Abstract
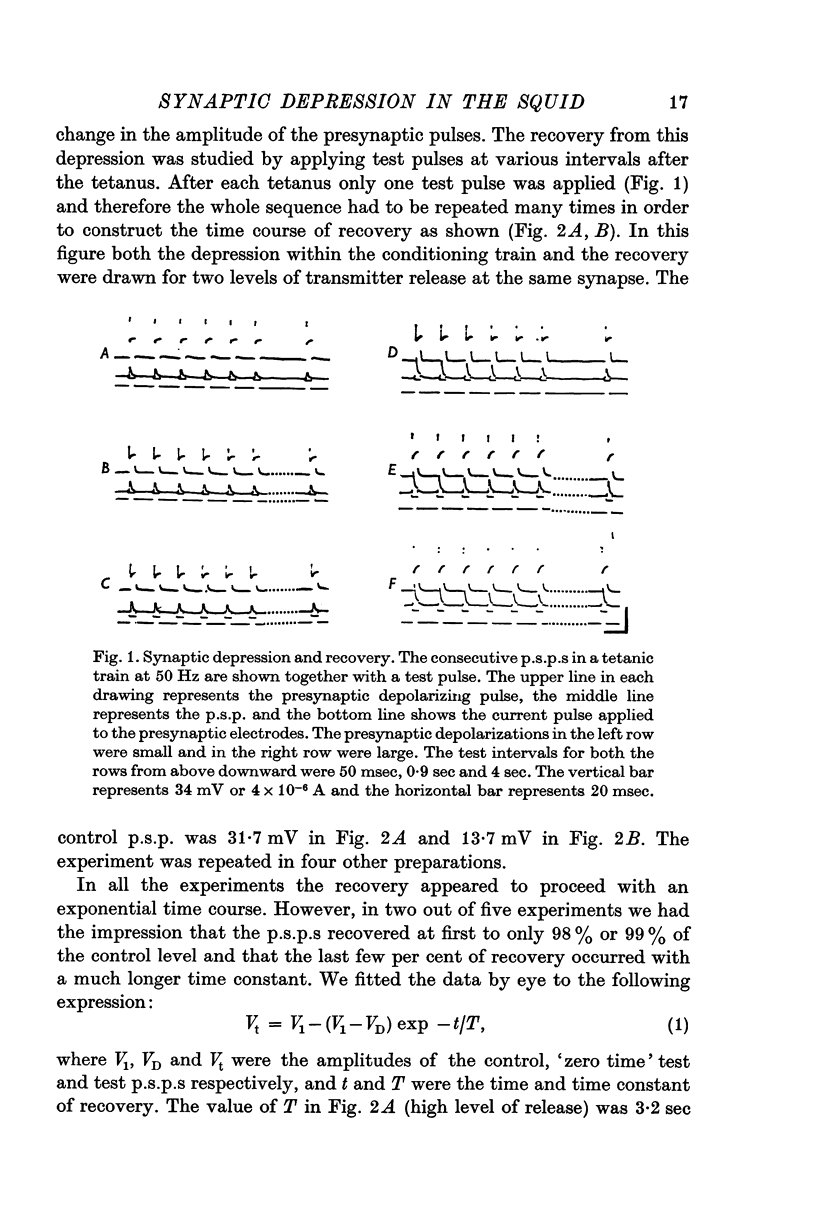
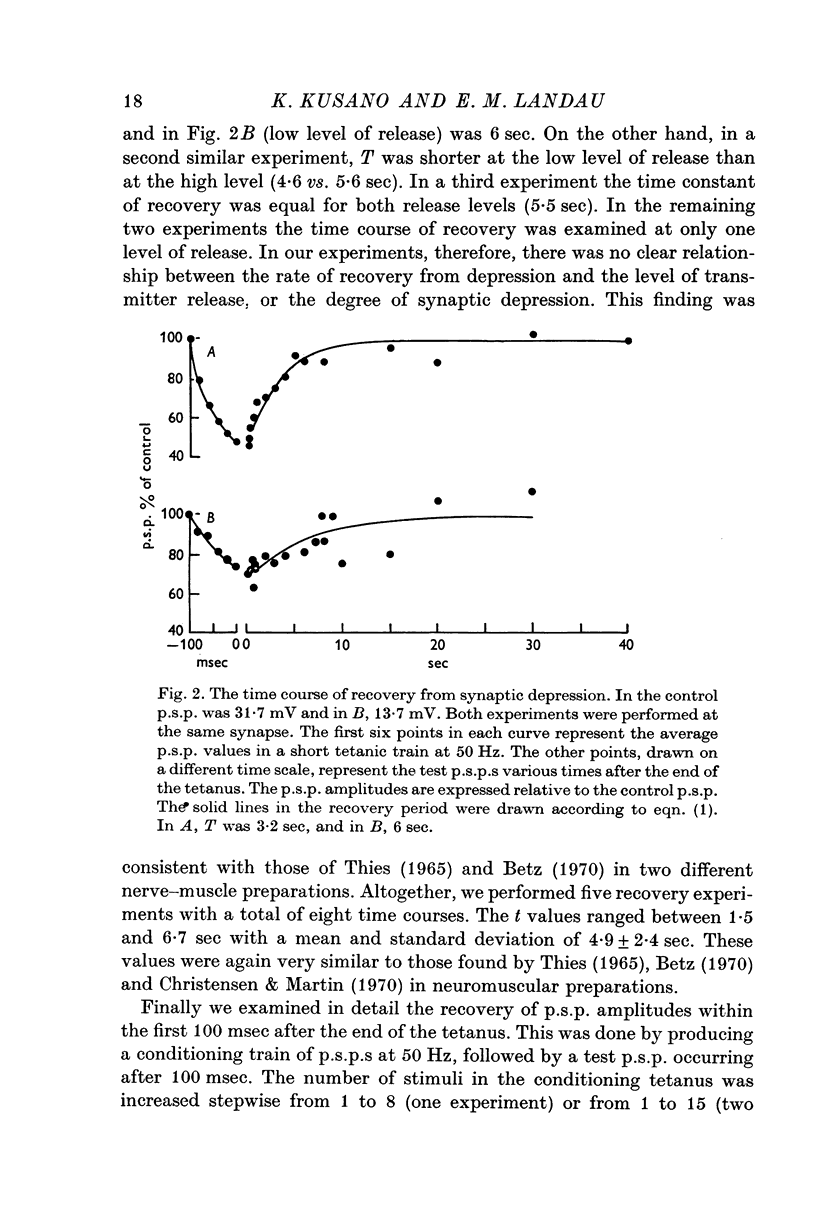
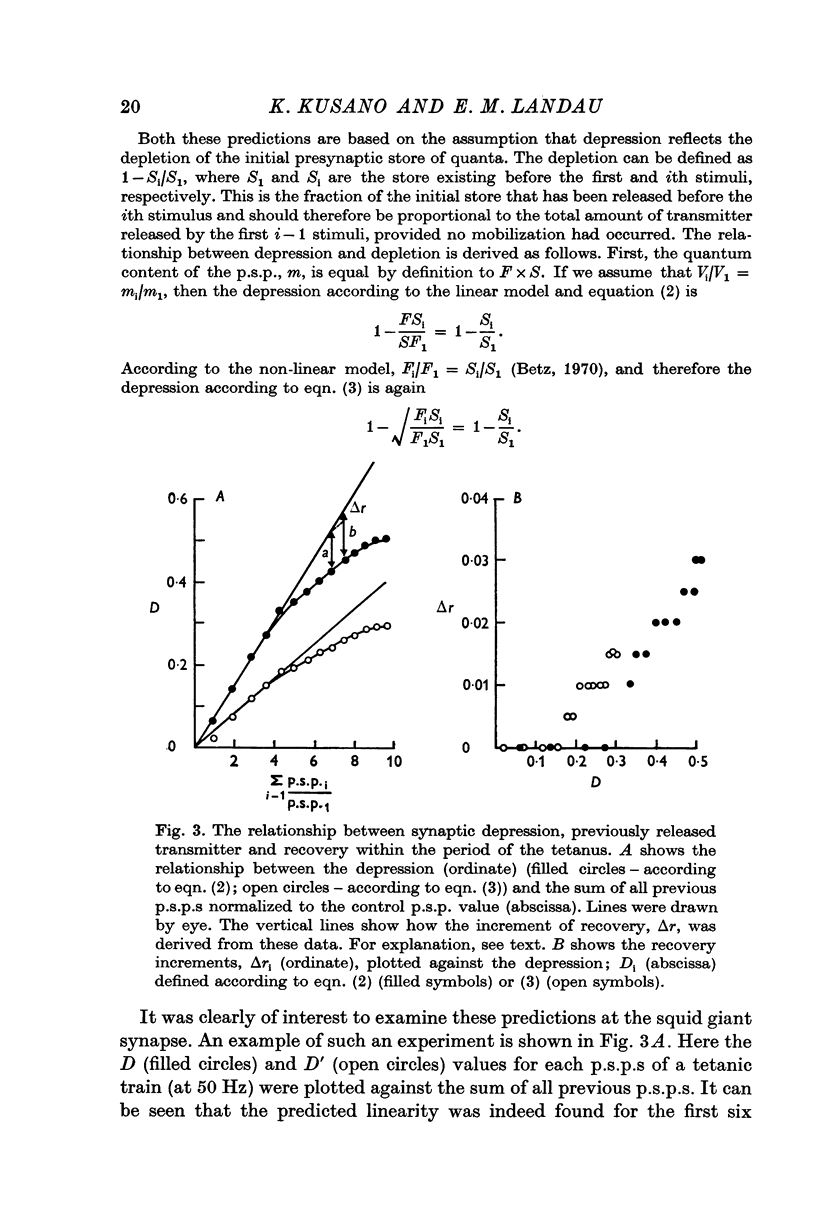
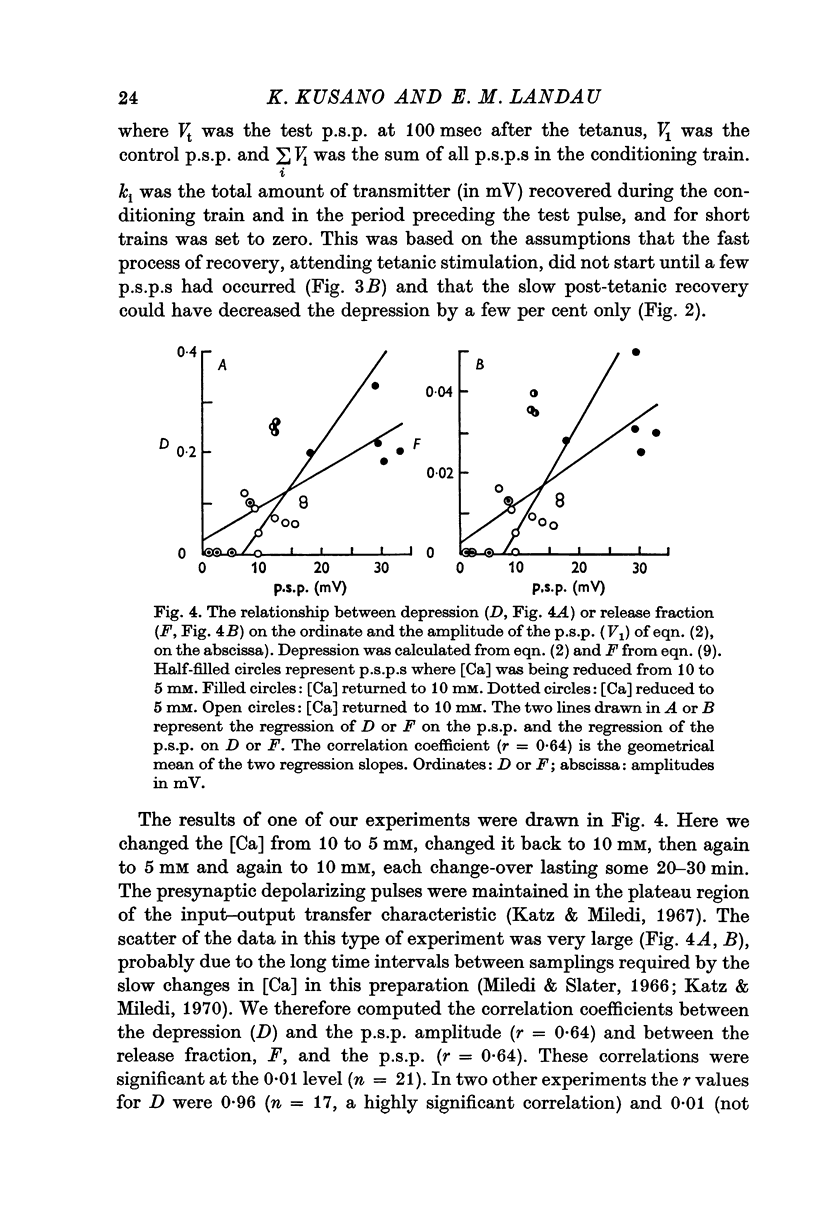
1. The process of synaptic depression and recovery were studied in the squid (Loligo pealii) giant synapse with intracellular recording and stimulating electrodes in the prescence of tetrodotoxin (10-minus 7 M). 2. When the synapse was stimulated at 50 Hz, depression occurred rapidly. Recovery after the tetanus was a first-order process with an average recovery time constant of 4-9 sec. The rate of recovery was independent of the amplitude of the post-synaptic potential (p.s.p.) or the degree of depression. 3. For the first five to seven p.s.p.s in the train there was a linear relationship between depression and the total amount of transmitter previously released. This may indicate that depression in this preparation was caused by the depletion of the presynaptic store of transmitter (S). 4. Assuming that this interpretation was correct, we could show that recovery from depression during the tetanus (i.e. 'mobilization') proceeded about 10 times faster than after the end of the tetanus. 5. When the amplitude of the p.s.p. was varied by changing the bathing calcium concentration, [Ca], the degree of depression was correlated to the amplitude of the p.s.p. 6. When the amplitude of the p.s.p. was increased by increasing pre-synaptic depolarization, synaptic depression was found to increase as well. However, synaptic depression increased less than the amplitude of the p.s.p., the relationship between these two measures being non-linear. 7. This finding is interpreted to indicate that the transmitter stores, S, are closely related to the area of the presynaptic membrane which is sufficiently depolarized to release transmitter.
