Abstract
This study reports for the first time that leptospires are killed by H2O2 and by low-molecular-weight primary granule components, which are agents normally released by neutrophils upon stimulation. Although both pathogenic and nonpathogenic strains were sensitive to H2O2-mediated killing, nonpathogenic organisms were found to be more susceptible. In addition, the killing of leptospires by H2O2 was found to be independent of the presence of the neutrophil primary granule component myeloperoxidase and therefore not a consequence of halogenation reactions. We have also determined that leptospires are significantly sensitive only to primary granule components and, among those, to proteins and/or peptides of less than 30 kDa.
Leptospires are the etiological agents of leptospirosis, a zooanthroponosis widespread throughout the world. The course of human leptospirosis ranges from mild to severe; some forms can be fatal, depending on the serovar of Leptospira involved and its virulence.
Although we are far from having a complete picture of the Leptospira pathogenic factors, it is clear that these organisms are invasive and that the success of their invasions is mainly due to their ability to survive and grow in tissues by escaping natural defense mechanisms such as phagocytic cells, the complement system, and pharmacologically active tissue peptides and mediators. In fact, pathogenic leptospires can survive in the nonimmune host by evading complement-mediated killing (6) and (probably) phagocytosis. Studies have shown that pathogenic leptospires bind nonopsonically to and are slowly internalized by guinea pig macrophages and human polymorphonuclear leucocytes (1, 5-7, 20). Although nonpathogenic spirochetes are rapidly engulfed and killed by human polymorphonuclear phagocytes, there is no clear evidence of the killing of pathogenic spirochetes other than that mediated by specific antibodies directed against some of their surface components (5). Moreover, live pathogenic leptospires can partially escape uptake by Kupffer cells from perfused rat liver (16). Overall, these data indicate that survival of leptospires is due primarily to scarce uptake by phagocytes. However, whether those leptospires that have been engulfed through nonopsonic phagocytosis are susceptible to intracellular killing is not known.
The bactericidal systems activated after uptake by phagocytes include the generation of oxygen metabolites (O2-dependent killing) and the release of granule components such as proteins and peptides with microbicidal activity (10, 15) into the phagocytic vacuole (O2-independent killing) and combinations of the two. Since it is not known which among the killing mechanisms of phagocytes are effective toward leptospires, we have investigated the in vitro sensitivity of pathogenic and nonpathogenic strains of leptospires to the O2-dependent and -independent killing machinery of neutrophils.
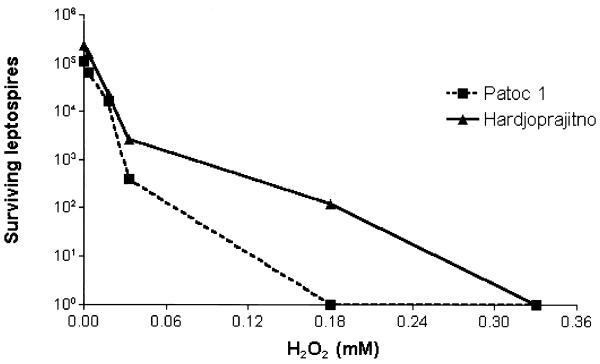
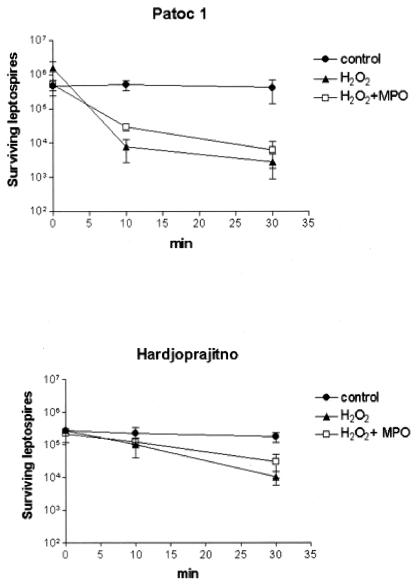
The oxygen-dependent chlorinating system constitutes an important microbicidal strategy employed by neutrophils (13), and we tested its efficacy with respect to leptospires by means of a myeloperoxidase (MPO)-H2O2-Cl− cell-free assay. As previously reported, the complete chlorinating system kills Escherichia coli K-12 (strain AB 1157) very efficiently (reference 11 and data not shown) whereas neither MPO nor H2O2 is effective on its own. However, we found a pronounced killing effect with H2O2 on both the pathogenic Leptospira interrogans strain Hardjoprajitno (serovar hardjo) and the nonpathogenic Leptospira biflexa strain Patoc1 (serovar patoc), regardless of the presence of MPO (Table 1). Both saprophytic and pathogenic leptospires were very sensitive to 0.33 mM H2O2 in the presence or absence of MPO, with the decrease of viable leptospires being at least 2 logarithms. The leptospiracidal activity of H2O2 alone was confirmed by using other pathogenic strains such as 142 (serovar icterohaemorrhagiae), Wijmberg (serovar copenhageni), and Ballico (serovar australis), with the microorganism