Abstract
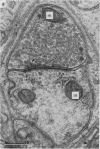
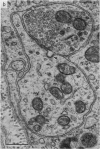
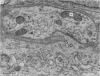
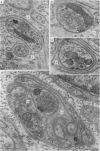
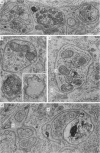
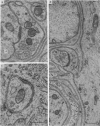
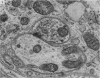
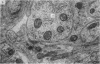
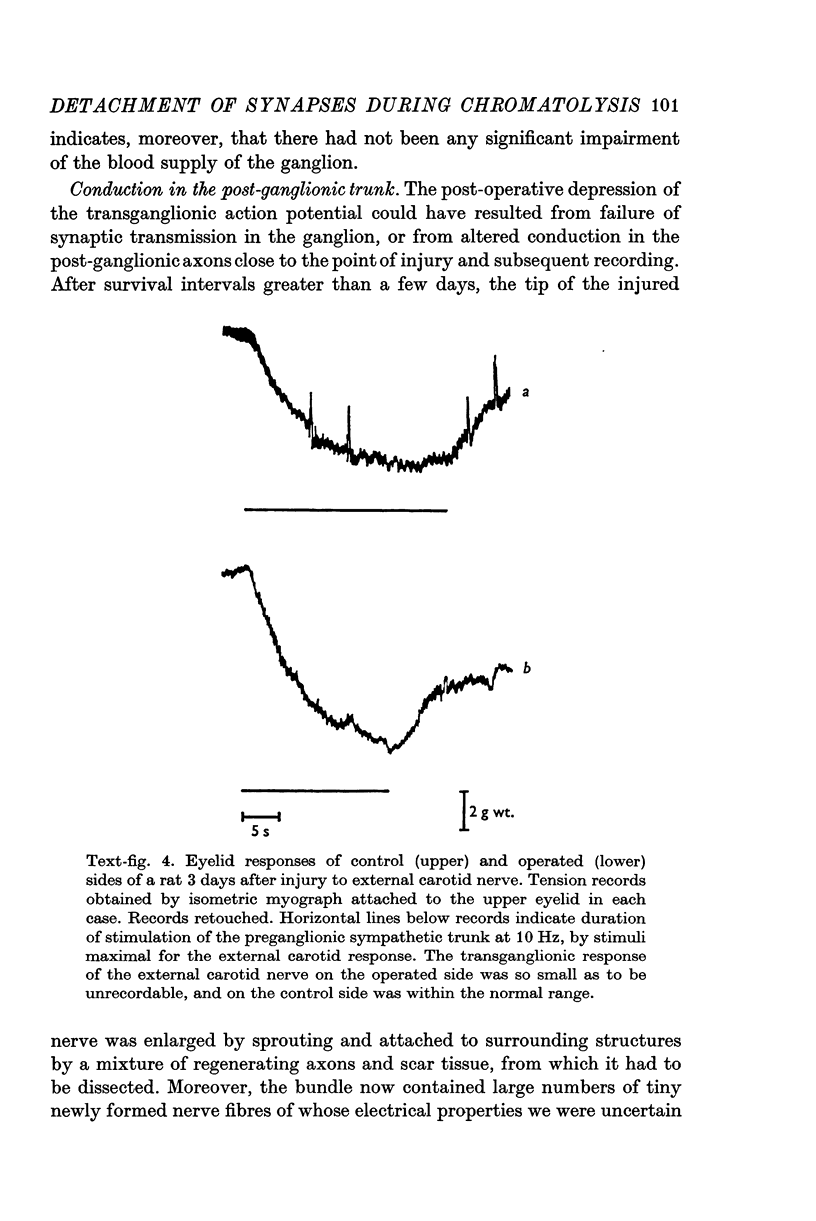
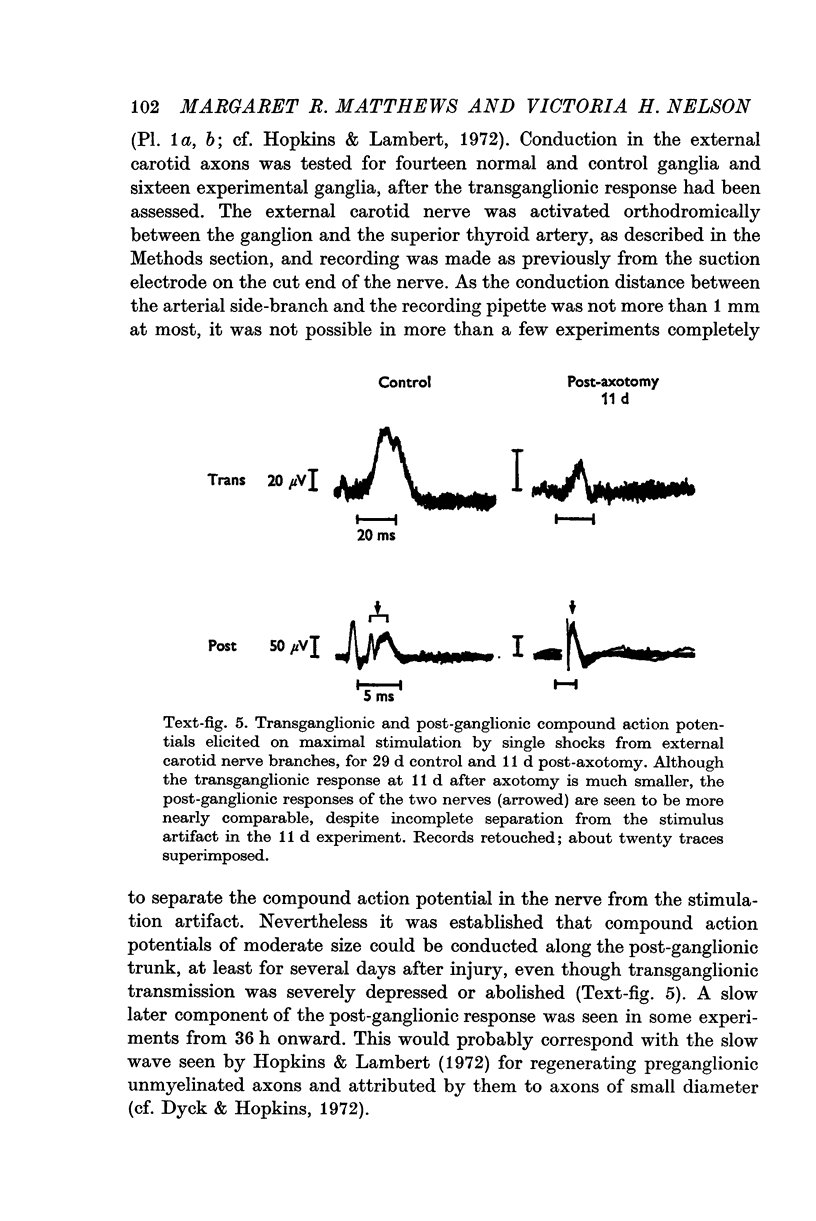
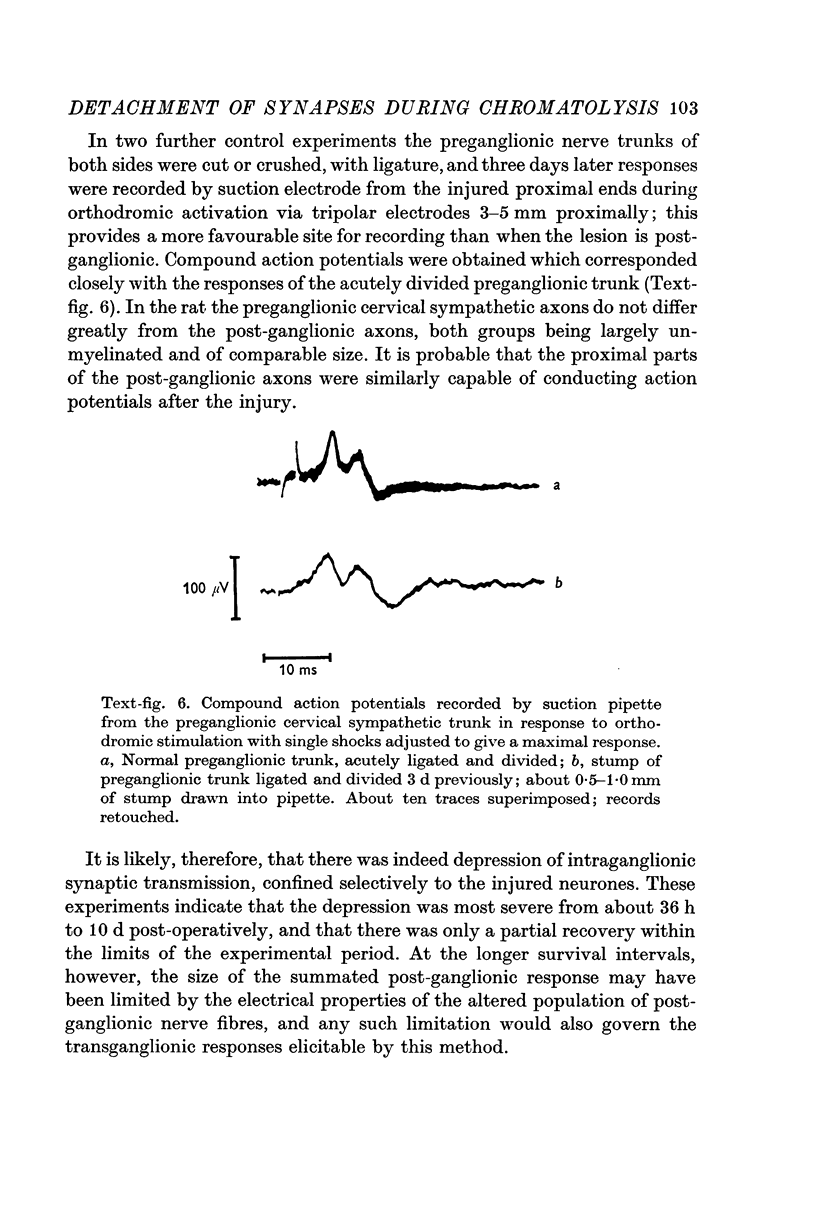
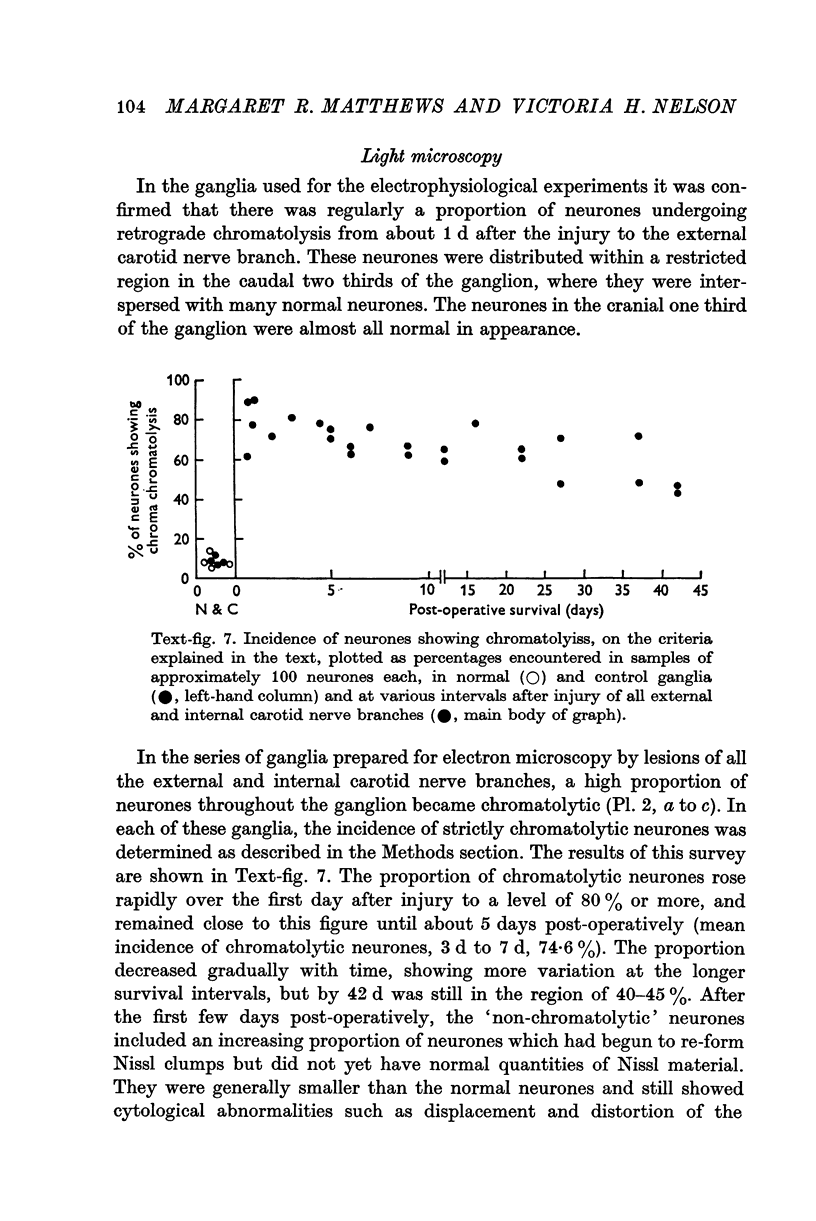
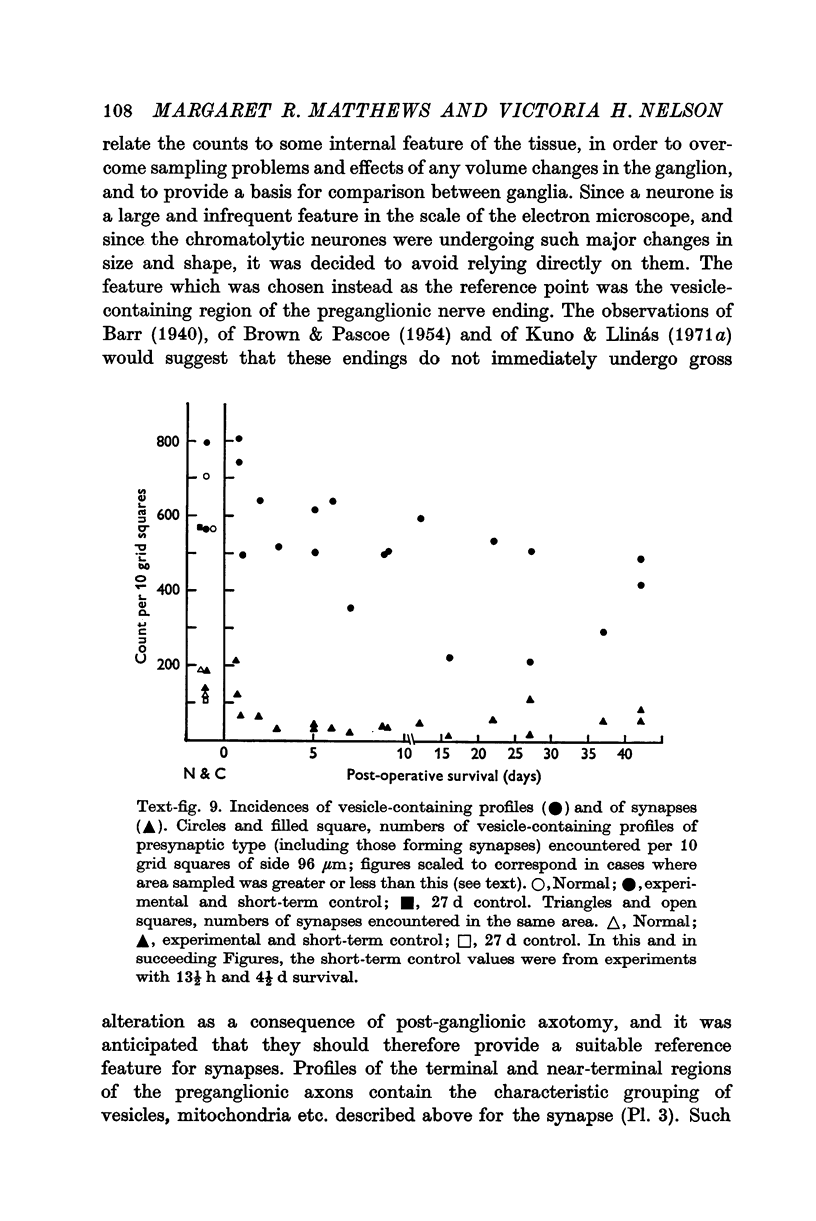
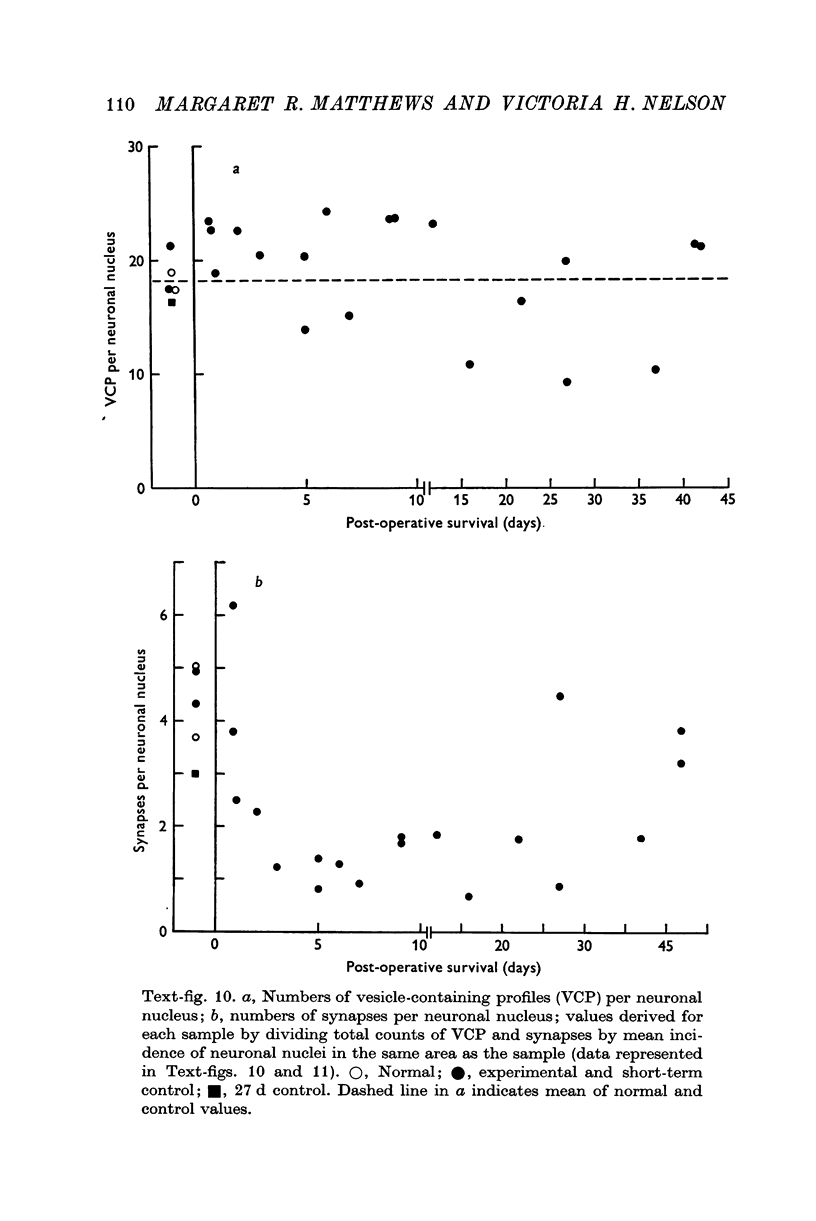
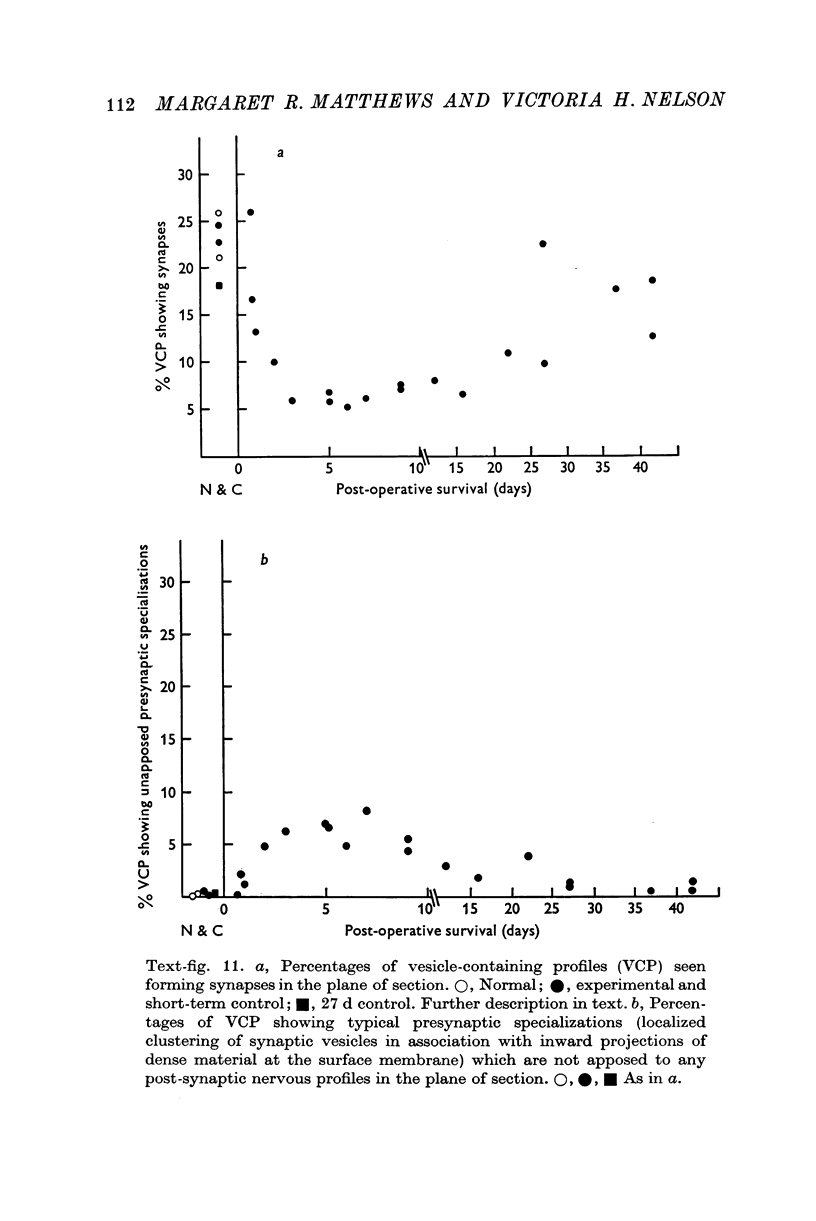
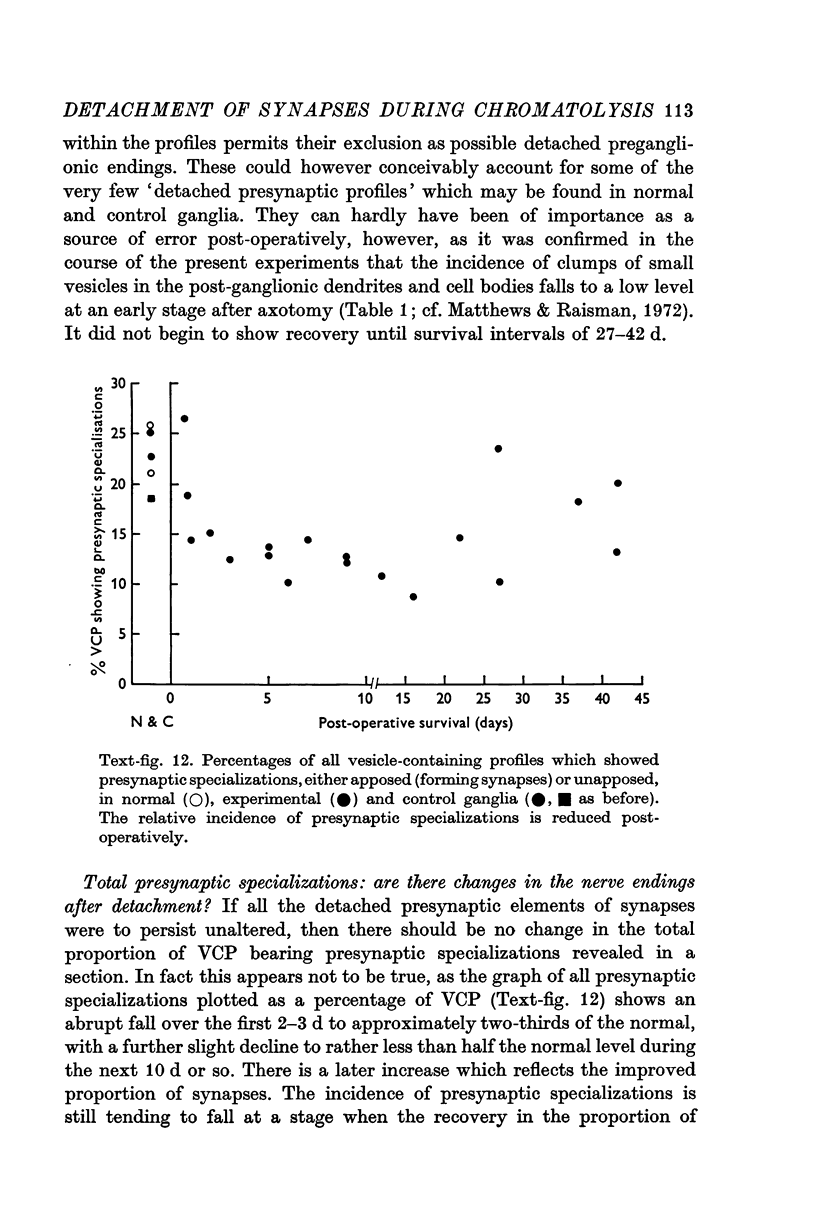
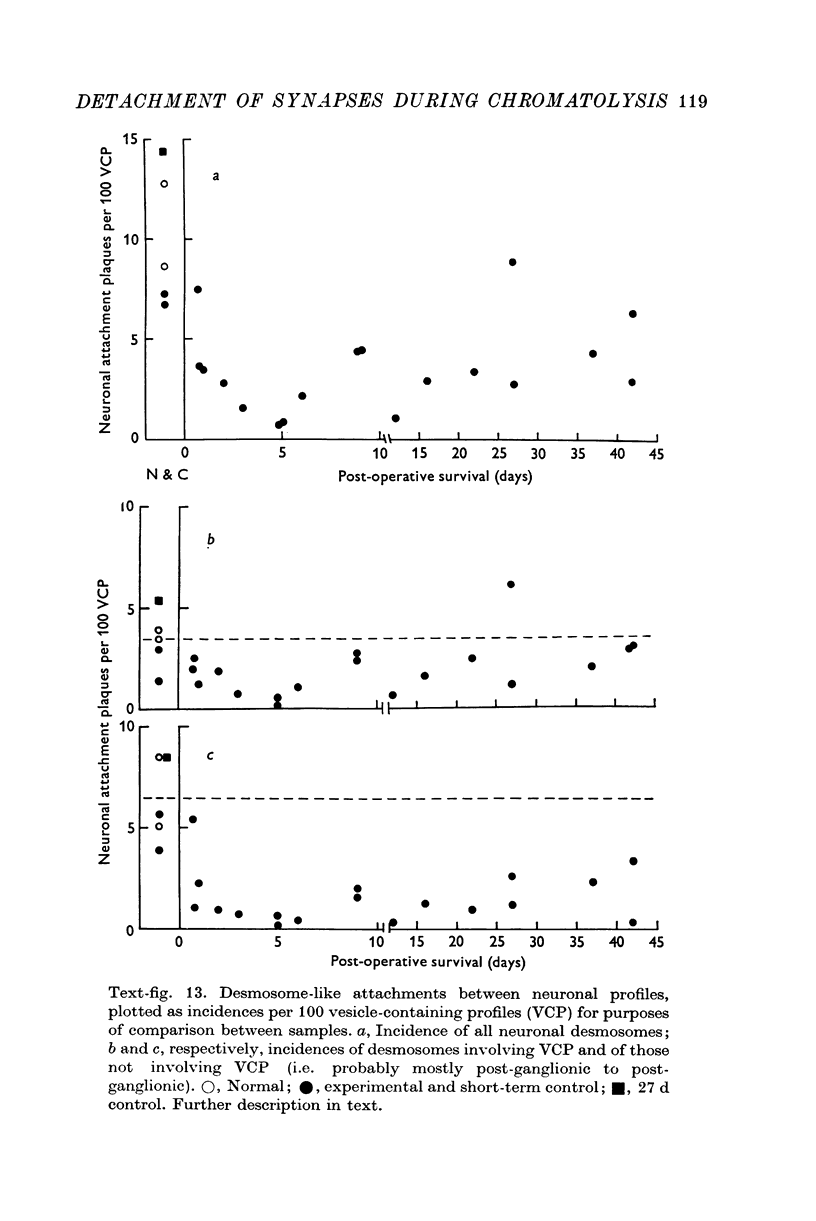
