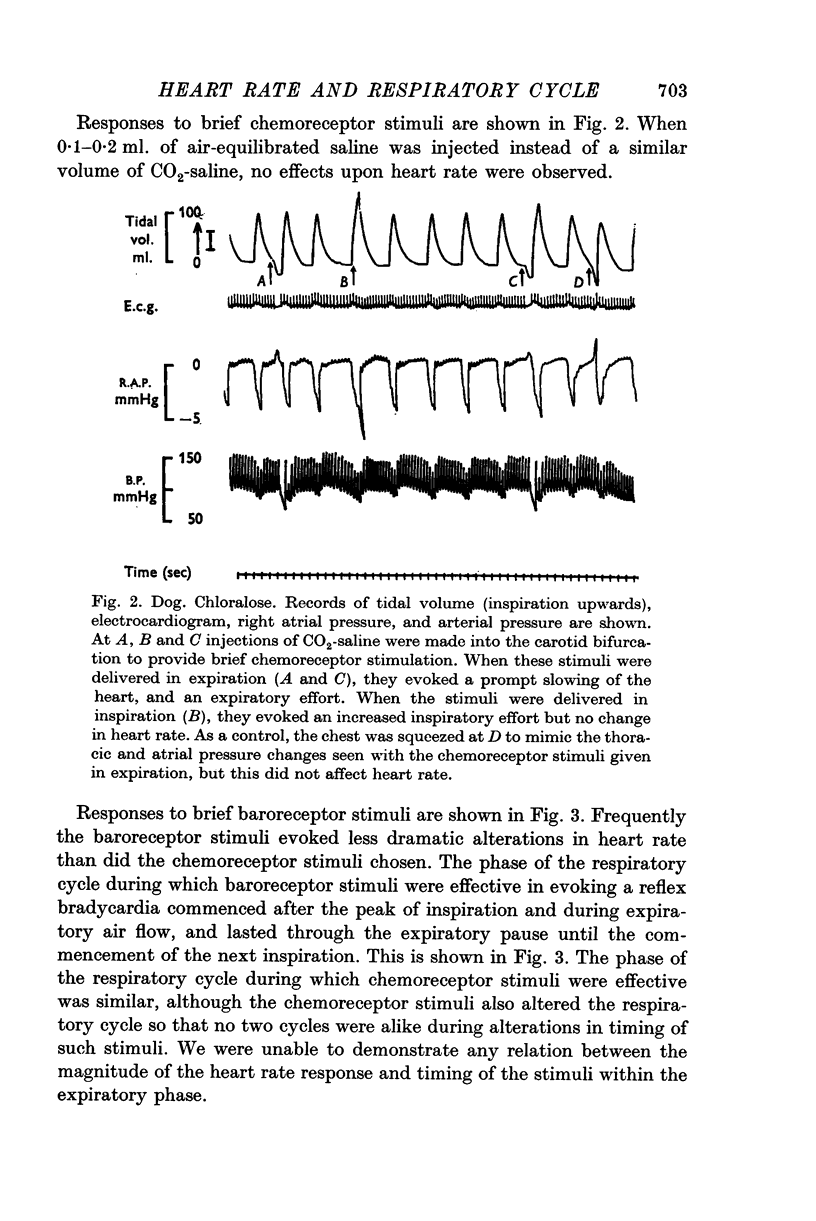
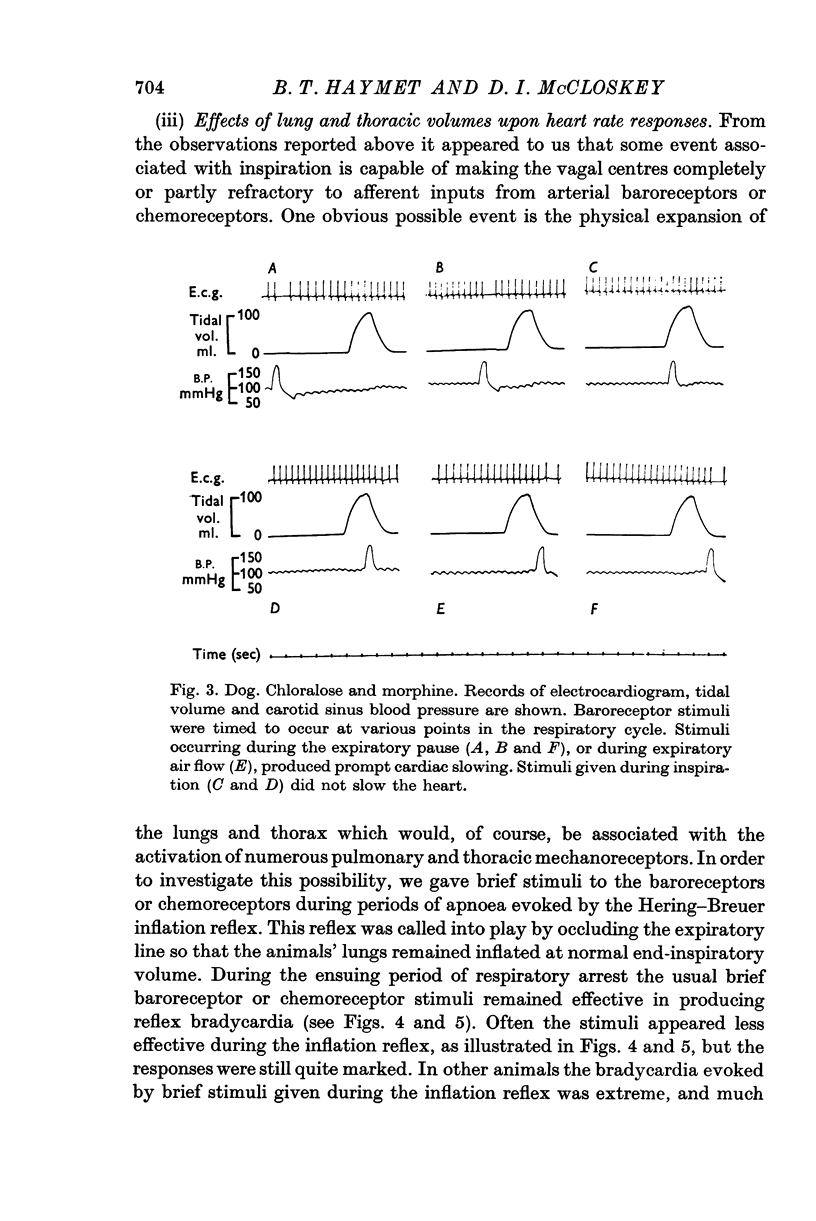
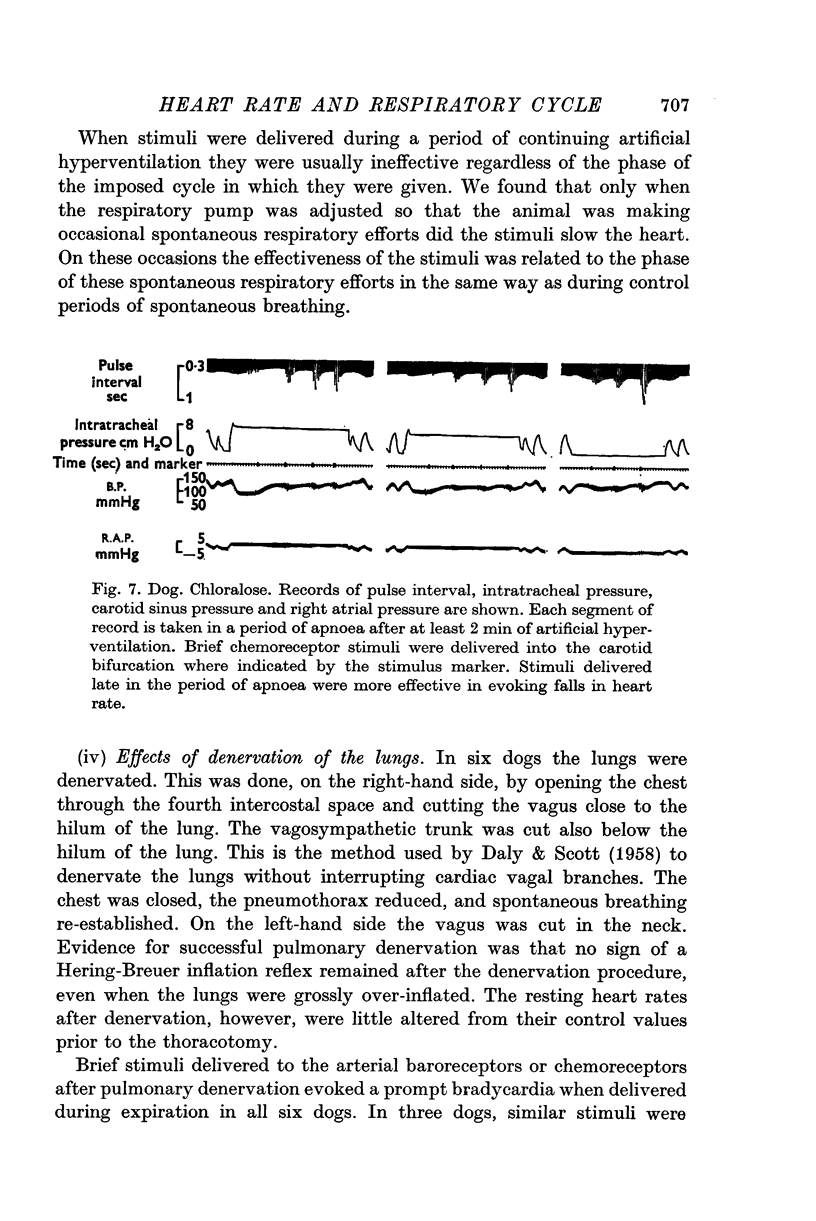
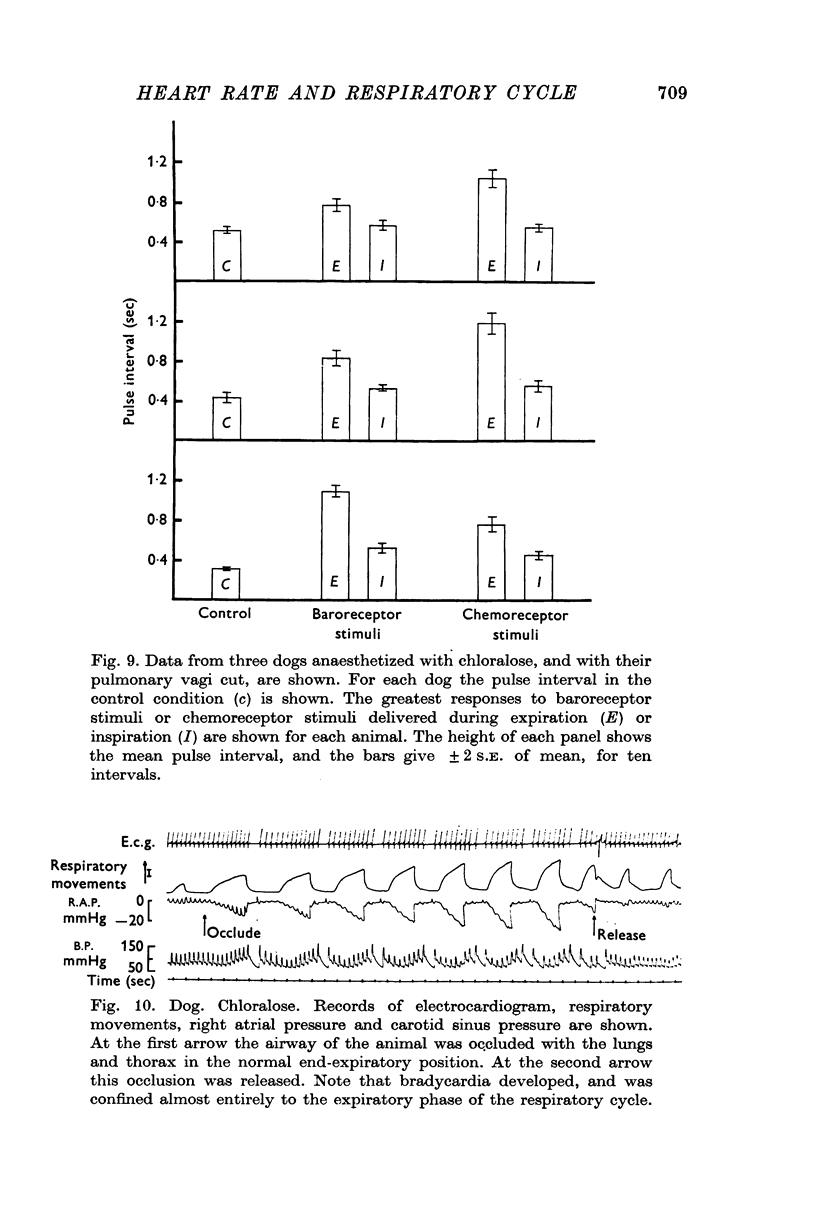
1. Brief stimuli were delivered to the carotid chemoreceptors or baroreceptors in dogs anaesthetized with pentobarbitone or chloralose. Chemoreceptor stimulation was achieved by rapid retrograde injections of 0-2-0-5 ml. warmed, CO2-equilibrated saline through a cannula in the external carotid artery. Baroreceptor stimulation was achieved by forceful retrograde injection of 2-5 ml. air-equilibrated saline, or of freshly drawn arterial blood, into the external carotid artery after first clamping the common carotid artery. 2. Brief baroreceptor stimuli had no noticeable effect on breathing. Brief chemoreceptor stimuli had no effect on breathing in some dogs, but in many produced a reflex increase in the depth of inspiration when delivered during inspiration. In