Full text
PDF







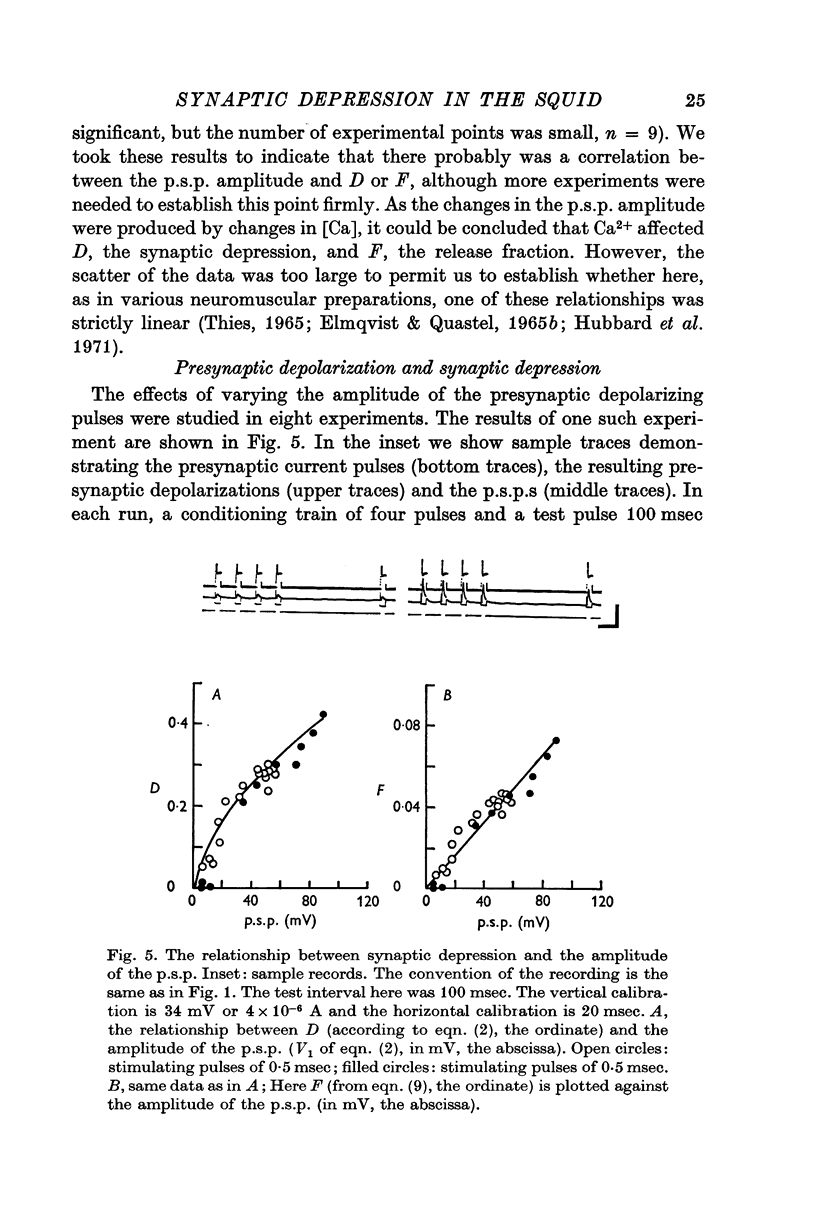
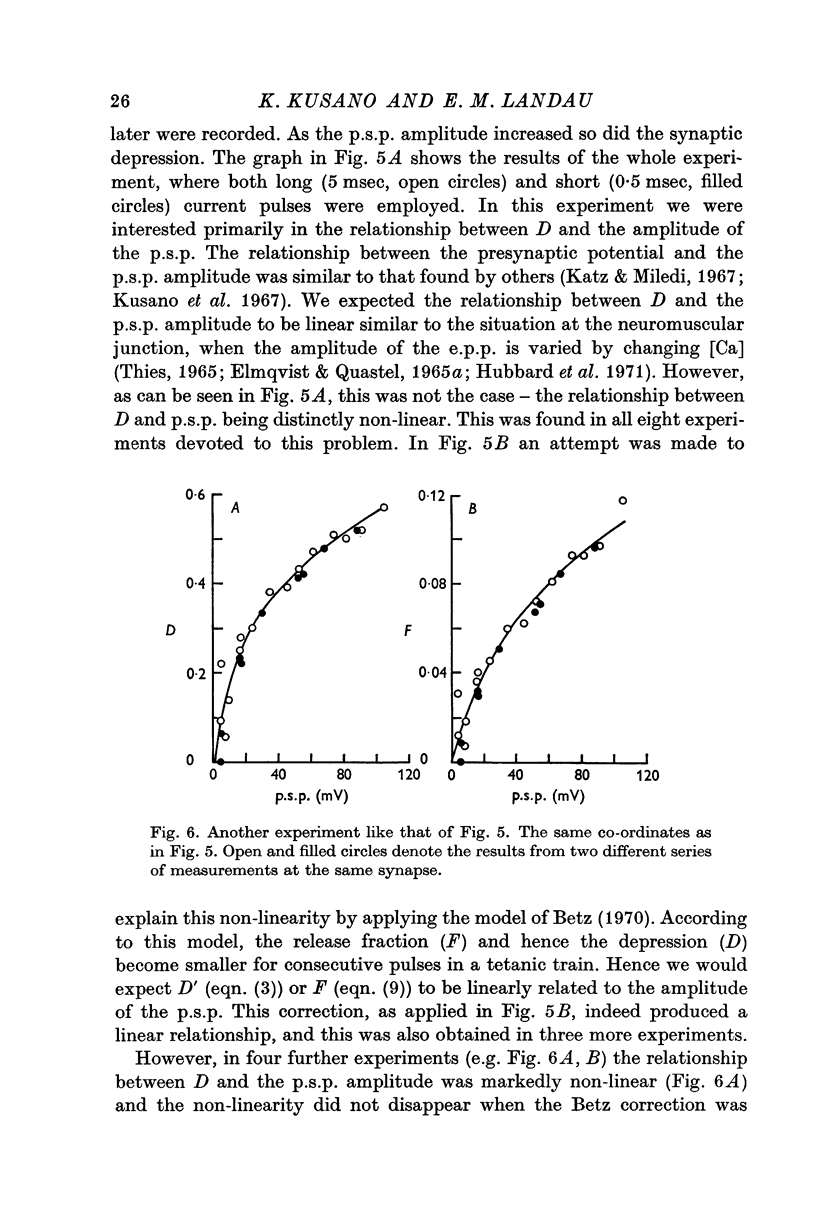






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A. A., Bennett M. V. Chemically mediated transmission at a giant fiber synapse in the central nervous system of a vertebrate. J Gen Physiol. 1969 Feb;53(2):183–210. doi: 10.1085/jgp.53.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., HAYHOW W. R. Repetitive stimulation of optic nerve and lateral geniculate synapses. Exp Neurol. 1959 Dec;1:534–555. doi: 10.1016/0014-4886(59)90016-0. [DOI] [PubMed] [Google Scholar]
- BRYANT S. H. Transmission in squid giant synapses: the importance of oxygen supply and the effects of drugs. J Gen Physiol. 1958 Jan 20;41(3):473–484. doi: 10.1085/jgp.41.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLOCK T. H., HAGIWARA S. Intracellular recording from the giant synapse of the squid. J Gen Physiol. 1957 Mar 20;40(4):565–577. doi: 10.1085/jgp.40.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLOCK T. H. Properties of a single synapse in the stellate ganglion of squid. J Neurophysiol. 1948 Jul;11(4):343–364. doi: 10.1152/jn.1948.11.4.343. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M. An electrophysiological analysis of the storage of acetylcholine in preganglionic nerve terminals. J Physiol. 