Abstract
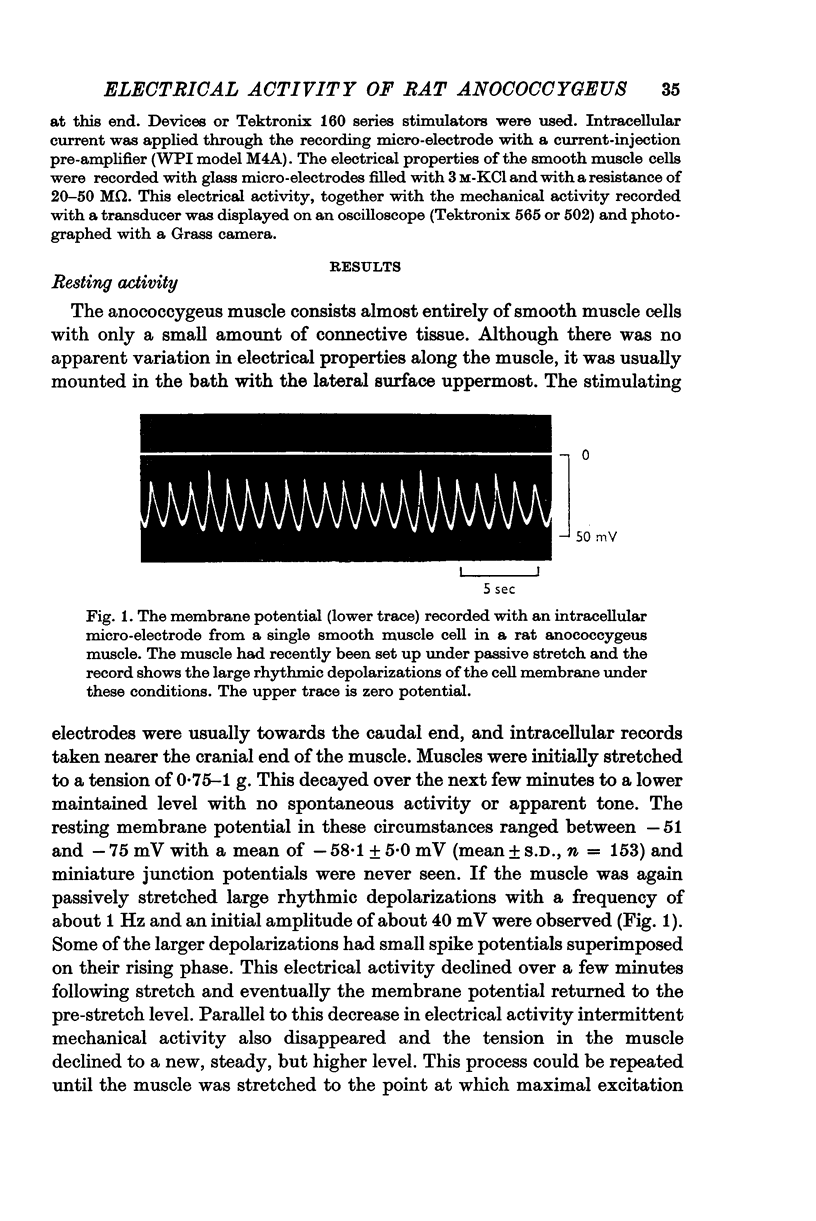
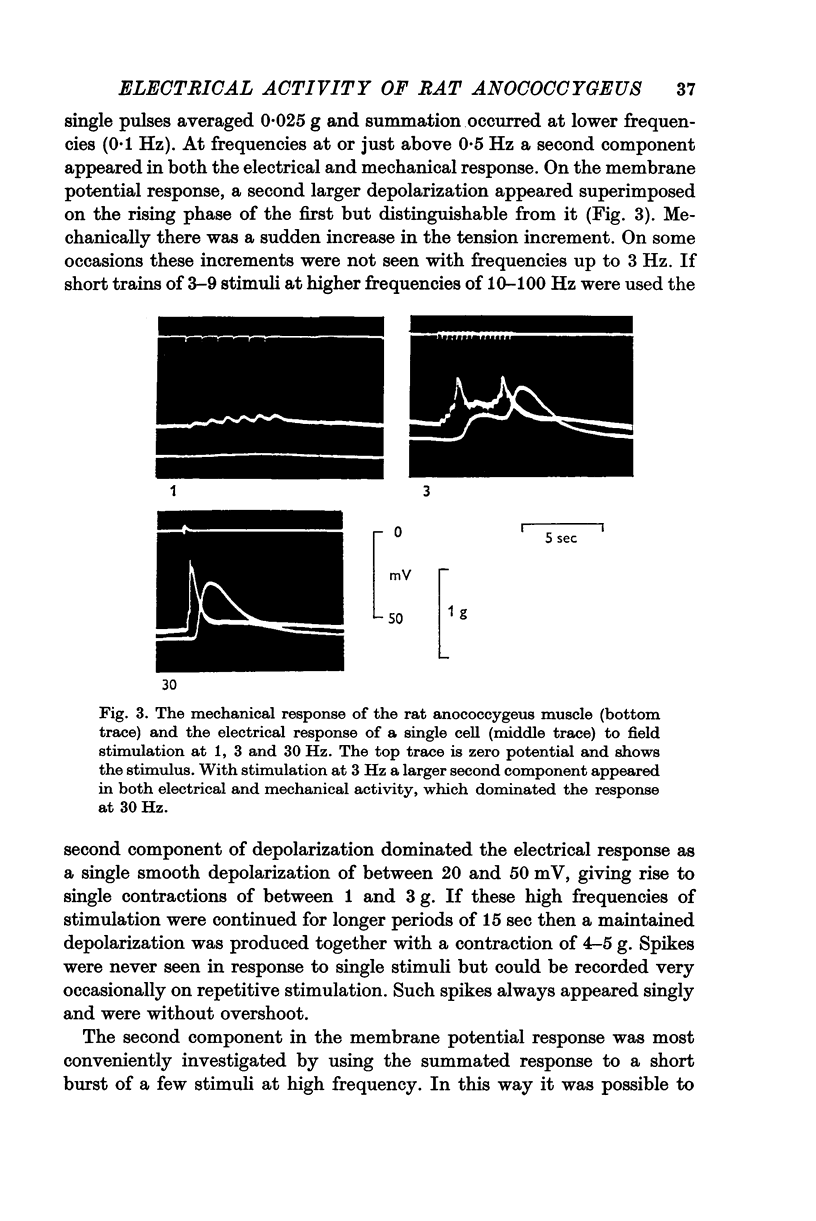
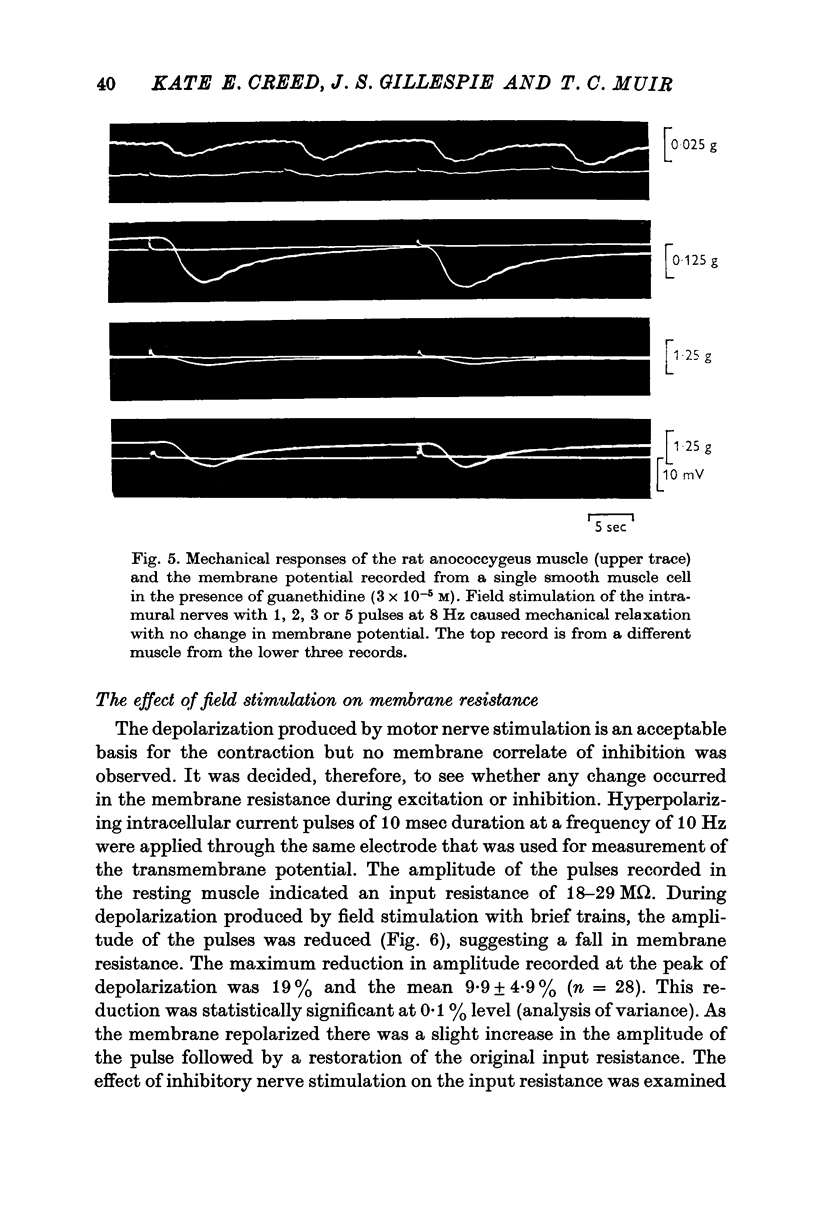
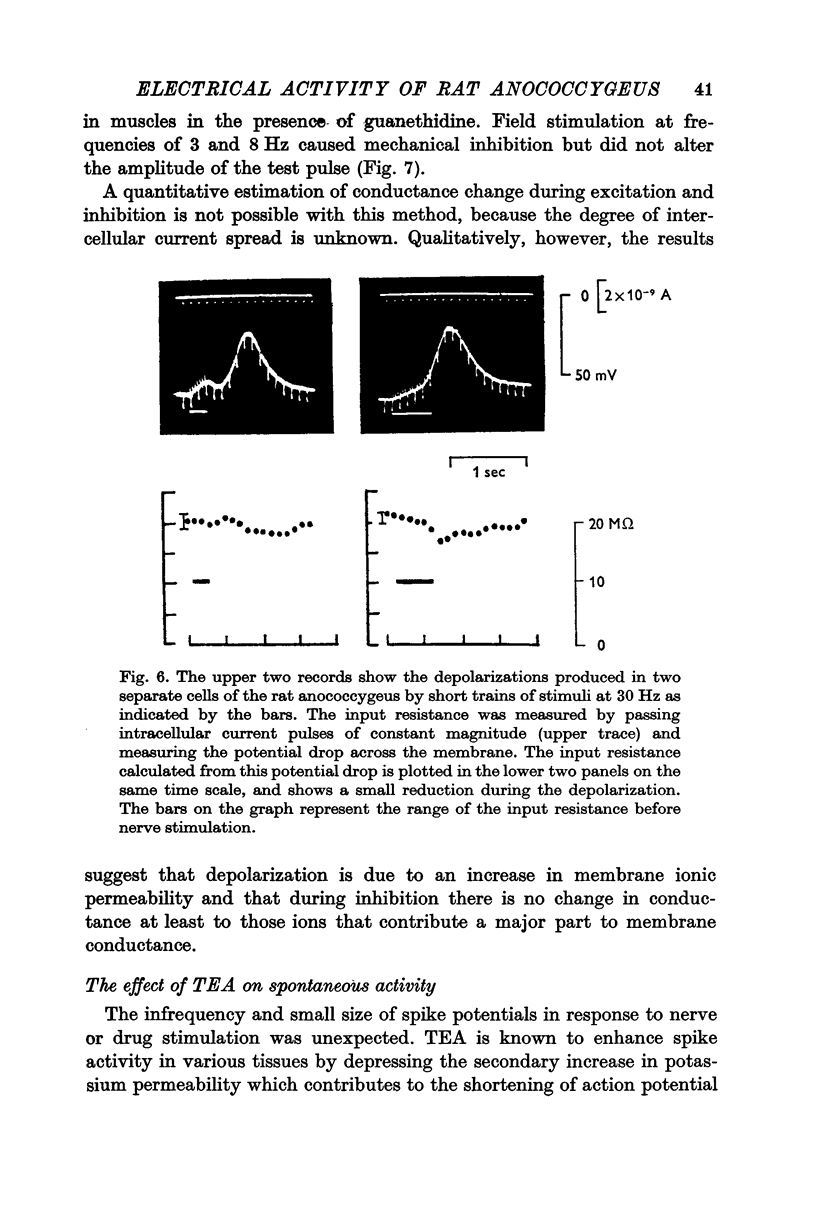
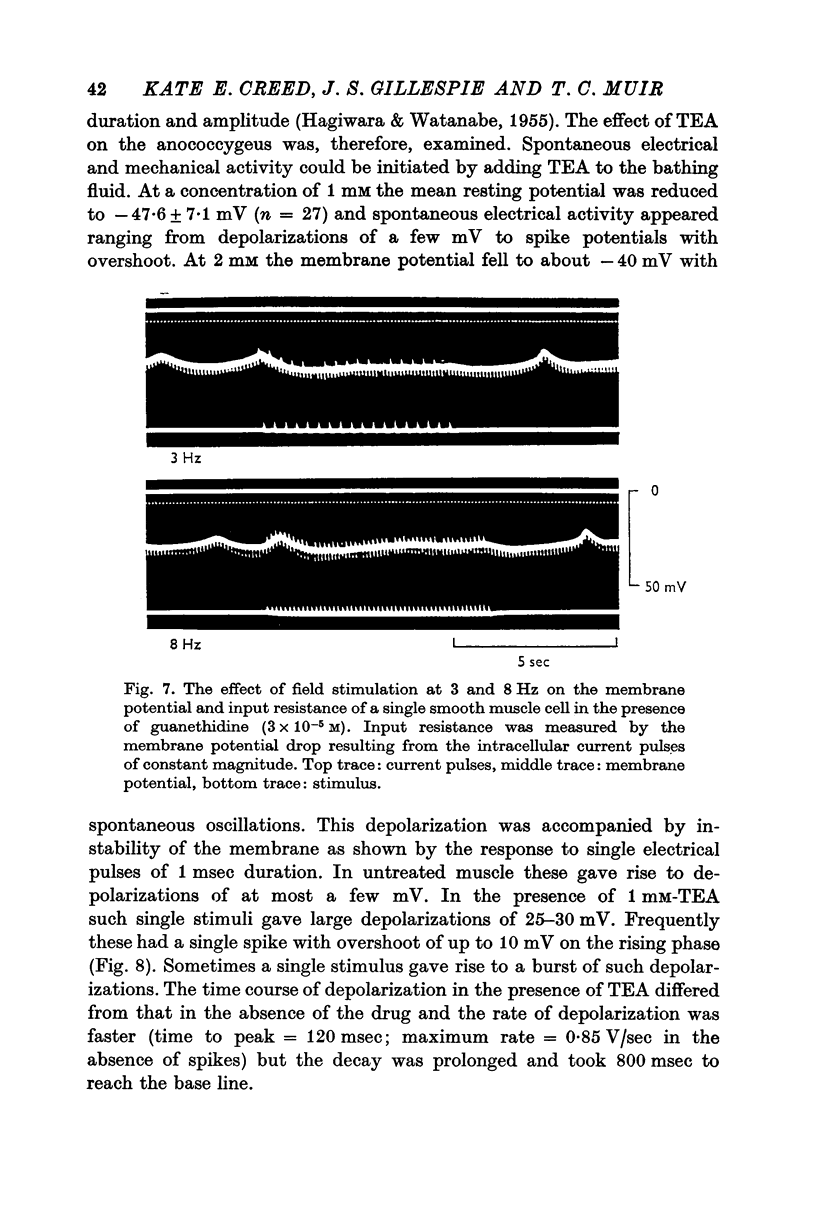
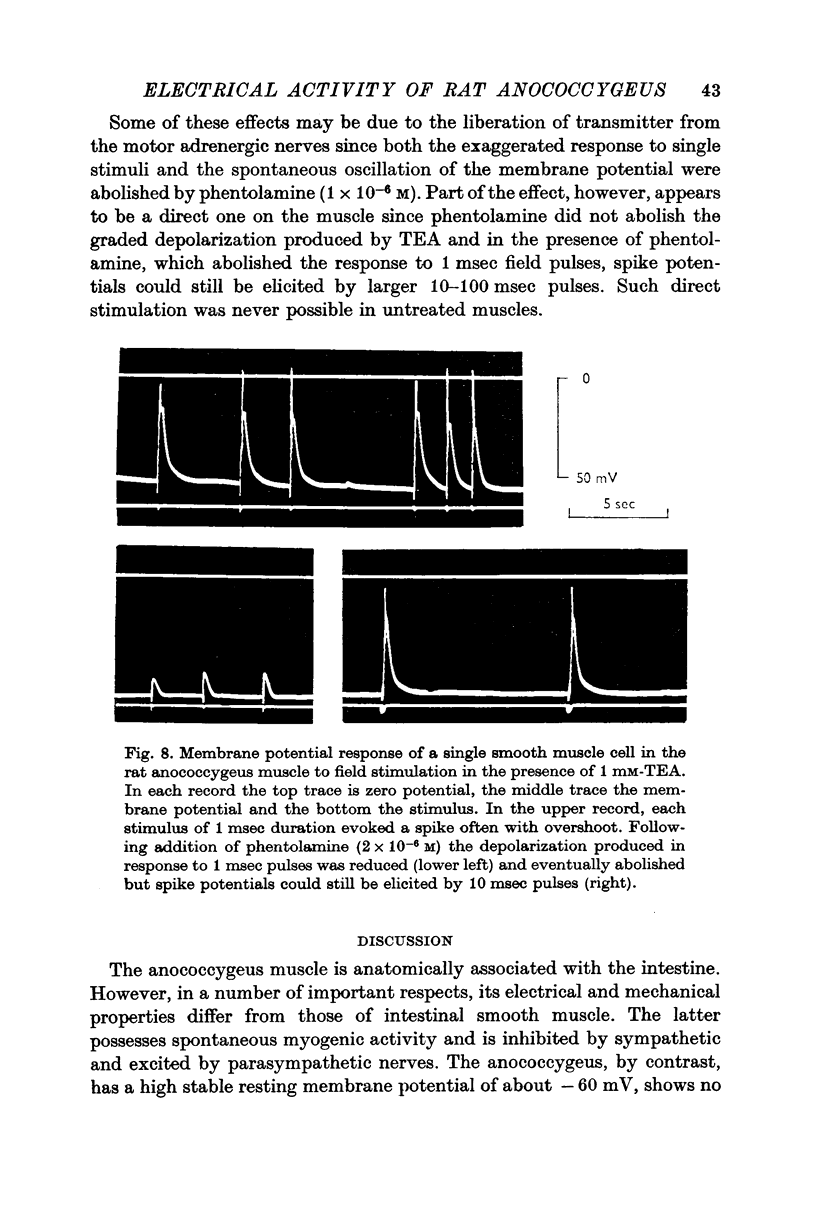
1. The transmembrane potential of smooth muscle cells of the rat anococcygeus muscle was studied with micro-electrodes. The muscle had a mean resting membrane potential of --61-5 mV and normally lacked spontaneous mechanical and electrical activity. 2. Stimulation of intramural nerves with single pulses produced a small depolarization and contraction. At frequencies greater than 0.5 Hz a second component occurred which had a maximum value of 35--51 mV with short trains at 30 Hz. Spike potentials were rarely seen. Depolarization was accompanied by a decrease in membrane resistance. 