survival level being at least 2 logarithms lower than that of the controls. We then tested the effect of lower H2O2 concentrations on the Hardjoprajitno and Patoc1 strains, starting with 0.0018 mM (Fig. 1). The dose response curves showed that nonpathogenic Patoc1 was more sensitive than Hardjoprajitno to H2O2, with the level of killing 1 order of magnitude higher at 0.03 mM and 3 orders of magnitude higher at 0.18 mM, which is the concentration at which complete killing was achieved for Patoc1. The apparent absence of a contribution of MPO to the level of killing could have been due to too high a concentration of H2O2, to which these leptospires are particularly sensitive. Therefore, we investigated the possible contribution of MPO (0.1 guaiacol unit [1 guaiacol unit is the amount of enzyme which oxidizes 1 μmol of guaiacol per min]) to the level of killing at 0.033 mM H2O2, which is a concentration that results in only partial killing of the leptospires. As shown in Fig. 2, the presence of MPO did not increase the efficiency of H2O2-mediated killing after 10 or 30 min of incubation. As observed previously, Patoc1 was demonstrated to be the more susceptible of the two strains, with a decrease of viable leptospires on the order of 2 log against a decrease of 1 log for Hardjoprajitno. Altogether, leptospires were found to be sensitive to H2O2 independently of MPO, i.e., not as a consequence of halogenation reactions. This H2O2 sensitivity can be postulated to be due to a scarce or absent production of catalase. In fact, no catalase activity has been detected in strain Patoc1, and strain Hardjoprajitno hydrolyzes only 16 μmol of H2O2 per min per 109 cells (2). This is likely to be the reason that leptospires show a higher susceptibility to H2O2 toxicity than other bacteria such as Staphylococcus aureus (21), Legionella pneumophila (12), Listeria monocytogenes (4), and E. coli (19). That H2O2 is less reactive than other products of oxygen metabolism such as O2−, OH, and hypohalous acid allows it to pass intact through cell membranes (8), diffuse within biological fluids containing little or no catalase, and act as an oxidizing agent (18). The lack of a role for MPO in the killing of both pathogenic and nonpathogenic leptospires is in keeping with results previously obtained with Borrelia species (11). We have demonstrated here that MPO is not only not bactericidal but it is also unable to increase the microbicidal action of H2O2. MPO is a cationic protein and, as such, is generally thought to adhere to cell surfaces and result in cell injury (in the presence of H2O2) by increasing the local concentration of hypohalous acid at the target membrane (13). Therefore, it can be speculated that in the case of spirochetes in particular, MPO is not able to bind to the outer membranes and therefore chlorination reactions cannot take place. According to this hypothesis, the lack of such reactions excludes the possibility that the halogenation of Leptospira membrane proteins is the cause of functional damage leading to the killing of spirochetes.
TABLE 1.
Oxygen-dependent killing of leptospiresa
| Treatment | Mean bacterial count (105 MPN) ± SD forb:
|
|
|---|---|---|
| Hardjoprajitno | Patoc1 | |
| None | 4.4 ± 2.1 | 2.2 ± 2.9 |
| MPO | 3.7 ± 3.7 | 1.3 ± 1.8 |
| H2O2 | <0.01 | <0.01 |
| MPO + H2O2 | <0.01 | <0.01 |
Leptospires (strain Hardjoprajitno or Patoc1) were incubated for 30 min at 37°C in MEM (containing 0.126 M chloride), with or without the addition of 0.1 or 1.0 guaiacol unit of MPO (kindly provided by G. Zabucchi) and with different amounts of H2O2 as indicated. Incubation mixtures were tested for bacterial viability by using the microdilution test described previously for Borrelia burgdorferi (11), with the statistical elaboration described by Meynell and Meynell (17) for the calculations. As a positive control, E. coli bacteria were treated with MPO, H2O2, or MPO + H2O2, resulting in levels of viability of 100, 100, and 0%, respectively, as determined by measurements of the number of CFU. Viability counts were performed in triplicate in all cases.