these same dogs, brief chemoreceptor stimuli delivered in expiration either prolonged the expiratory pause or evoked an active expiratory effort. 3. Prompt decreases in heart rate were elicited by brief sudden chemoreceptor or baroreceptor stimuli when these were delivered during the expiratory phase of respiration. The stimuli did not modify the control heart rate pattern when delivered during inspiration. If the carotid sinus nerve or the vagus nerves were cut the responses were abolished. 4. Brief chemoreceptor or baroreceptor stimuli remained effective in evoking prompt decreases in heart rate during periods of apnoea in the end-inspiratory position (Hering-Breuer inflation reflex). In periods of apnoea after prolonged artificial hyperventilation the stimuli were sometimes ineffective at first, but were always effective late in the period of apnoea, again producing prompt cardiac slowing. 5. After denervation of the lungs, brief baroreceptor and chemoreceptor stimuli continued to evoke prompt falls in heart rate when given during expiration. When delivered during inspiration the same stimuli were either ineffective, or less effective.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biscoe T. J., Sampson S. R. An analysis of the inhibition of phrenic motoneurones which occurs on stimulation of some cranial nerve afferents. J Physiol. 1970 Aug;209(2):375–393. doi: 10.1113/jphysiol.1970.sp009170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black A. M., Torrance R. W. Respiratory oscillations in chemoreceptor discharge in the control of breathing. Respir Physiol. 1971 Nov;13(2):221–237. doi: 10.1016/0034-5687(71)90092-2. [DOI] [PubMed] [Google Scholar]
- Boushey H. A., Richardson P. S., Widdicombe J. G., Wise J. C. The response of laryngeal afferent fibres to mechanical and chemical stimuli. J Physiol. 1974 Jul;240(1):153–175. doi: 10.1113/jphysiol.1974.sp010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DALY M. B., SCOTT M. J. The effects of stimulation of the carotid body chemoreceptors on heart rate in the dog. J Physiol. 1958 Nov 10;144(1):148–166. doi: 10.1113/jphysiol.1958.sp006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L. The importance of timing on the respiratory effects of intermittent carotid body chemoreceptor stimulation. J Physiol. 1972 Apr;222(2):319–333. doi: 10.1113/jphysiol.1972.sp009799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRIUCHIJIMA J., KUMADA M. ACTIVITY OF SINGLE VAGAL FIBERS EFFERENT TO THE HEART. Jpn J Physiol. 1964 Oct 15;14:479–487. doi: 10.2170/jjphysiol.14.479. [DOI] [PubMed] [Google Scholar]
- Levy M. N., DeGeest H., Zieske H. Effects of respiratory center activity on the heart. Circ Res. 1966 Jan;18(1):67–78. doi: 10.1161/01.res.18.1.67. [DOI] [PubMed] [Google Scholar]