3. Noradrenaline (3 times 10-minus 5 M) and guanethidine (3 times 10-minus 5 M) both depolarized the membrane and produced contraction. Initially, oscillations in the membrane potential were oftern seen. 4. Intramural nerve stimulation in the presence of guanethidine damped the oscillations and produced relaxation. This was accompanied by neither hyperpolarization nor a change in membrane resistance. 5. TEA (1 mM) depolarized the membrane (--47-6 mV) and initiated spontaneous activity. Field stimulation evoked spikes with overshoot.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnstock G., Holman M. E. Junction potentials at adrenergic synapses. Pharmacol Rev. 1966 Mar;18(1):481–493. [PubMed] [Google Scholar]
- Furness J. B., Costa M. The nervous release and the action of substances which affect intestinal muscle through neither adrenoreceptors nor cholinoreceptors. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):123–133. doi: 10.1098/rstb.1973.0015. [DOI] [PubMed] [Google Scholar]
- GILLESPIE J. S. Spontaneous mechanical and electrical activity of stretched and unstretched intestinal smooth muscle cells and their response to sympathetic-nerve stimulation. J Physiol. 1962 Jun;162:54–75. doi: 10.1113/jphysiol.1962.sp006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Gillespie J. S. The effect of immunosympathectomy and of 6-hydroxydopamine on the responses of the rat anococcygeus to nerve stimulation and to some drugs. Br J Pharmacol. 1973 Feb;47(2):261–267. doi: 10.1111/j.1476-5381.1973.tb08323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Creed K. E., Muir T. C. The mechanisms of action of neurotransmitters. Electrical changes underlying excitation and inhibition in intestinal and related smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):95–106. doi: 10.1098/rstb.1973.0012. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Lüllmann-Rauch R. On the ultrastructure of the rat anococcygeus muscle. Cell Tissue Res. 1974;149(1):91–104. doi: 10.1007/BF00209052. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Maxwell J. D. Adrenergic innervation of sphincteric and nonsphincteric smooth muscle in the rat intestine. J Histochem Cytochem. 1971 Nov;19(11):676–681. doi: 10.1177/19.11.676. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., McGrath J. C. The response of the cat anococcygeus muscle to nerve or drug stimulation and a comparison with the rat anococcygeus. Br J Pharmacol. 1974 Jan;50(1):109–118. doi: 10.1111/j.1476-5381.1974.tb09597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., McGrath J. C. The spinal origin of the motor and inhibitory innervation of the rat anococcygeus muscles. J Physiol. 1973 May;230(3):659–672. doi: 10.1113/jphysiol.1973.sp010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S. The rat anococcygeus muscle and its response to nerve stimulation and to some drugs. Br J Pharmacol. 1972 Jul;45(3):404–416. doi: 10.1111/j.1476-5381.1972.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., WATANABE A. The effect of tetraethylammonium chloride on the muscle membrane examined with an intracellular microelectrode. J Physiol. 1955 Sep 28;129(3):513–527. doi: 10.1113/jphysiol.1955.sp005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMPHREY J. H., JAQUES R. The release of histamine and 5-hydroxytryptamine (serotonin) from platelets by antigen-antibody reactions (in vitro). J Physiol. 1955 Apr 28;128(1):9–27. doi: 10.1113/jphysiol.1955.sp005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H., Sakamoto Y. Effects of tetraethylammonium chloride on the membrane activity of guinea-pig stomach smooth muscle. J Physiol. 1970 Dec;211(2):445–460. doi: 10.1113/jphysiol.1970.sp009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Tasaki H. Electrophysiological studies of the antrum muscle fibers of the guinea pig stomach. J Gen Physiol. 1970 Jan;55(1):48–62. doi: 10.1085/jgp.55.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurszewski J. H., Bülbring E. The stimulant action of acetylcholine and catecholamines on the uterus. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):149–156. doi: 10.1098/rstb.1973.0017. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Haefely W., Staehelin H. Potentiation by tetraethylammonium of the response of the cat spleen to postganglionic sympathetic nerve stimulation. J Pharmacol Exp Ther. 1967 Sep;157(3):532–540. [PubMed] [Google Scholar]
- Tomita T. Spike propagation in the smooth muscle of the guinea-pig taenia coli. J Physiol. 1967 Aug;191(3):517–527. doi: 10.1113/jphysiol.1967.sp008265. [DOI] [PMC free article] [PubMed] [Google Scholar]