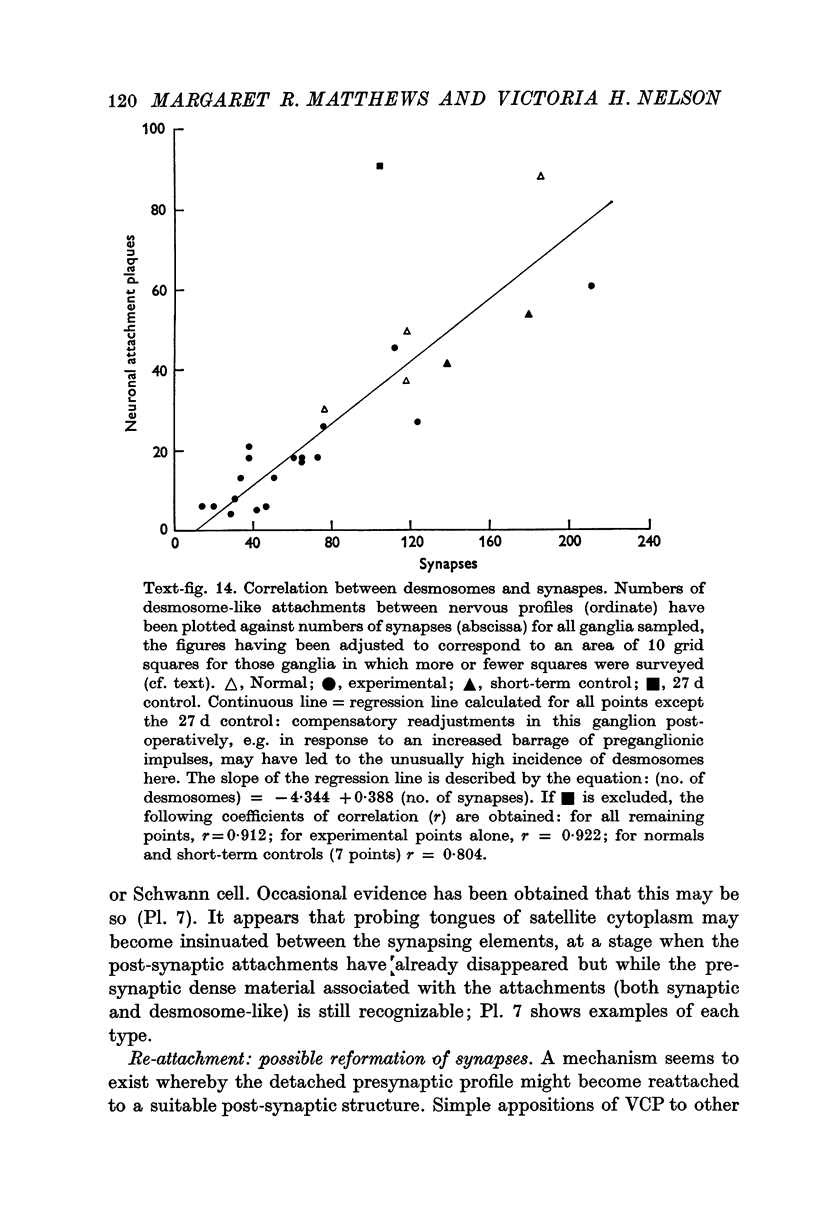
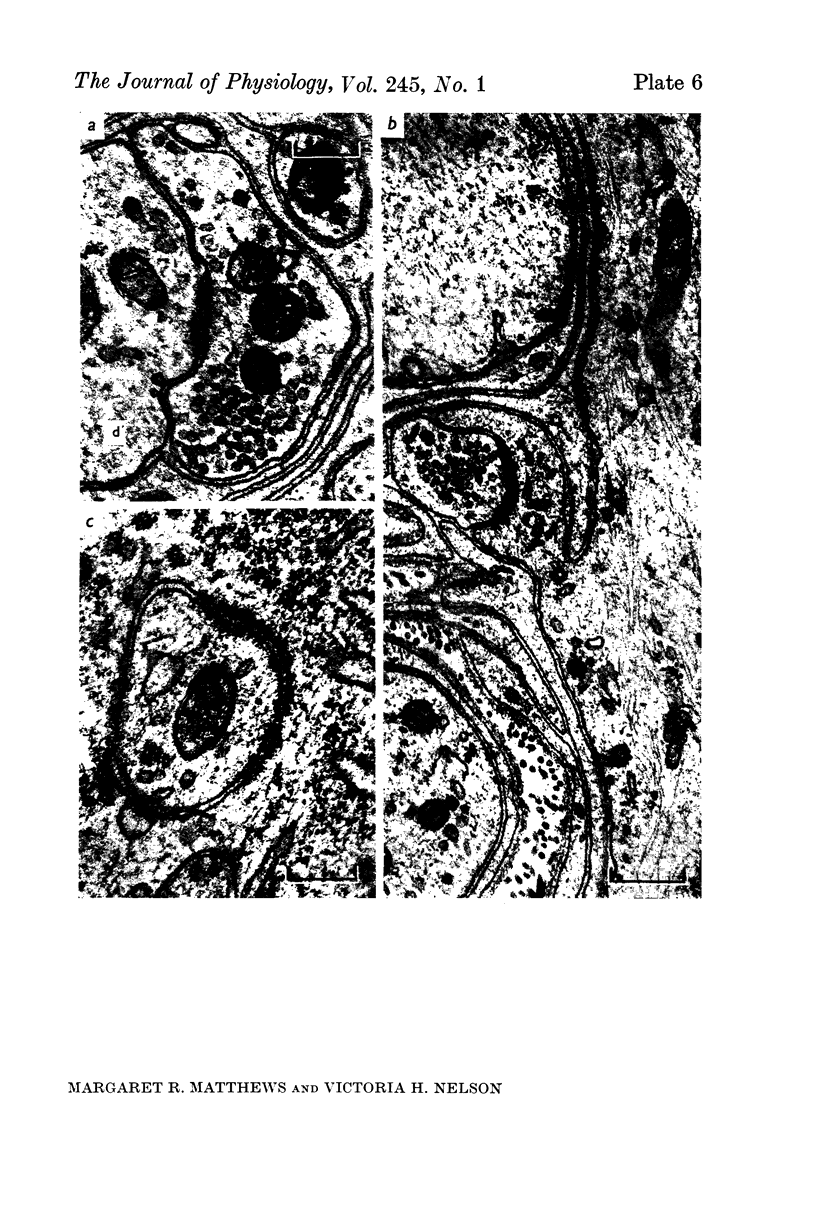
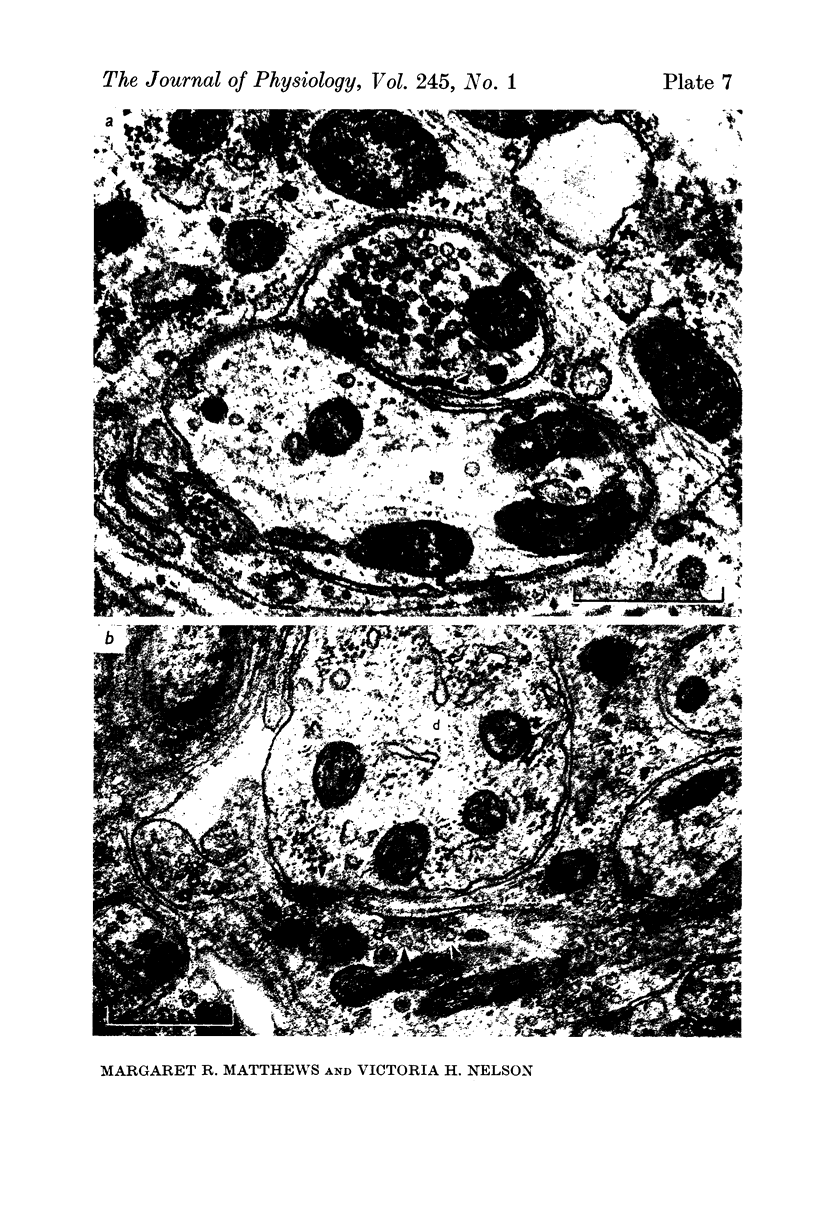
1. Electrophysiological studies showed that injury of post-ganglionic nerve fibres leads to severe and prolonged depression of synaptic transmission through the rat superior cervical ganglion, beginning within 24 h. This is in line with the results of previous studies in other species and upon other neurones. 2. electron microscopy after post-ganglionic axotomy revealed nerve endings of presynaptic type with all the specialized membrane-related features of a synaptic zone, but which were not apposed to any post-synaptic nervous element. These umusual profiles were interpreted as detached presynaptic