Abstract
1. Variations in the output of glucocorticoids and catecholamines from the right adrenal gland, in response to insulin hypoglycaemia, have been investigated in calves 2-5 weeks after birth. These have been correlated with changes in the concentration of glucocorticoids and glucagon in arterial plasma. 2. Moderate hypoglycaemia for a limited period (0-1 u. insulin/kg), elicited a prompt increase in steroid output from the adrenal gland followed by a significant rise in plasma glucagon concentration. By comparison, changes in both catecholamine output and peripheral plasma glucocorticoid concentrations were found to be trivial in this group of animals. 3. Administration of a larger dose of insulin (0-5 u./kg) produced a more substantial fall in plasma glucose concentration followed by spontaneous recovery within 2-3 hr. This stimulus elicited the release of greater amounts of both cortisol and corticosterone, followed by a significant increase both in the output of adrenaline and in plasma glucagon concentration. Increase in steroid output was accompanied by an increase in adrenal blood flow and was associated with elevated concentrations of both steroids in arterial plasma. 4. The adrenal cortical response and associated changes in plasma steroid concentration were found to be transient even in response to persistent and intense hypoglycaemia (4 u. insulin/kg). The increase in plasma glucagon concentration in this group of animals was not significantly greater than that produced by smaller doses of insulin. However, substantial amounts of adrenaline (78 plus or minus 14 ng. kg-minus 1 min-minus 1; maximum; n equals 9) together with a little noradrenaline (10 plus or minus 3 ng.kg-minus 1 min-minus 1; maximum; n equals 9) were released from the right adrenal gland under these conditions. 5. Changes in adrenal blood flow could be related to adrenal glucocorticoid output in calves given 0-1 or 0-5 u. insulin/kg. In animals given the largest dose of insulin adrenal blood flow was found to increase coincidentally with rising steroid output but this hyperaemia then persisted after steroid output had subsided to values within the normal range. 