MPN, most probable number.
FIG. 1.
Dose response of H2O2 leptospiracidal activity. Leptospires (105) were incubated at 37°C for 30 min with different concentrations of H2O2 in a final volume of 100 μl of minimal essential medium (MEM). Bactericidal activity was evaluated as described in Table 1, footnote a. Values are the means of two representative experiments.
FIG. 2.
Bactericidal effect of H2O2-MPO-Cl− on nonpathogenic and pathogenic leptospires. Leptospira strain Patoc1 (nonpathogenic) or Hardjoprajitno (pathogenic) was incubated at 37°C in the presence of 0.033 mM H2O2, with or without 0.1 guaiacol unit of MPO, in minimal essential medium. At different incubation times of up to 30 min, microbicidal activities were evaluated as described in Table 1; footnote a. Data shown are means ± standard deviations (n = 3).
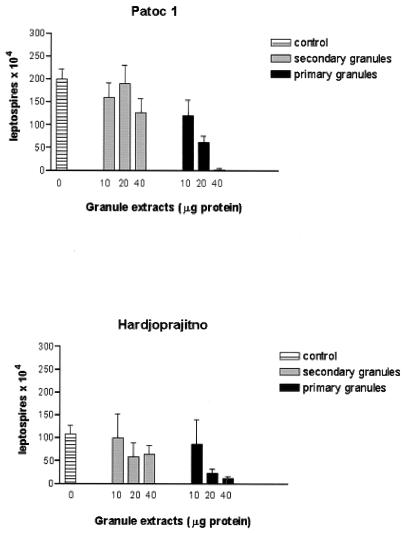
The potential leptospiracidal contribution of oxygen-independent mechanisms was studied using different subcellular fractions from neutrophils isolated from the peripheral blood of healthy donors. Cytosolic, membrane (low and high density), and secondary and primary granule fractions were obtained by Percoll gradient fractionation of cells disrupted by nitrogen cavitation, as previously described (3, 11, 14). A very low level of cross-contamination between primary and secondary granules was demonstrated by using determinations of the ratios of MPO to lysozyme (11). Acid extracts from membranes and primary or secondary granules were prepared as previously described (11). We found that no leptospiracidal activity was found to be associated with cytosolic or membrane fractions. A very modest, not significant level of leptospiracidal activity was observed for both strains by using specific granule extracts, while a substantial level of killing of leptospires was found by using the same amounts of primary granule extracts (Table 2). A dose-response study (Fig. 3) confirmed that secondary granule extracts did not contribute in any significant proportion to levels of killing, while primary granule extracts were clearly effective. The relative difference between the effects of secondary and primary granules was found to be maximal at a dose of 40 μg.
TABLE 2.
Oxygen-independent killing of leptospiresa
| Subcellular fraction | Mean bacterial count (105 MPN) ± SD (n = 3) forb:
|
|
|---|---|---|
| Patoc1 | Hardjoprajitno | |
| Control (buffer) | 17.0 ± 2.5 | 18.5 ± 5.3 |
| Cytosol | 16.5 ± 2.6 | 25.2 ± 2.0 |
| Low-density membranes | 19.1 ± 3.0 | 26.4 ± 3.2 |
| High-density membranes | 18.2 ± 0.7 | 25.2 ± 1.4 |
| Secondary granules | 15.2 ± 0.7 | 14.5 ± 6.0 |
| Primary granules | 4.6 ± 1.5 | 5.5 ± 2.0 |
Leptospira killing assays were performed by using 20 μg of protein from either cytosolic fractions or acid extracts from membrane and granule fractions. Control assays using the same amount of acid buffer were performed simultaneously. Viability was determined as described in Table 1, footnote a.
MPN, most probable number.
FIG. 3.
Dose dependency of the microbicidal activity of primary or secondary neutrophil granule extracts toward leptospires. Nonpathogenic (Patoc1) and pathogenic (Hardjoprajitno) strains of leptospires were incubated at 37°C for 30 min in the presence of different amounts of granule extracts. Controls were incubated for 30 min with the same amount of acetate buffer (pH 4.2) as that present in the granule extracts. Microbicidal activities were evaluated as described in Table 1, footnote a and are expressed as means ± standard deviations (n = 3).