nerve endings. In normal and control ganglia, such profiles formed at most 0-5% of all vesicle-containing profiles of presynaptic type; in ganglia with all major post-ganglionic branches cut the proportion rose to approximately 7%, between 3 and 7 d post-operatively. Over this period, the mean incidence of chromatolytic neurones was 74-6%. 3. Concomitantly, the incidence of synapses within the ganglion fell by about 75%, reaching its lowest levels between 3 and 7 d post-operatively. There was strikingly little evidence of persistence of post-synaptic membrane specializations ('membrane thickenings') following detachment of synapses. 4. At longer survival intervals the incidence of synapses gradually increased, and that of detached nerve endings gradually decreased; recovery was well advanced by 42 d. 5. The fall in the incidence of synapses was closely paralleled by a fall in the incidence of desmosome-like attachments in the ganglion; the incidence of such attachments was found to be correlated to a significant degree with that of synapses. 6. It is concluded that most or all of the synapses upon sympathetic neurones become physically dissociated during the chromatolytic reaction of these neurones to axotomy. The failure to persist of ultrastructurally specialized post-synaptic sites, and the loss of desmosomes (particularly marked for those involving purely post-ganglionic nervous elements) suggest that the post-ganglionic neurone is losing all its specializations for attachment. 7. Some evidence suggests that the satellite cells may effect the final separation between pre- and post-synaptic structures.
Full text
PDF






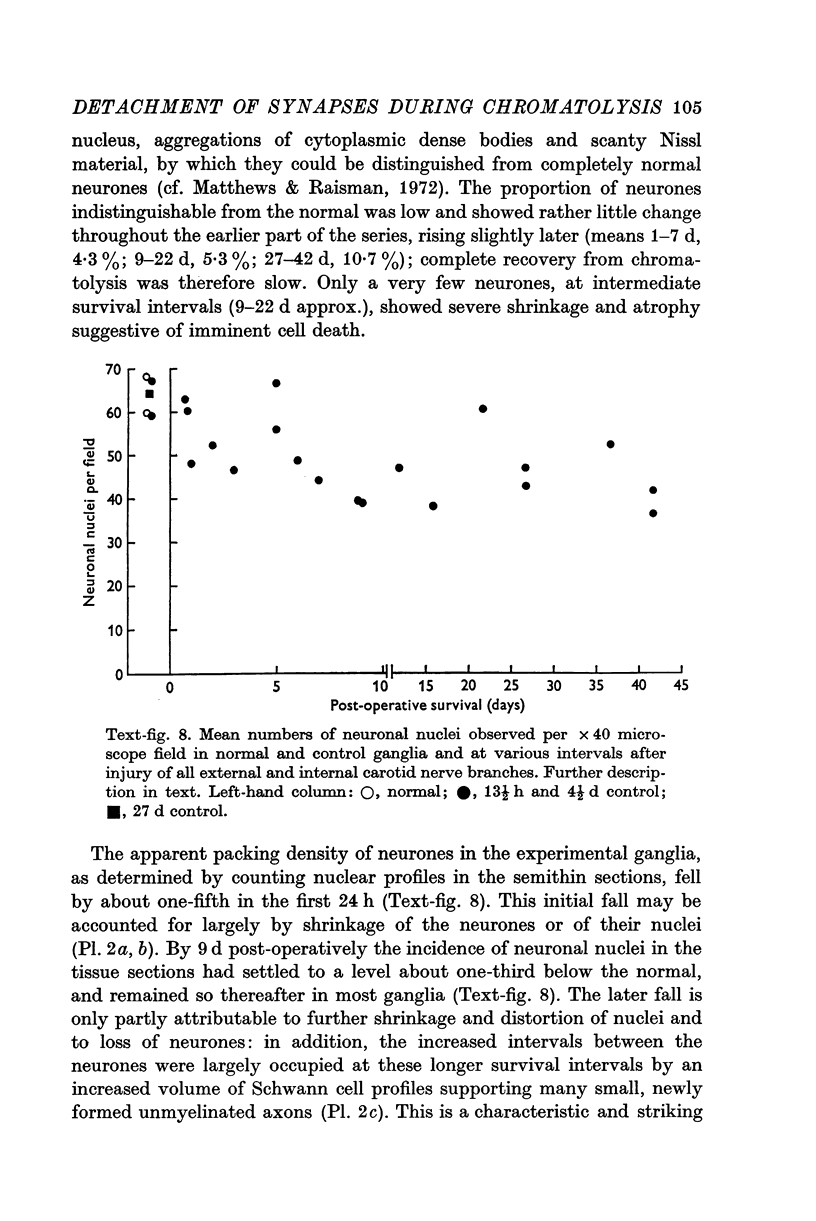























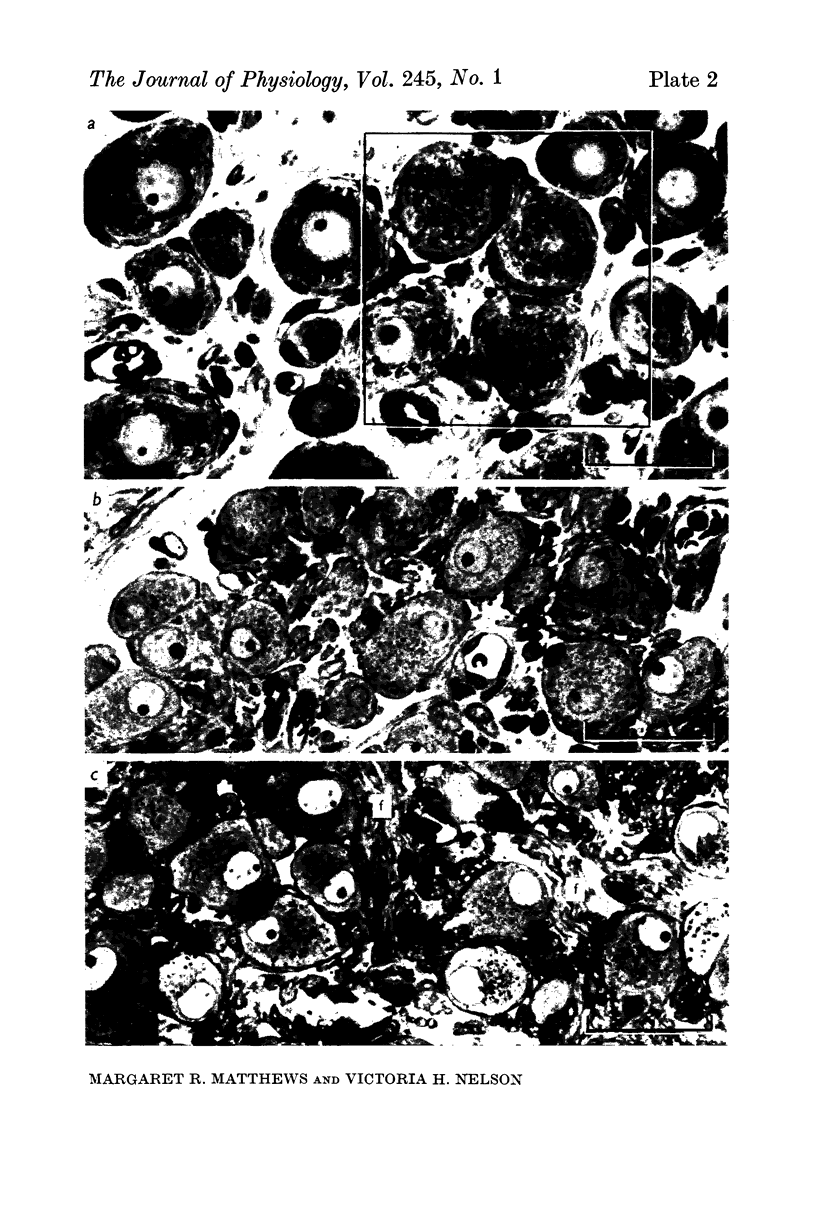
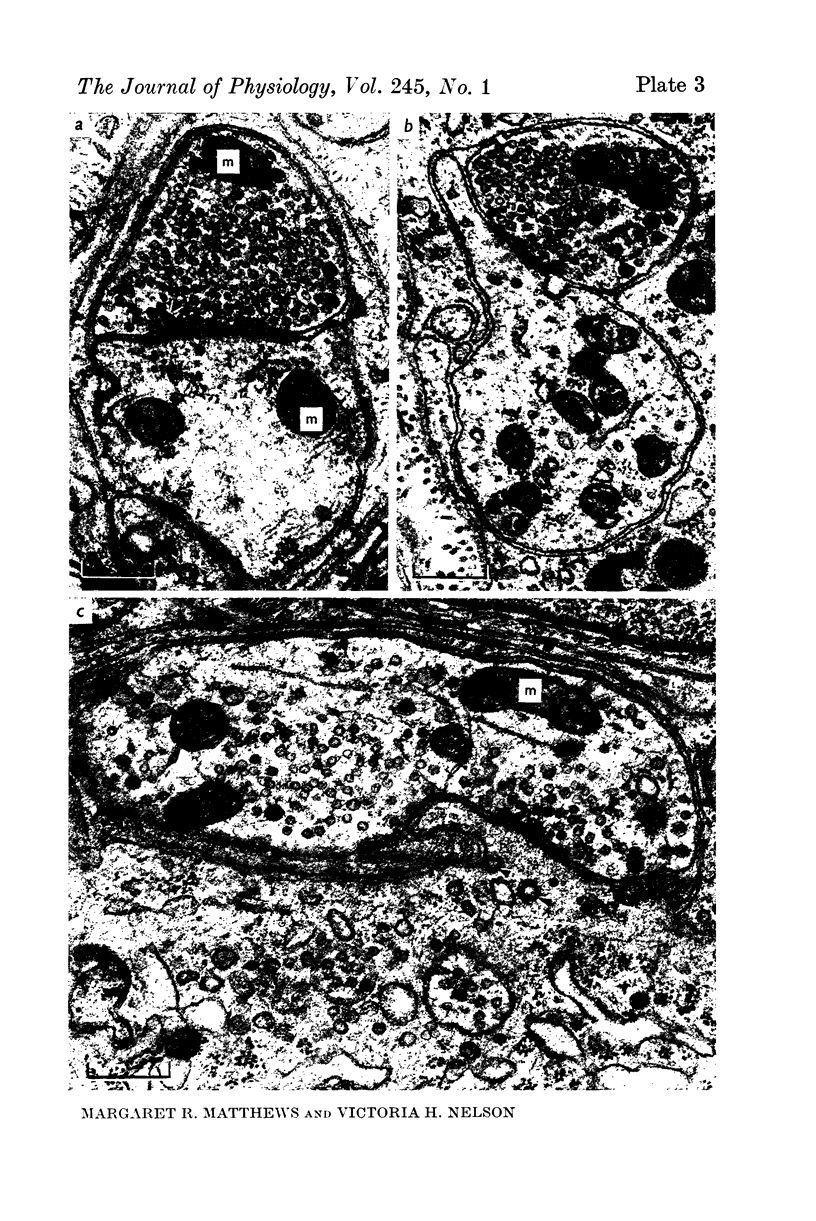
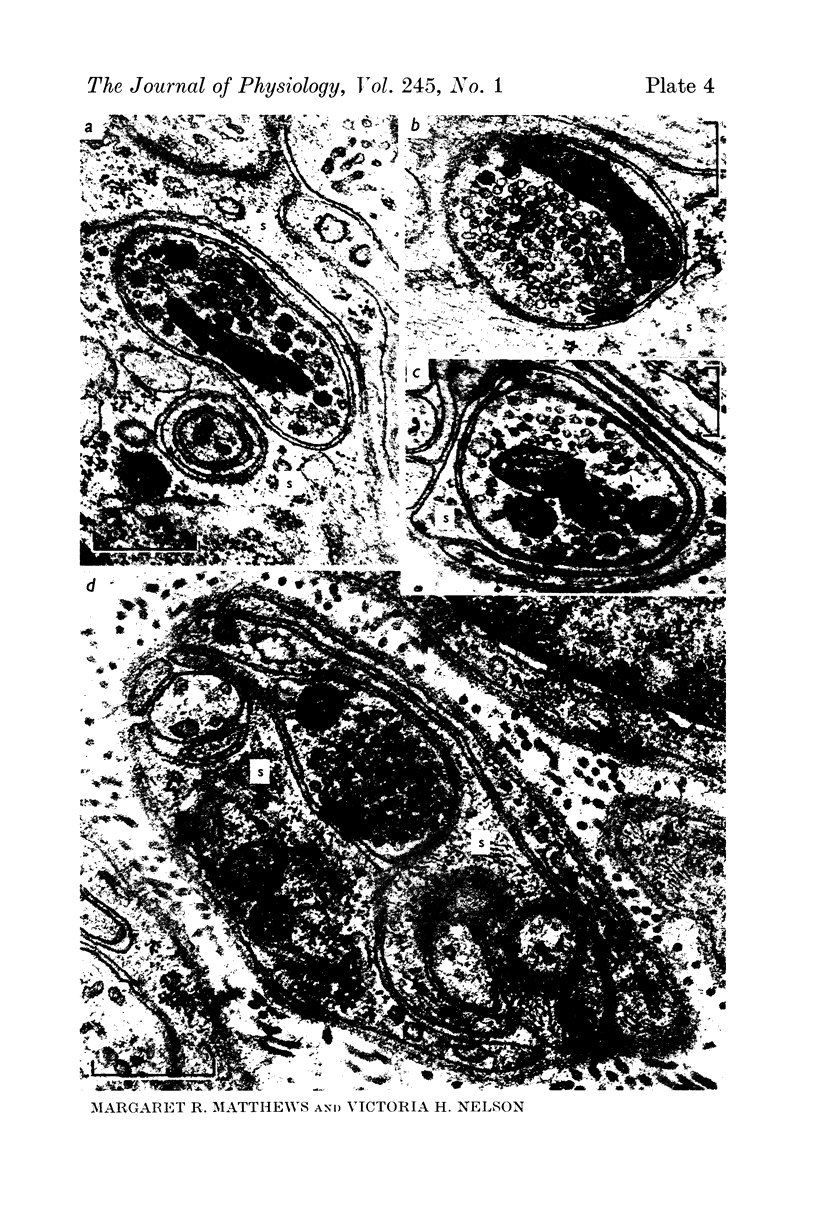
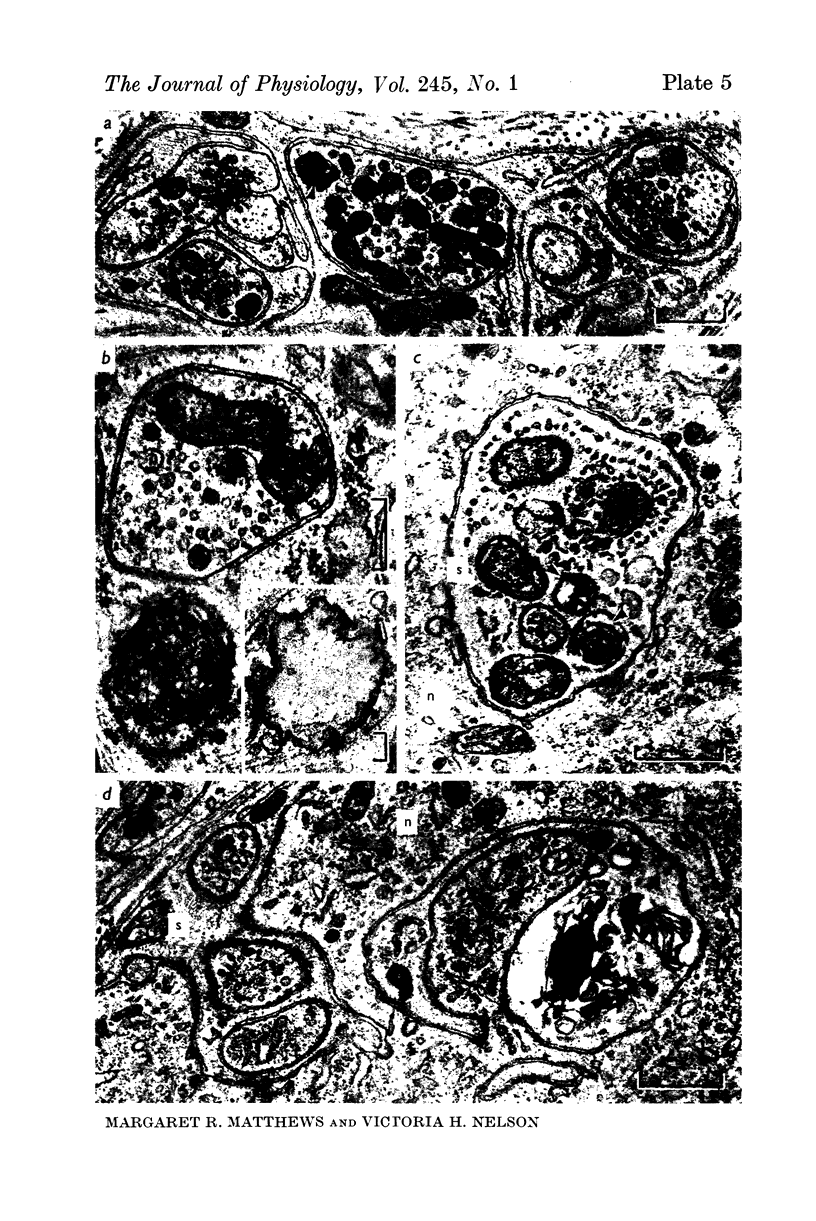

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHESON G. H., REMOLINA J. The temporal course of the effects of post-ganglionic axotomy on the inferior mesenteric ganglion of the cat. J Physiol. 