Abstract
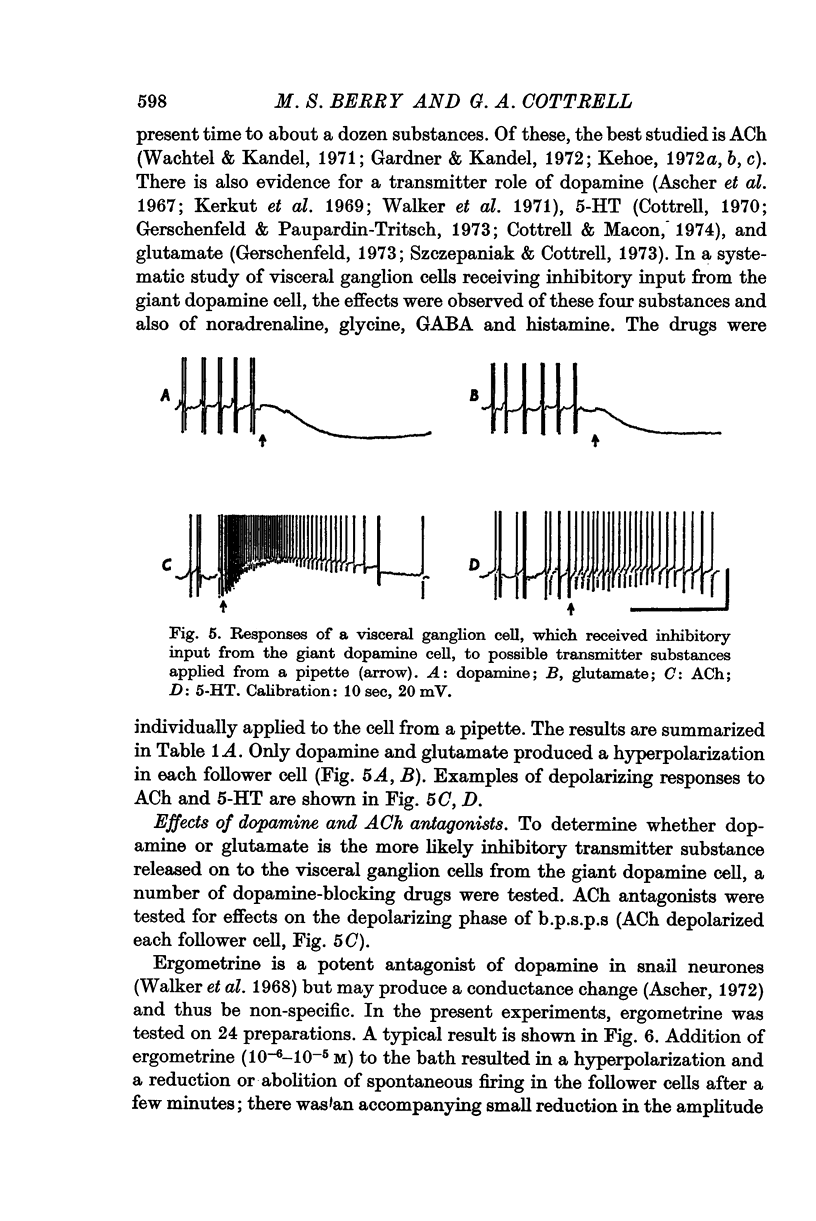
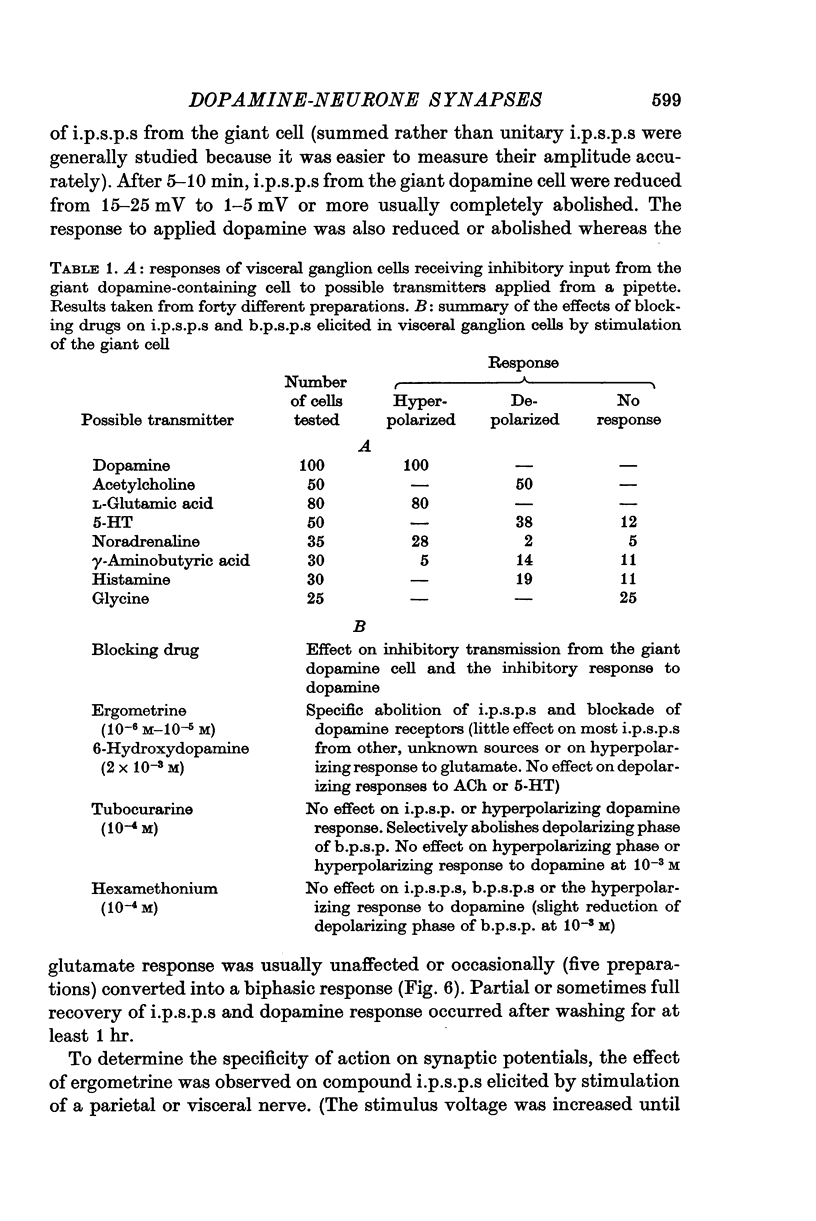
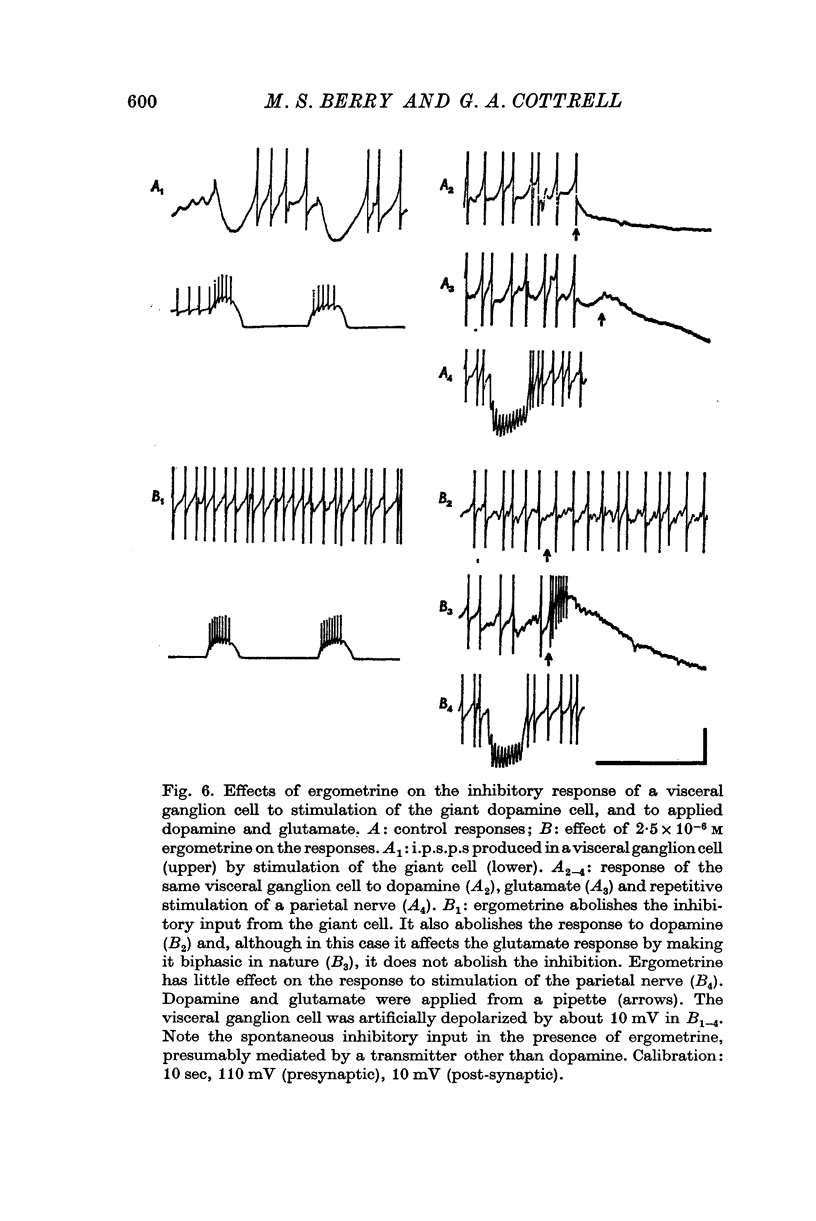
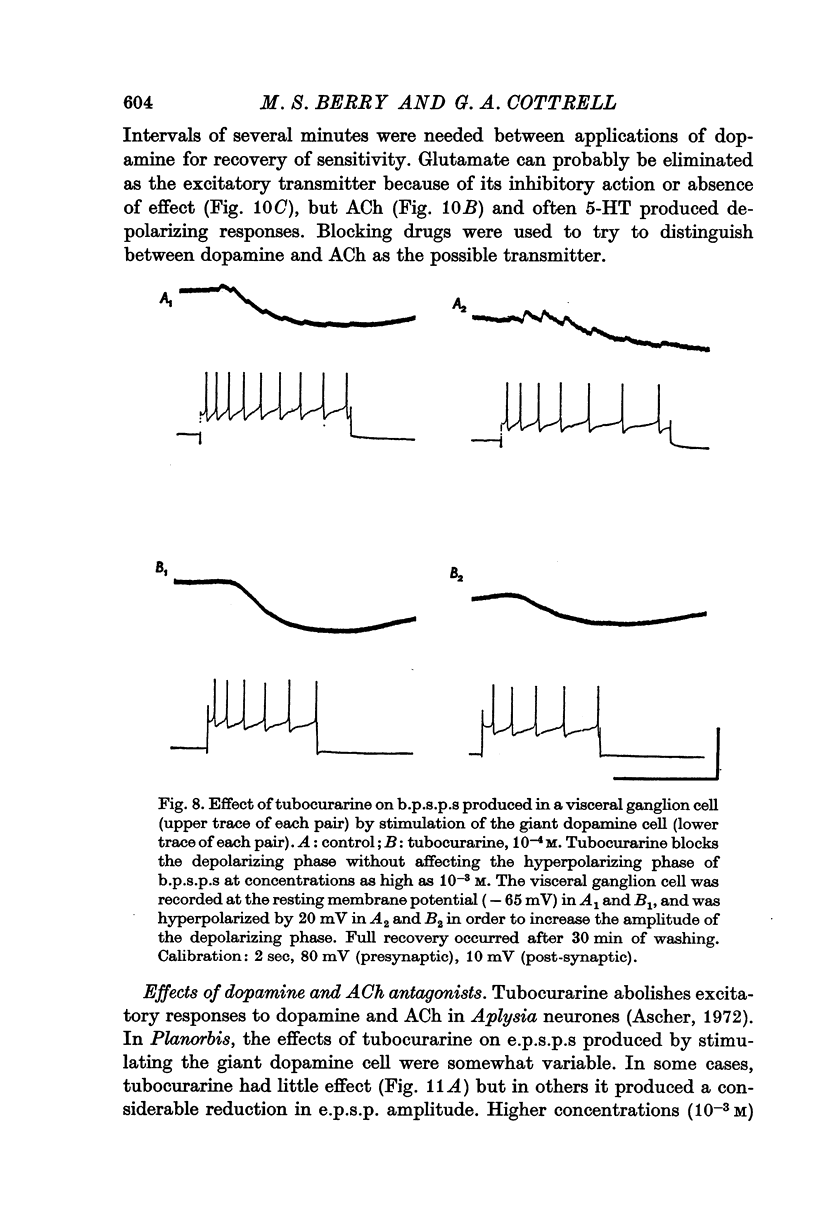
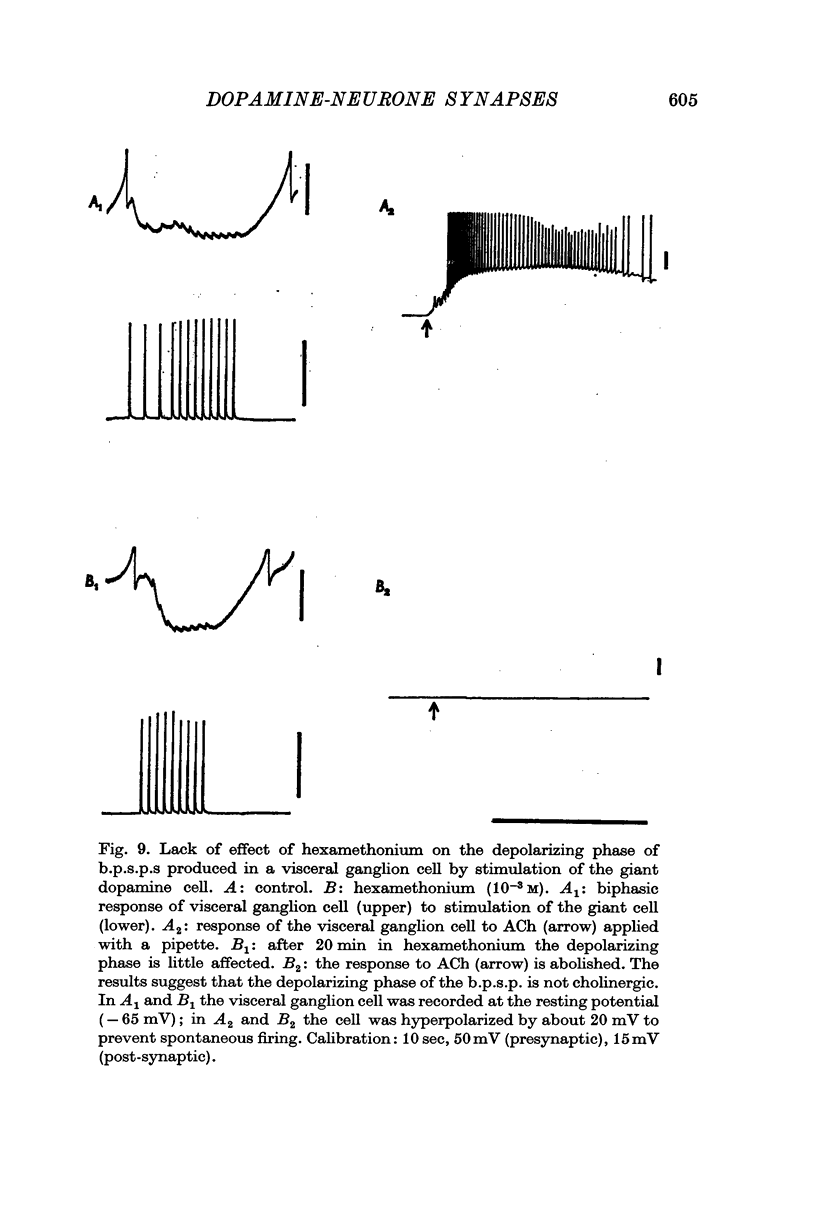
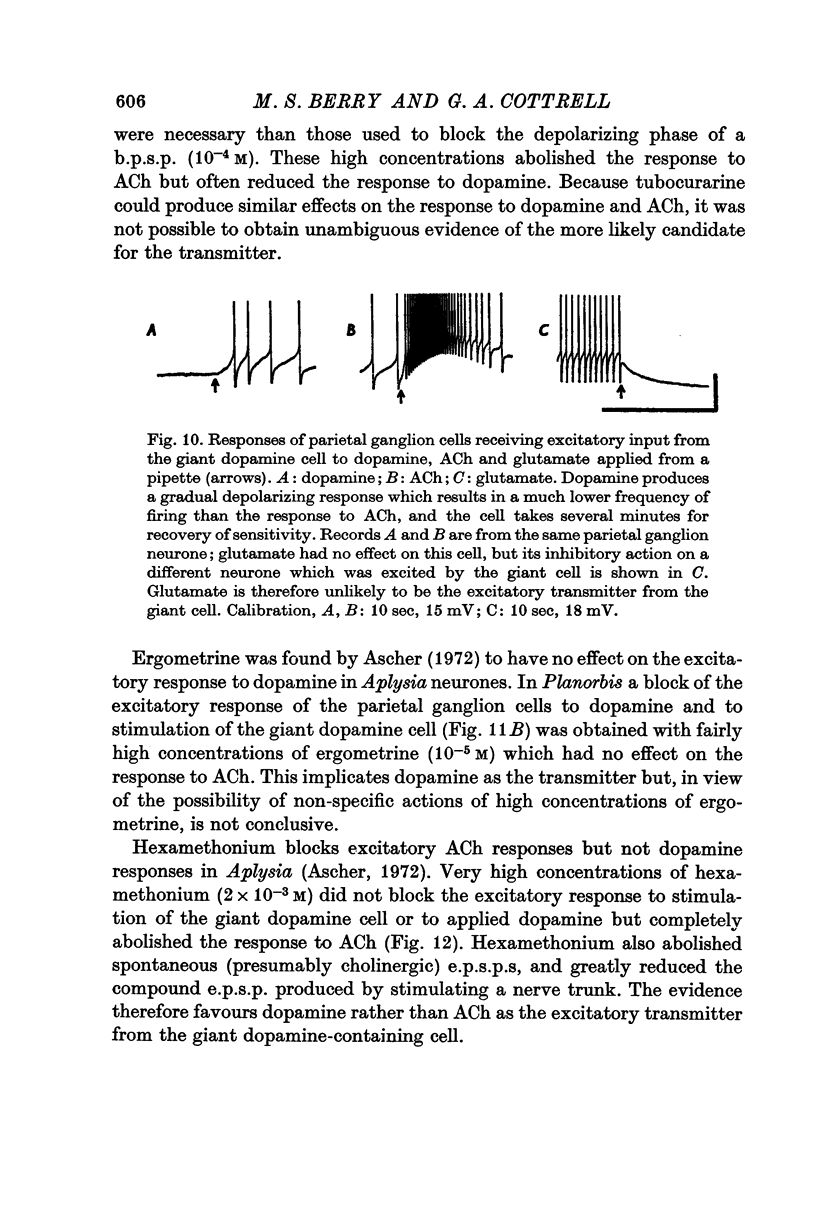
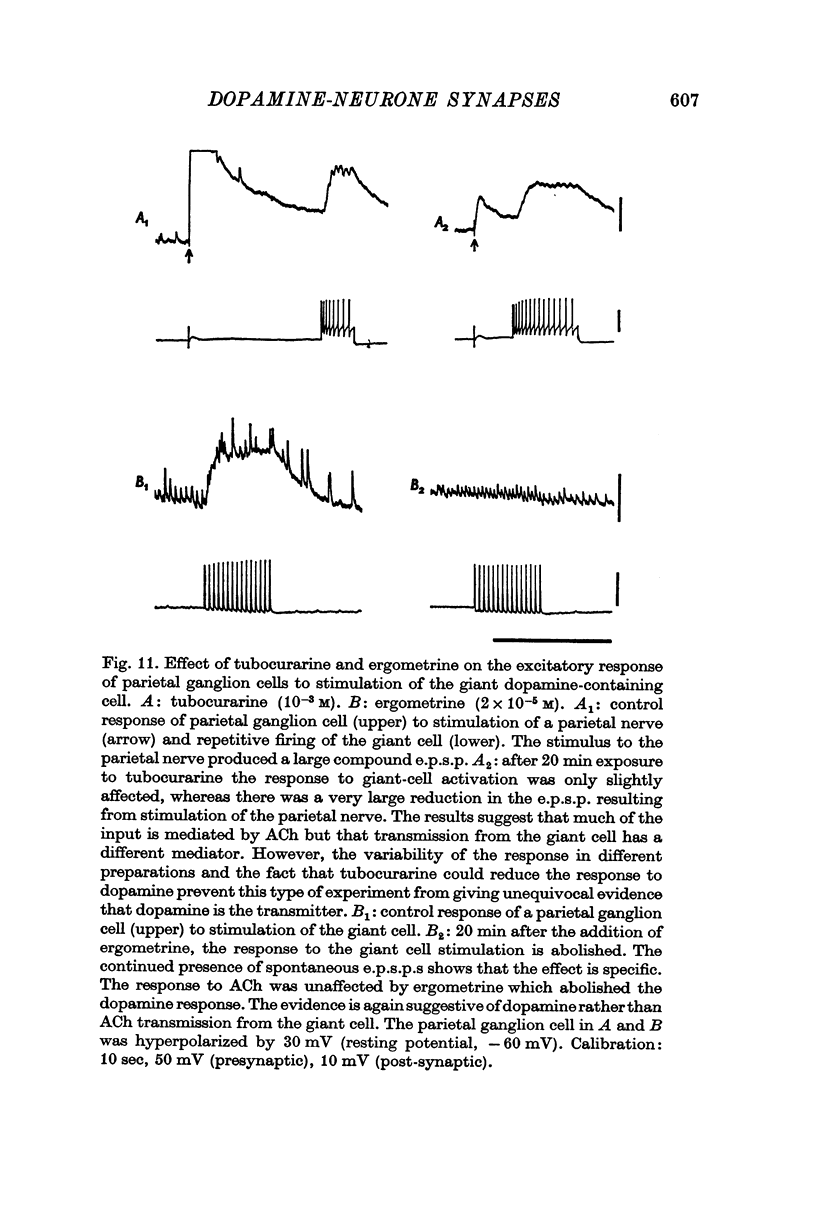
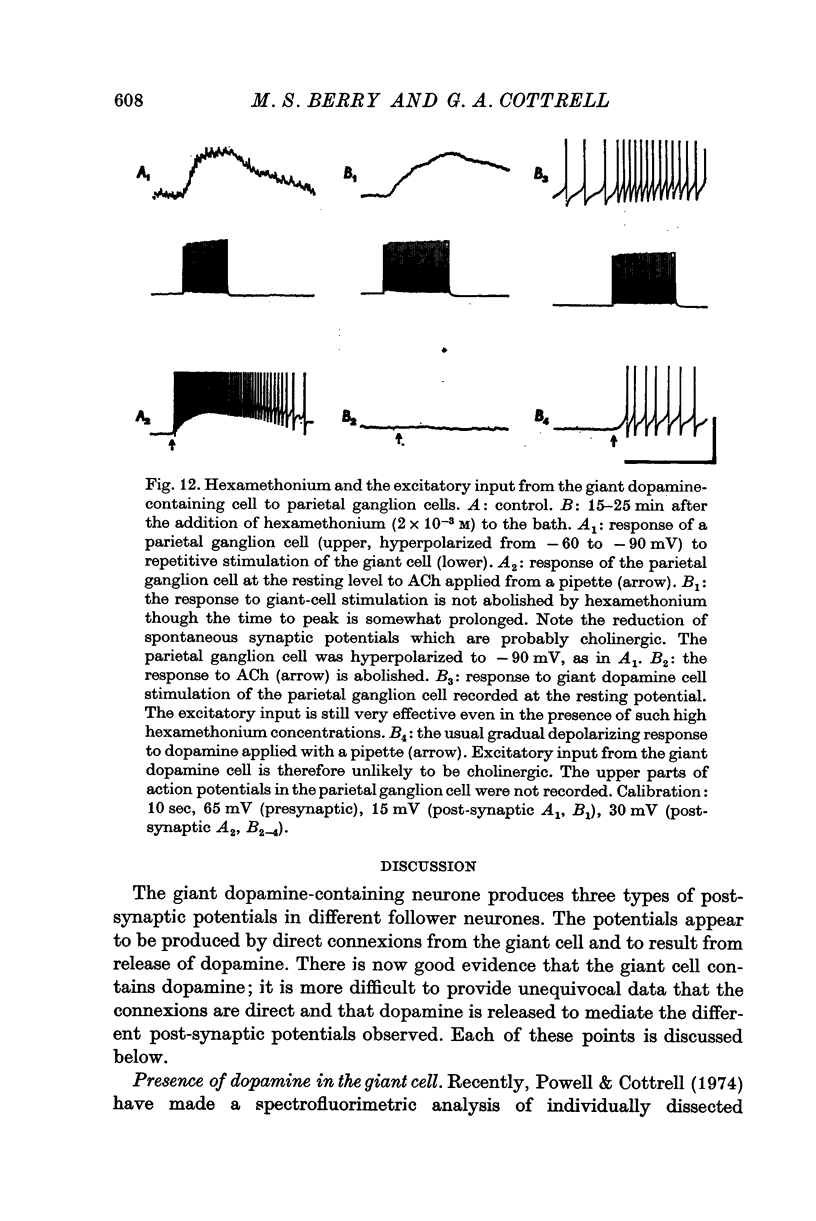
1. A giant dopamine-containing cell, situated in the left pedal ganglion of the water snail Planorbis corneus, was identified in isolated living preparations of the central nervous system. Spectrophotofluorimetric analysis confirms that the cell contains dopamine, whereas noradrenaline appears to be absent. The cell is unique in being a repeatedly identifiable dopamine-containing neurone. 2. Stimulation of the giant dopamine-containing cell resulted in excitatory, inhibitory or biphasic (depolarizing-hyperpolarizing) synaptic potentials in a number of follower neurones. The duration of the e.p.s.p.s and i.p.s.p.s was 0-3-5 sec; they ranged from barely detectable responses to ones 7 mV in amplitude in