1972 Mar;221(3):657–668. doi: 10.1113/jphysiol.1972.sp009774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M. An electrophysiological analysis of the synthesis of acetylcholine in preganglionic nerve terminals. J Physiol. 1972 Mar;221(3):669–682. doi: 10.1113/jphysiol.1972.sp009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970 Mar;206(3):629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloedel J., Gage P. W., Llinás R., Quastel D. M. Transmitter release at the squid giant synapse in the presence of tetrodotoxin. Nature. 1966 Oct 1;212(5057):49–50. doi: 10.1038/212049a0. [DOI] [PubMed] [Google Scholar]
- Bruner J., Dkennedy D. Habituation: occurrence at a neuromuscular junction. Science. 1970 Jul 3;169(3940):92–94. doi: 10.1126/science.169.3940.92. [DOI] [PubMed] [Google Scholar]
- Bruner J., Tauc L. Habituation at the synaptic level in Aplysia. Nature. 1966 Apr 2;210(5031):37–39. doi: 10.1038/210037a0. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callec J. J., Guillet J. C., Pichon Y., Boistel J. Further studies on synaptic transmission in insects. II. Relations between sensory information and its synaptic integration at the level of a single giant axon in the cockroach. J Exp Biol. 1971 Aug;55(1):123–149. doi: 10.1242/jeb.55.1.123. [DOI] [PubMed] [Google Scholar]
- Castellucci V., Pinsker H., Kupfermann I., Kandel E. R. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970 Mar 27;167(3926):1745–1748. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- Christensen B. N., Martin A. R. Estimates of probability of transmitter release at the mammalian neuromuscular junction. J Physiol. 