6. Calves given the largest dose of insulin (4-0 u./kg) invariably collapsed and convulsed after 2-3 hr, but these symptoms could not be related to any particular endocrine response. No clinical signs of hypoglycaemia were observed in the other animals. 7. The results are discussed in relation to previous studies of adrenal function in this and other species.
Full text
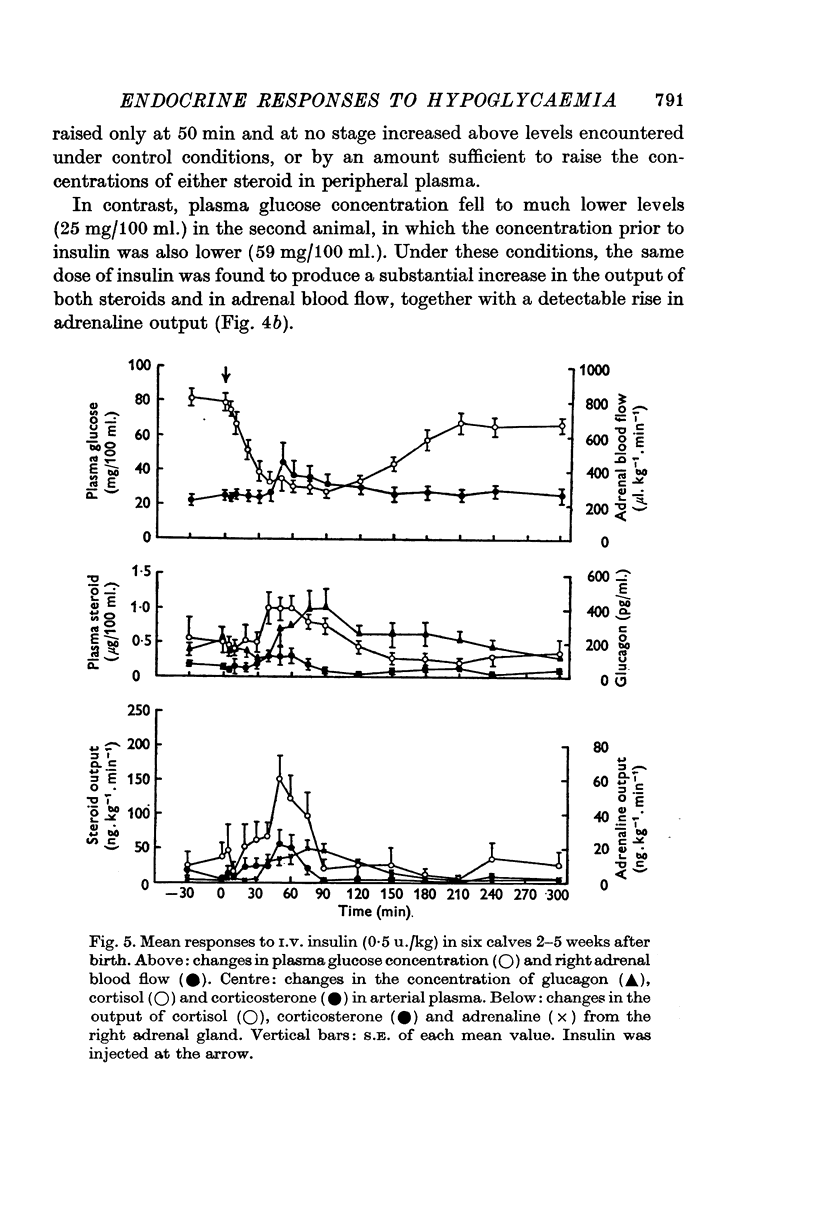
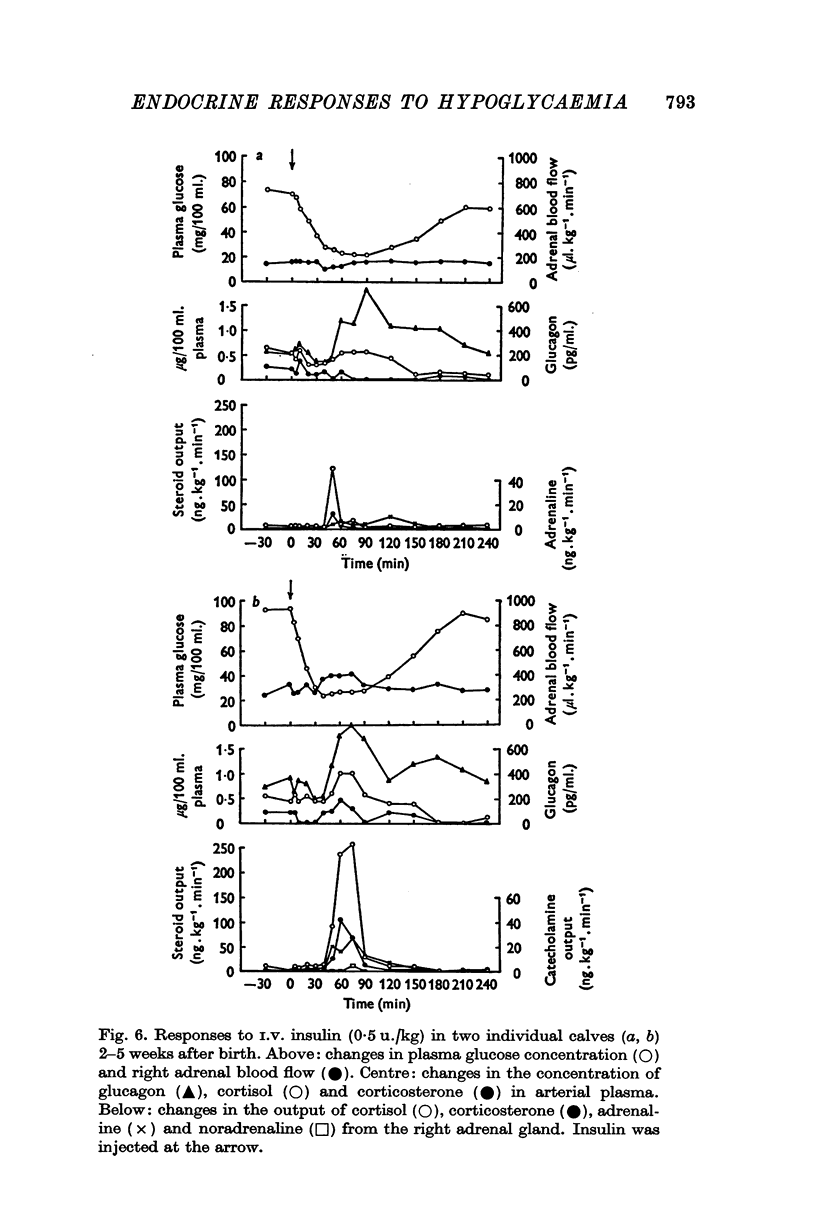
PDF


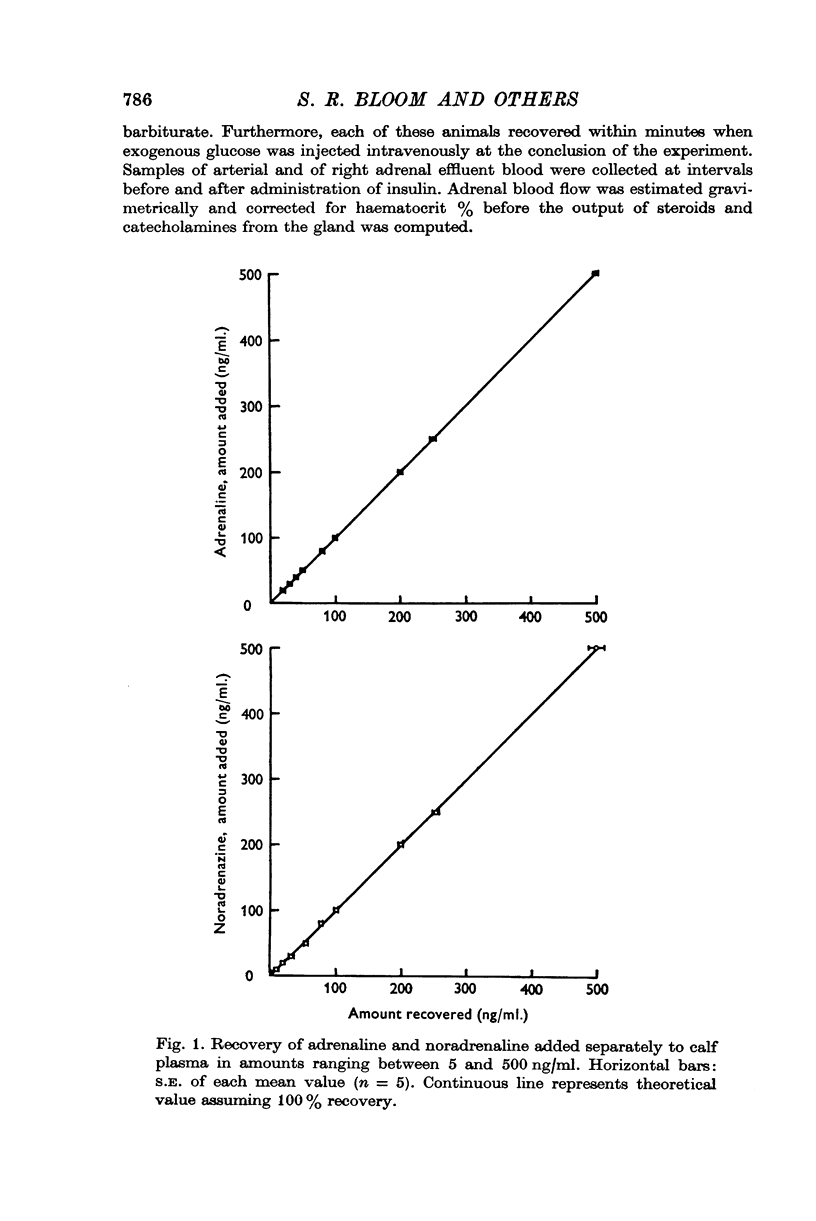
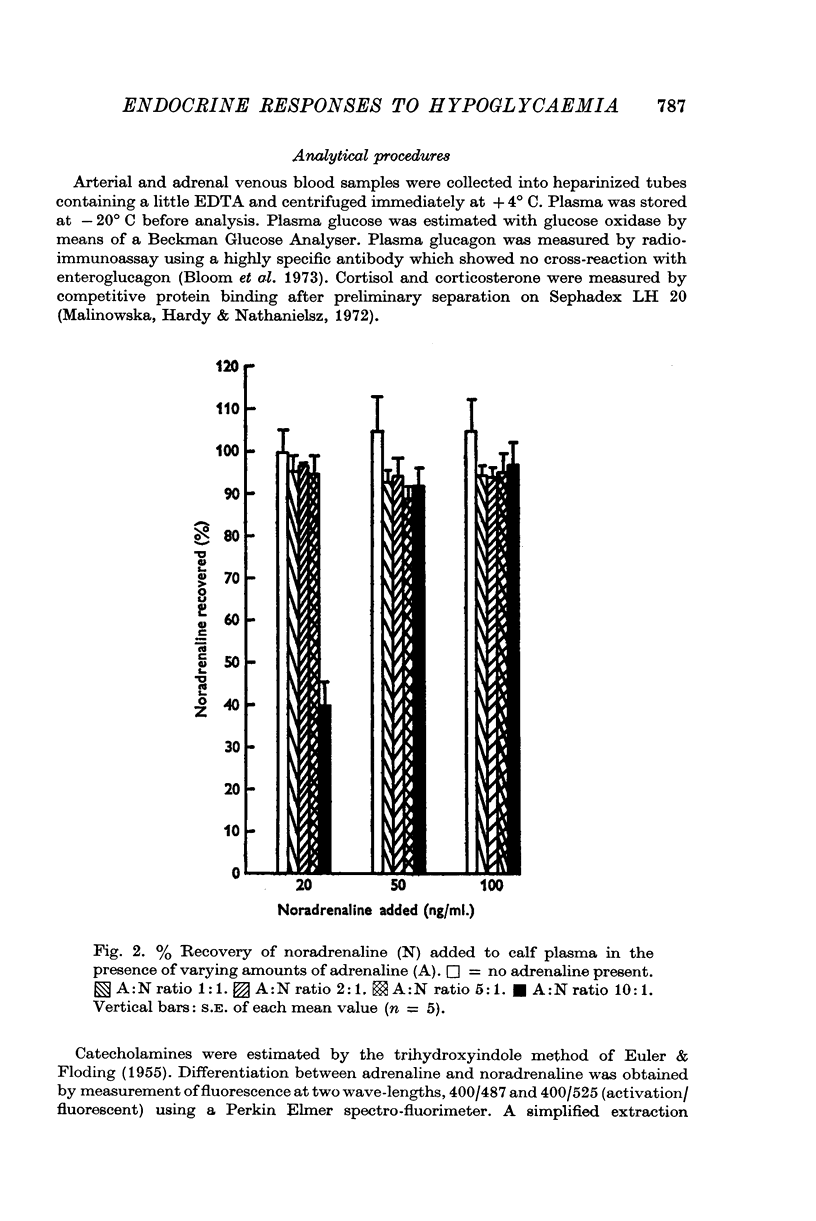

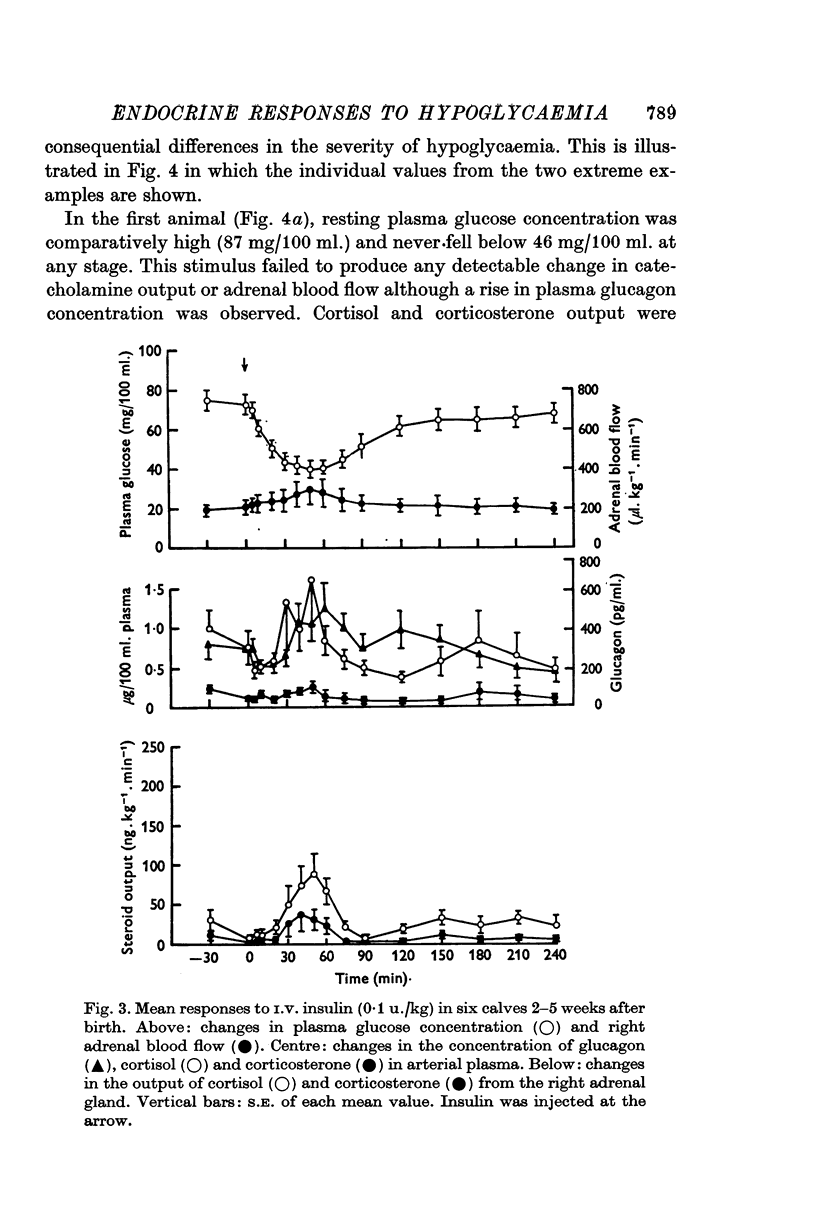
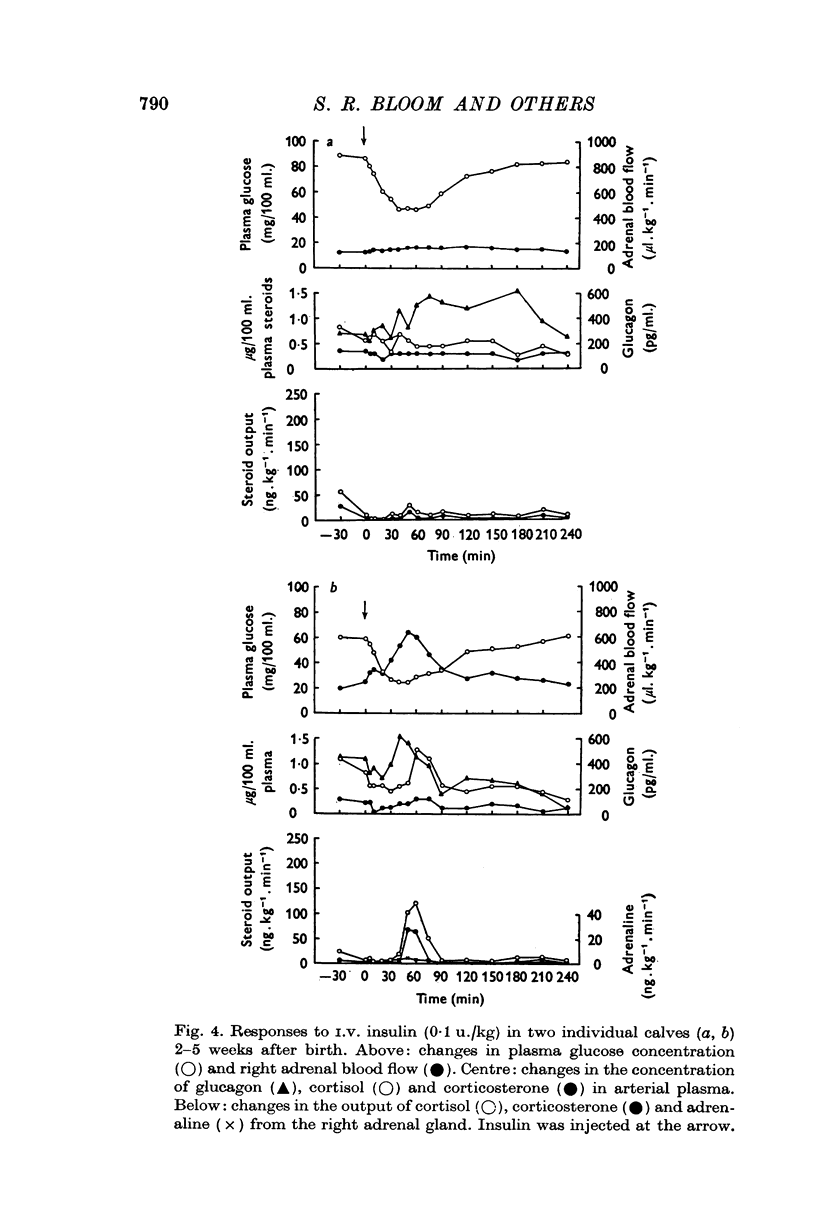

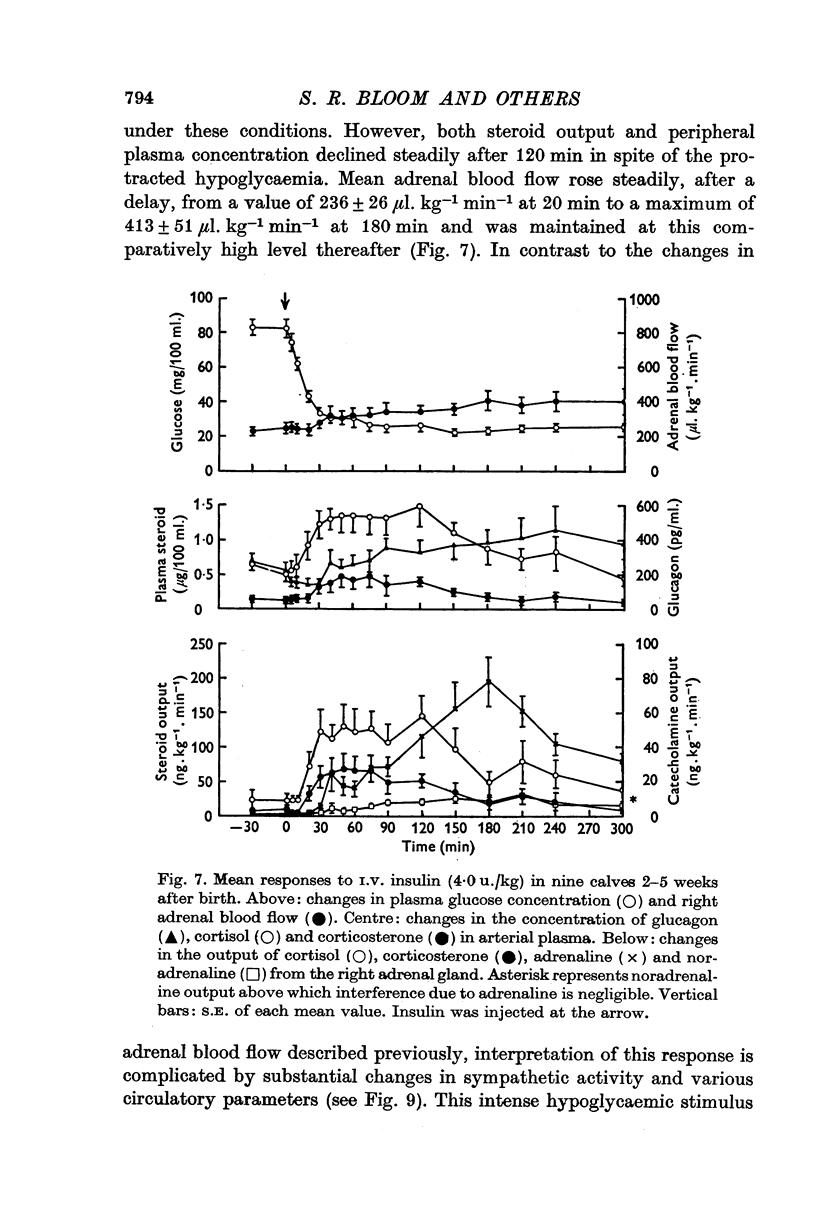
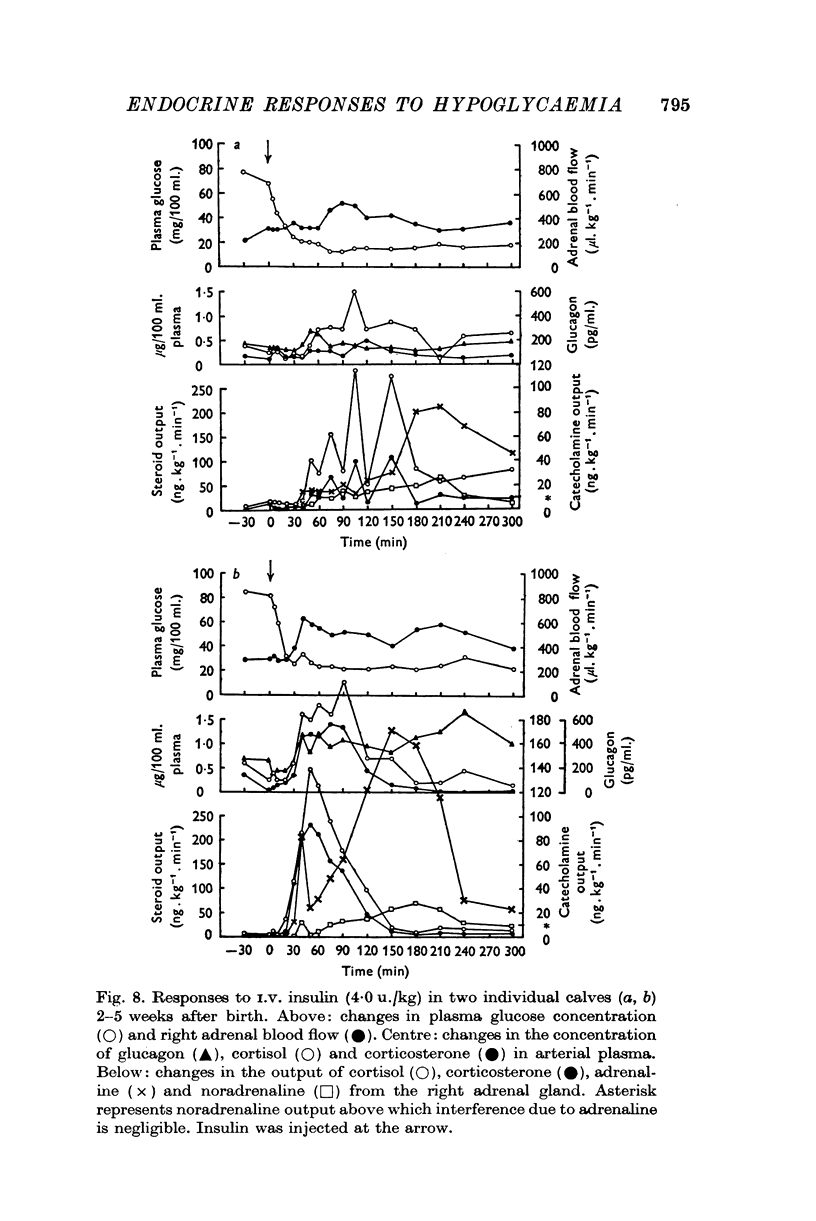







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMIN J., GRANT R. T. Adrenaline release during insulin hypoglycaemia in the rabbit. J Physiol. 1959 Dec;149:228–249. doi: 10.1113/jphysiol.1959.sp006337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTRAND J., LORAS B., FREDERICH A. [Study, in the child, of adrenal cortex participation in the correction of insulin hypoglycemia by the determination of plasma 17-hydroxycorticosteroids]. C R Seances Soc Biol Fil. 