different cells. The depolarizing phase of a biphasic synaptic potential (b.p.s.p.) was usually less than 1 mV in amplitude (max. 3mV) and lasted 40-400 msec. The latency of i.p.s.p.s was long (70-120 msec) compared with that of e.p.s.p.s and b.p.s.p.s (20 msec). Abolition of the depolarizing phase of b.p.s.ps. by tubocurarine left a long-latency (70-120 msec) i.p.s.p. All responses showed summation and marked facilitation. 3. Evidence is presented that the post-synaptic potentials are produced by direct connections from the giant cell and result from a release of dopamine. Of eight putative transmitter substances tested on these different groups of neurones, only dopamine produced a potential change which in each case was of the same polarity as the post-synaptic potential when this was monophasic. However, generally applied dopamine produced only a hyperpolarization in follower cells showing b.p.s.p.s. This result is probably partly due to rapid desensitization of the receptors mediating the depolarization and also to a masking of the depolarization by the more effective hyperpolarizing response. 4. Erogometrine and 6-hydroxydopamine specifically antagonized the i.p.s.p.s and dopamine receptors mediating inhibition. Neither the e.p.s.p.s nor the excitatory dopamine response were blocked by high concentrations of hexamethonium. Hexamethonium was also ineffective in blocking the depolarizing phase of a b.p.s.p., which was, however, selectively eliminated by tubocurarine. 5. It is suggested that dopamine is the transmitter released from the giant cell and that it can mediate excitatory, inhibitory or biphasic responses in different follower neurones.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P. Inhibitory and excitatory effects of dopamine on Aplysia neurones. J Physiol. 1972 Aug;225(1):173–209. doi: 10.1113/jphysiol.1972.sp009933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Kehoe J. S., Tauc L. Effets d'injections électrophorétiques de dopamine sur les neurones d'aplysie. J Physiol (Paris) 1967;59(4 Suppl):331–332. [PubMed] [Google Scholar]