1970 Nov;210(4):933–945. doi: 10.1113/jphysiol.1970.sp009250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., OSCARSSON O., WILLIS W. D. Synaptic action of group I and II afferent fibres of muscle on the cells of the dorsal spinocerebellar tract. J Physiol. 1961 Oct;158:517–543. doi: 10.1113/jphysiol.1961.sp006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M. Intracellular potentials recorded from a mammalian sympathetic ganglion. J Physiol. 1955 Dec 29;130(3):572–584. doi: 10.1113/jphysiol.1955.sp005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Quastel D. M. A quantitative study of end-plate potentials in isolated human muscle. J Physiol. 1965 Jun;178(3):505–529. doi: 10.1113/jphysiol.1965.sp007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D., Kandel E. R. Diphasic postsynaptic potential: a chemical synapse capable of mediating conjoint excitation and inhibition. Science. 1972 May 12;176(4035):675–678. doi: 10.1126/science.176.4035.675. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., BULLOCK T. H. Intracellular potentials in pacemaker and integrative neurons of the lobster cardiac ganglion. J Cell Physiol. 1957 Aug;50(1):25–47. doi: 10.1002/jcp.1030500103. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., TASAKI I. A study on the mechanism of impulse transmission across the giant synapse of the squid. J Physiol. 1958 Aug 29;143(1):114–137. doi: 10.1113/jphysiol.1958.sp006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., WILLIS W. D. Hyperpolarization of mammalian motor nerve terminals. J Physiol. 1962 Aug;163:115–137. doi: 10.1113/jphysiol.1962.sp006961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn G., Wright M. J. Characteristics of transmission ilure in the squid stellate ganglion: a study of a simple habituating system. J Exp Biol. 1970 Feb;52(1):217–231. doi: 10.1242/jeb.52.1.217. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. The effect of temperature change upon transmitter release, facilitation and post-tetanic potentiation. J Physiol. 1971 Aug;216(3):591–609. doi: 10.1113/jphysiol.1971.sp009542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Kwanbunbumpen S. Evidence for the vesicle hypothesis. J Physiol. 1968 Feb;194(2):407–420. doi: 10.1113/jphysiol.1968.sp008415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Willis W. D. The effects of depolarization of motor nerve terminals upon the release of transmitter by nerve impulses. J Physiol. 1968 Feb;194(2):381–405. doi: 10.1113/jphysiol.1968.sp008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F. Neuromuscular transmission in a mammalian preparation in the absence of blocking drugs and the effect of D-tubocurarine. J Physiol. 1973 Jan;228(2):307–325. doi: 10.1113/jphysiol.1973.sp010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F. Reduction of the quantum content of endplate potentials by atropine. Experientia. 1970 Nov 15;26(11):1234–1235. doi: 10.1007/BF01897985. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol. 1970 May;207(3):789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Livengood D. R., Werman R. Correlation of transmitter release with membrane properties of the presynaptic fiber of the squid giant synapse. J Gen Physiol. 1967 Dec;50(11):2579–2601. doi: 10.1085/jgp.50.11.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W., NORTH K. A. An electrical investigation of effects of repetitive stimulation on mammalian neuromuscular junction. J Neurophysiol. 1953 Sep;16(5):509–527. doi: 10.1152/jn.1953.16.5.509. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., QUILISCH H. Presynaptic potentiation and depression of neuromuscular transmission in frog and rat. Acta Physiol Scand Suppl. 1953;111:111–120. [PubMed] [Google Scholar]
- Lass Y., Halevi Y., Landau E. M., Gitter S. A new model for transmitter mobilization in the frog neuromuscular junction. Pflugers Arch. 1973 Oct 17;343(2):157–163. doi: 10.1007/BF00585711. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L. The effect of repetitive stimulation on facilitation of transmitter release at the frog neuromuscular junction. J Physiol. 1973 Oct;234(2):327–352. doi: 10.1113/jphysiol.1973.sp010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. The relation between quantum content and facilitation at the neuromuscular junction of the frog. J Physiol. 1968 Jun;196(3):593–604. doi: 10.1113/jphysiol.1968.sp008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless D. L., Zablocka-Esplin B., Esplin D. W. Rates of transmitter turnover in the cat superior cervical ganglion estimated by electrophysiological techniques. J Neurophysiol. 1971 Sep;34(5):817–830. doi: 10.1152/jn.1971.34.5.817. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. The action of calcium on neuronal synapses in the squid. J Physiol. 1966 May;184(2):473–498. doi: 10.1113/jphysiol.1966.sp007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter action in the giant synapse of the squid. Nature. 1969 Sep 20;223(5212):1284–1286. doi: 10.1038/2231284a0. [DOI] [PubMed] [Google Scholar]