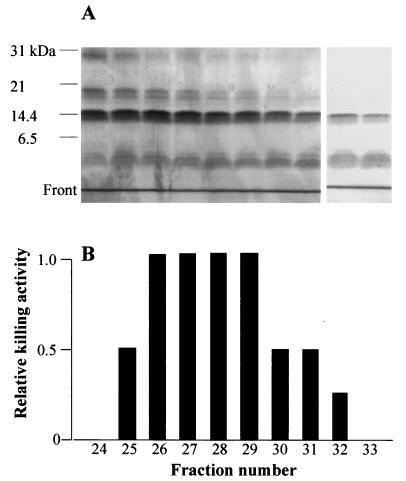
Since MPO, a major primary granule component, had been found not to be microbicidal towards leptospires (Table 1), the possibility that other granule constituents are responsible for the killing activity was examined. Acid extracts from primary granules were therefore subjected to gel filtration through a Sephadex G-75 column (11), and the eluted fractions were tested for leptospiracidal activity as well as by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Protein electrophoresis showed the presence of MPO in fractions 2 to 8, of serprocidins (9) in fractions 10 to 27, and of lysozyme in fractions 20 to 34. Leptospiricidal activity was found associated to fractions 25 to 32, with a peak from 26 to 29 (Fig. 4B). Figure 4A shows that after silver staining, the fractions with killing activity showed a protein doublet at 30 kDa (indicating the presence of serprocidins), a weak band at 20 kDa, a broad band at 15 kDa (lysozyme), and a doublet consisting of a minor and a broad band well below the 6.5-kDa molecular-mass marker, in a position corresponding to approximately 3 to 1 kDa. The latter, low-molecular-weight components are very likely to be defensins, the most abundant primary granule constituents, and/or cathelicidin-derived peptides. Both defensins and cathelicidins have been found to be leptospiracidal (22; V. Sambri, A. Marangoni, L. Giacani, R. Gennaro, R. Murgia, R. Cevenini, and M. Cinco, submitted for publication) when the purified molecules are used at high concentrations. However, we did not find any direct relationship between the concentration of the proteins detected in the G-75 fractions and the level of killing activity. For instance, fraction 24 shows no killing activity in spite of the fact that this fraction contains the same proteins as and in amounts similar to those in successive fractions, which are indeed microbicidal. This finding indicates that the leptospiracidal activity may be due to the presence of molecules not detected even after silver nitrate staining. Alternatively, killing may be the result of a more complex interaction of factors such as, for instance, a combination of killing enhancers and inhibitors whose concentration balance would determine the outcome of the microbicidal process or a synergy between peptides and microbicidal proteins, the latter being able to reach targets at the bacterial peptidoglycan or inner membrane level only after permeabilization by the peptides.
FIG. 4.
Leptospira killing by neutrophil primary granule components. (A) SDS-14% PAGE and sequential Coomassie and silver nitrate staining of Sephadex G-75 fractions showing microbicidal activity. No bands were detected above 31 kDa. (B) Killing activity of the fractions whose SDS-PAGE profiles are shown in panel A, determined as described in Table 1, footnote a. A decrease of at least 1 log in the number of surviving bacteria is referred to as relative activity 1. No microbicidal effect was found before fraction 25 or after fraction 32.
In brief, our results indicate that leptospires can be killed by both oxygen-dependent (H2O2) and -independent (primary granule components) mechanisms. Therefore, the poor in vivo clearance of phagocytosed, nonopsonized pathogenic leptospires is likely to be due to a modest uptake by neutrophils rather than to an intrinsic resistance of the microorganisms to killing.