- Berry M. S., Cottrell G. A. Dopamine: excitatory and inhibitory transmission from a giant dopamine neurone. Nat New Biol. 1973 Apr 25;242(121):250–253. doi: 10.1038/newbio242250a0. [DOI] [PubMed] [Google Scholar]
- Berry M. S., Cottrell G. A., Pentreath V. W., Powell B. Catecholamine microanalysis and morphology of a giant dopamine-containing neurone. J Physiol. 1974 Mar;237(2):19P–20P. [PubMed] [Google Scholar]
- Berry M. S., Pentreath V. W., Turner J. D., Cottrell G. A. Effects of 6-hydroxydopamine on an identified dopamine-containing neuron in the central nervous system of Planorbis corneus. Brain Res. 1974 Aug 16;76(2):309–324. doi: 10.1016/0006-8993(74)90462-4. [DOI] [PubMed] [Google Scholar]
- Cottrell G. A. Direct postsynaptic responses to stimulation of serotonin-containing neurones. Nature. 1970 Mar 14;225(5237):1060–1062. doi: 10.1038/2251060a0. [DOI] [PubMed] [Google Scholar]
- Cottrell G. A., Macon J. B. Synaptic connexions of two symmetrically placed giant serotonin-containing neurones. J Physiol. 1974 Jan;236(2):435–464. doi: 10.1113/jphysiol.1974.sp010445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSCHENFELD H. M. A NON-CHOLINERGIC SYNAPTIC INHIBITION IN THE CENTRAL NERVOUS SYSTEM OF A MOLLUSC. Nature. 1964 Jul 25;203:415–416. doi: 10.1038/203415a0. [DOI] [PubMed] [Google Scholar]
- Gardner D. Bilateral symmetry and interneuronal organization in the buccal ganglia of Aplysia. Science. 1971 Aug 6;173(3996):550–553. doi: 10.1126/science.173.3996.550. [DOI] [PubMed] [Google Scholar]
- Gardner D., Kandel E. R. Diphasic postsynaptic potential: a chemical synapse capable of mediating conjoint excitation and inhibition. Science. 1972 May 12;176(4035):675–678. doi: 10.1126/science.176.4035.675. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Gerschfeld H. M., Paupardin-Tritsch D. Proceedings: Excitatory and inhibitory monosynaptic actions mediated by a serotonin containing neurone in Aplysia californica. J Physiol. 1973 Oct;234(2):28P–29P. [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966 Jun;18(2):925–964. [PubMed] [Google Scholar]