- Nicholls J. G., Purves D. A comparison of chemical and electrical synaptic transmission between single sensory cells and a motoneurone in the central nervous system of the leech. J Physiol. 1972 Sep;225(3):637–656. doi: 10.1113/jphysiol.1972.sp009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D. Potentiation and depression of synaptic transmission in the olfactory cortex of the guinea-pig. J Physiol. 1972 Apr;222(1):209–231. doi: 10.1113/jphysiol.1972.sp009794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Electrical changes in pre- and postsynaptic axons of the giant synapse of Loligo. J Gen Physiol. 1962 Jul;45:1181–1193. doi: 10.1085/jgp.45.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIES R. E. NEUROMUSCULAR DEPRESSION AND THE APPARENT DEPLETION OF TRANSMITTER IN MAMMALIAN MUSCLE. J Neurophysiol. 1965 May;28:428–442. doi: 10.1152/jn.1965.28.3.427. [DOI] [PubMed] [Google Scholar]
- Wachtel H., Kandel E. R. A direct synaptic connection mediating both excitation and inhibition. Science. 1967 Dec 1;158(3805):1206–1208. doi: 10.1126/science.158.3805.1206. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Changes in the statistics of transmitter release during facilitation. J Physiol. 1973 Mar;229(3):787–810. doi: 10.1113/jphysiol.1973.sp010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Crayfish escape behavior and central synapses. II. Physiological mechanisms underlying behavioral habituation. J Neurophysiol. 1972 Sep;35(5):621–637. doi: 10.1152/jn.1972.35.5.621. [DOI] [PubMed] [Google Scholar]