Editor: B. B. Finlay
REFERENCES
- 1.Banfi, E., M. Cinco, M. Bellini, and M. R. Soranzo. 1982. The role of antibodies and complement in the interaction between macrophages and leptospires. J. Gen. Microbiol. 128:813-816. [DOI] [PubMed] [Google Scholar]
- 2.Banfi, E., M. Cinco, and P. Dri. 1981. Catalase activity among leptospires. Experientia 37:147-148. [DOI] [PubMed] [Google Scholar]
- 3.Borregaard, N., J. M. Heiple, E. R. Simons, and R. A. Clark. 1983. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase. J. Cell Biol. 97:52-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bortolussi, R., C. M. Vandenbroucke-Grauls, B. S. van Asbeck, and J. Verhoef. 1987. Relationship of bacterial growth phase to killing of Listeria monocytogenes by oxidative agents generated by neutrophils and enzyme systems. Infect. Immun. 55:3197-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinco, M., and E. Banfi. 1983. Interactions between human polymorphonuclear leukocytes and one strain of pathogenic Leptospira (L. interrogans sp.) and one strain of saprophytic Leptospira (L. biflexa sp.). FEMS Microbiol. Lett. 19:51-54. [Google Scholar]
- 6.Cinco, M., and E. Banfi. 1983. Activation and bactericidal activity of complement by leptospires. Zentbl. Bakteriol. Hyg. Abt. 1 Orig. A 254:261-265. [PubMed] [Google Scholar]
- 7.Cinco, M., E. Banfi, and M. R. Soranzo. 1981. Studies on the interaction between macrophages and leptospires. J. Gen. Microbiol. 124:409-413. [DOI] [PubMed] [Google Scholar]
- 8.Frimer, A. A., A. Forman, and D. C. Borg. 1983. H2O2 diffusion through lysosome. Isr. J. Chem. 23:442-445. [Google Scholar]
- 9.Gabay, J. E. 1994. Antimicrobial proteins with homology to serine proteases. Ciba Found. Symp. 186:237-247. [DOI] [PubMed] [Google Scholar]
- 10.Ganz, T., and R. I. Lehrer. 1998. Antimicrobial peptides of vertebrates. Curr. Opin. Immunol. 10:41-44. [DOI] [PubMed] [Google Scholar]
- 11.Garcia, R., L. Gusmani, R. Murgia, C. Guarnaccia, M. Cinco, and G. Rottini. 1998. Elastase is the only human neutrophil granule protein that alone is responsible for in vitro killing of Borrelia burgdorferi. Infect. Immun. 66:1408-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jepras, R. I., and R. B. Fitzgeorge. 1986. The effect of oxygen-dependent antimicrobial systems on strains of Legionella pneumophila of different virulence. J. Hyg. Camb. 97:61-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klebanoff, S. J. 1988. Phagocytic cells: products of oxygen metabolism, p. 391-444. In J. Gallin, I. M. Goldstein, and R. Snyderman (ed.), Inflammation: basic principles and clinical correlates. Raven Press Ltd., New York, N.Y.
- 14.Klempner, M. S., R. B. Mikkelsen, D. H. Corfman, and J. Andre-Schwartz. 1980. Neutrophil plasma membranes. I. High-yield purification of human neutrophil plasma membrane vesicles by nitrogen cavitation and differential centrifugation. J. Cell Biol. 86:21-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehrer, R. I., and T. Ganz. 1990. Antimicrobial peptides of human neutrophils. Blood 76:2169-2181. [PubMed] [Google Scholar]
- 16.Marangoni, A., R. Aldini, V. Sambri, M. Montagnani, G. Ballardini, E. Sturni, and R. Cevenini. 2000. Uptake and killing of Leptospira interrogans and Borrelia burgdorferi, spirochetes pathogenic for humans, by reticuloendothelial cells in perfused rat liver. Infect. Immun. 68:5408-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meynell, G. G., and E. Meynell. 1970. Theory and practice in experimental bacteriology, p. 231-232. Cambridge University Press, Cambridge, United Kingdom.
- 18.Miller, R. A., and B. E. Britigan. 1997. Role of oxidants in microbial pathophysiology. Clin. Microbiol. Rev. 10:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacelli, R., D. A. Wink, J. A. Cook, M. C. Krisha, W. De Graff, N. Friedman, M. Tsokos, A. Samuni, and J. B. Mitchell. 1995. Nitric oxide potentiates hydrogen peroxide-induced killing of Escherichia coli. J. Exp. Med. 182:1469-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, B., J. Sullivan, G. W. Sullivan, and G. Mandell. 1984. Interaction of leptospires with human polymorphonuclear neutrophils. Infect. Immun. 44:459-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson, C. B., and W. M. Weaver. 1985. Comparative susceptibility of group B streptococci and Staphylococcus aureus to killing by oxygen metabolites. J. Infect. Dis. 152: 323-329. [DOI] [PubMed] [Google Scholar]
- 22.Wu, Q., L. Xu, X. Wang, S. Li, and B. Wang. 1992. Investigation of microbicidal activity of neutrophil defensins against leptospires. Huaxi Yike Daxue Xuebao 23:126-129. [PubMed] [Google Scholar]