- Hornykiewicz O. Pharmacology and pathophysiology of dopaminergic neurons. Adv Cytopharmacol. 1971 May;1:369–377. [PubMed] [Google Scholar]
- Kandel E. R., Frazier W. T., Waziri R., Coggeshall R. E. Direct and common connections among identified neurons in Aplysia. J Neurophysiol. 1967 Nov;30(6):1352–1376. doi: 10.1152/jn.1967.30.6.1352. [DOI] [PubMed] [Google Scholar]
- Kandel E. R. The organization of subpopulations in the abdominal ganglion of Aplysia. UCLA Forum Med Sci. 1969;11:71–111. [PubMed] [Google Scholar]
- Kehoe J. Ionic mechanisms of a two-component cholinergic inhibition in Aplysia neurones. J Physiol. 1972 Aug;225(1):85–114. doi: 10.1113/jphysiol.1972.sp009930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. The physiological role of three acetylcholine receptors in synaptic transmission in Aplysia. J Physiol. 1972 Aug;225(1):147–172. doi: 10.1113/jphysiol.1972.sp009932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkut G. A., Horn N., Walker R. J. Long-lasting synaptic inhibition and its transmitter in the snail Helix aspersa. Comp Biochem Physiol. 1969 Sep 15;30(6):1061–1074. doi: 10.1016/0010-406x(69)91044-5. [DOI] [PubMed] [Google Scholar]