1962;156:694–698. [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Vaughan N. J. The role of the autonomic innervation in the control of glucagon release during hypoglycaemia in the calf. J Physiol. 1974 Feb;236(3):611–623. doi: 10.1113/jphysiol.1974.sp010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V., Vaughan N. J. The role of the sympathetic innervation in the control of plasma glucagon concentration in the calf. J Physiol. 1973 Sep;233(2):457–466. doi: 10.1113/jphysiol.1973.sp010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRONE C. THE SECRETION OF ADRENAL MEDULLARY HORMONES DURING HYPOGLYCEMIA IN INTACT, DECEREBRATE AND SPINAL SHEEP. Acta Physiol Scand. 1965 Mar;63:213–224. doi: 10.1111/j.1748-1716.1965.tb04061.x. [DOI] [PubMed] [Google Scholar]
- Comline R. S., Edwards A. V. The effects of insulin of the new-born calf. J Physiol. 1968 Sep;198(2):383–404. doi: 10.1113/jphysiol.1968.sp008613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comline R. S., Silver M. The development of the adrenal medulla of the foetal and new-born calf. J Physiol. 1966 Mar;183(2):305–340. doi: 10.1113/jphysiol.1966.sp007868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMOOR P., HINNEKENS M., STEENO O., DECKX R., DELAERE K., MEULEP AS E. Corticosteroid metabolism during the combined administration of ACTH and catecholamines. J Lab Clin Med. 1962 Jul;60:138–149. [PubMed] [Google Scholar]
- DUNER H. The effect of insulin hypoglycemia on the secretion of adrenaline and noradrenaline from the suprarenal of cat. Acta Physiol Scand. 1954 Oct 20;32(1):63–68. doi: 10.1111/j.1748-1716.1954.tb01155.x. [DOI] [PubMed] [Google Scholar]
- Donald R. A. Plasma immunoreactive corticotrophin and cortisol response to insulin hypoglycemia in normal subjects and patients with pituitary disease. J Clin Endocrinol Metab. 1971 Feb;32(2):225–231. doi: 10.1210/jcem-32-2-225. [DOI] [PubMed] [Google Scholar]
- Edwards A. V., Hardy R. N., Malinowska K. W. The effects of infusions of synthetic adrenocorticotrophin in the conscious calf. J Physiol. 1974 Jun;239(3):477–498. doi: 10.1113/jphysiol.1974.sp010579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOKFELT B., BYDGEMAN S. Increased adrenaline production following administration of 2-deoxy-D-glucose in the rat. Proc Soc Exp Biol Med. 1961 Mar;106:537–539. doi: 10.3181/00379727-106-26394. [DOI] [PubMed] [Google Scholar]