- Marsden C., Kerkut G. A. The occurrence of monoamines in Planorbis corneus: a fluorescence microscopic and microspectrometric study. Comp Gen Pharmacol. 1970 Mar;1(1):101–116. doi: 10.1016/0010-4035(70)90015-7. [DOI] [PubMed] [Google Scholar]
- Pentreath V. W., Berry M. S., Cottrell G. A. Anatomy of the giant dopamine-containing neurone in the left pedal ganglion of Planorbis corneus. Cell Tissue Res. 1974;151(3):369–384. doi: 10.1007/BF00224547. [DOI] [PubMed] [Google Scholar]
- Powell B., Cottrell G. A. Dopamine in an identified neuron of Planorbus corneus. J Neurochem. 1974 Apr;22(4):605–606. doi: 10.1111/j.1471-4159.1974.tb06902.x. [DOI] [PubMed] [Google Scholar]
- Szczepaniak A. C., Cottrell G. A. Biphasic action of glutamic acid and synpatic inhibition in an identified serotonin-containing neurone. Nat New Biol. 1973 Jan 10;241(106):62–64. doi: 10.1038/newbio241062a0. [DOI] [PubMed] [Google Scholar]
- Tauc L. Excitatory and inhibitory processes. UCLA Forum Med Sci. 1969;11:37–70. [PubMed] [Google Scholar]
- Wachtel H., Kandel E. R. A direct synaptic connection mediating both excitation and inhibition. Science. 1967 Dec 1;158(3805):1206–1208. doi: 10.1126/science.158.3805.1206. [DOI] [PubMed] [Google Scholar]
- Wachtel H., Kandel E. R. Conversion of synaptic excitation to inhibition at a dual chemical synapse. J Neurophysiol. 1971 Jan;34(1):56–68. doi: 10.1152/jn.1971.34.1.56. [DOI] [PubMed] [Google Scholar]
- Walker R. J., Ralph K. L., Woodruff G. N., Kerkut G. A. Evidence for a dopamine inhibitory post-synaptic potential in the brain of Helix aspersa. Comp Gen Pharmacol. 1971 Mar;2(5):15–26. doi: 10.1016/0010-4035(71)90063-2. [DOI] [PubMed] [Google Scholar]
- Walker R. J., Woodruff G. N., Glaizner B., Sedden C. B., Kerkut G. A. The pharmacology of Helix dopamine receptor of specific neurones in the snail, Helix aspersa. Comp Biochem Physiol. 1968 Feb;24(2):455–469. doi: 10.1016/0010-406x(68)90997-3. [DOI] [PubMed] [Google Scholar]