- Hardy R. N., Silver M., Addison K., Malinowska K. W., Edwards A. V. The response of the adrenal gland to hypoglycaemia in the conscious calf. Experientia. 1974 Jul 15;30(7):819–820. doi: 10.1007/BF01924206. [DOI] [PubMed] [Google Scholar]
- Himsworth R. L. Compensatory reactions to a lack of metabolizable glucose. J Physiol. 1968 Sep;198(2):451–465. doi: 10.1113/jphysiol.1968.sp008616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himsworth R. L. Interference with the metabolism of glucose by a non-metabolizable hexose (3-methylglucose). J Physiol. 1968 Sep;198(2):467–477. doi: 10.1113/jphysiol.1968.sp008617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGLE D. J. Permissive action of hormones. J Clin Endocrinol Metab. 1954 Oct;14(10):1272–1274. doi: 10.1210/jcem-14-10-1272. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y., Nishikai M., Kawagoe M., Yoshida K., Homma M. Plasma corticotropin, cortisol and growth hormone responses to hypoglycemia in the morning and in the evening. J Clin Endocrinol Metab. 1972 May;34(5):895–898. doi: 10.1210/jcem-34-5-895. [DOI] [PubMed] [Google Scholar]
- LANDON J., WYNN V., JAMES V. H. THE ADRENOCORTICAL RESPONSE TO INSULIN-INDUCED HYPOGLYCAEMIA. J Endocrinol. 1963 Nov;27:183–192. doi: 10.1677/joe.0.0270183. [DOI] [PubMed] [Google Scholar]
- Malinowska K. W., Hardy R. N., Nathanielsz P. W. Neonatal adrenocortical function and its possible relation to the uptake of macromolecules by the small intestine of the guinea-pig and rabbit. J Endocrinol. 1972 Nov;55(2):397–404. doi: 10.1677/joe.0.0550397. [DOI] [PubMed] [Google Scholar]
- Müller-Hess R., Geser C. A., Jéquier E., Felber J. P., Vannotti A. Effects of adrenaline on insulin-induced release of growth hormone and cortisol in man. Acta Endocrinol (Copenh) 1974 Feb;75(2):260–273. doi: 10.1530/acta.0.0750260. [DOI] [PubMed] [Google Scholar]
- SANDBERG A. A., NELSON D. H., PALMER J. G., SAMUELS L. T., TYLER F. H. The effects of epinephrine on the metabolism of 17-hydroxycorticosteroids in the human. J Clin Endocrinol Metab. 1953 Jun;13(6):629–647. doi: 10.1210/jcem-13-6-629. [DOI] [PubMed] [Google Scholar]
- SILVER M. The output of adrenaline and noradrenaline from the adrenal medulla of the calf. J Physiol. 1960 Jun;152:14–29. doi: 10.1113/jphysiol.1960.sp006466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub J. J., Jenkins J. S., Ratcliffe J. G., Landon J. Comparison of corticotrophin and corticosteroid response to lysine vasopressin, insulin, and pyrogen in man. Br Med J. 1973 Feb 3;1(5848):267–269. doi: 10.1136/bmj.1.5848.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON EULER U. S., FLODING I. A fluorimetric micromethod for differential estimation of adrenaline and noradrenaline. Acta Physiol Scand Suppl. 1955;33(118):45–56. [PubMed] [Google Scholar]
- VON EULER U. S., LUFT R. Effect of insulin on urinary excretion of adrenalin and noradrenalin; studies in ten healthy subjects and in six cases of acromegaly. Metabolism. 1952 Nov;1(6):528–532. [PubMed] [Google Scholar]
- Zukoski C. F. Mechanism of action of insulin hypoglycemia on adrenal cortical secretion. Endocrinology. 1966 Jun;78(6):1264–1267. doi: 10.1210/endo-78-6-1264. [DOI] [PubMed] [Google Scholar]