1955 Mar 28;127(3):603–616. doi: 10.1113/jphysiol.1955.sp005281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken J. T., Sharman M., Young J. Z. Maturation of regenerating nerve fibres with various peripheral connexions. J Anat. 1947 Jan;81(Pt 1):1–22.2. [PMC free article] [PubMed] [Google Scholar]
- BROWN G. L., PASCOE J. E. The effect of degenerative section of ganglionic axons on transmission through the ganglion. J Physiol. 1954 Mar 29;123(3):565–573. doi: 10.1113/jphysiol.1954.sp005071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTSON A. R. C. Regeneration of the cervical sympathetic. Br J Surg. 1950 Oct;38(150):223–239. doi: 10.1002/bjs.18003815011. [DOI] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. The role of post-synaptic neurones in the biochemical maturation of presynaptic cholinergic nerve terminals in a mouse sympathetic ganglion. J Physiol. 1972 Feb;221(1):149–159. doi: 10.1113/jphysiol.1972.sp009745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. Trans-synaptic regulation of growth and development of adrenergic neurones in a mouse sympathetic ganglion. Brain Res. 1971 Nov;34(2):229–240. doi: 10.1016/0006-8993(71)90278-2. [DOI] [PubMed] [Google Scholar]
- Blinzinger K., Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85(2):145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- Borysenko J. Z., Revel J. P. Experimental manipulation of desmosome structure. Am J Anat. 1973 Aug;137(4):403–421. doi: 10.1002/aja.1001370404. [DOI] [PubMed] [Google Scholar]
- Boyle F. C., Gillespie J. S. Accumulation and loss of noradrenaline central to a constriction on adrenergic nerves. Eur J Pharmacol. 1970 Sep 1;12(1):77–84. doi: 10.1016/0014-2999(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Brimble M. J., Wallis D. I., Woodward B. Facilitation and inhibition of cell groups within the superior cervical ganglion of the rabbit. J Physiol. 1972 Nov;226(3):629–652. doi: 10.1113/jphysiol.1972.sp010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley J. H., Mark G. E., Burnstock G. Sympathetic ganglia in culture. II. Accessory cells. Z Zellforsch Mikrosk Anat. 1972;135(3):315–327. doi: 10.1007/BF00307179. [DOI] [PubMed] [Google Scholar]
- Cragg B. G. What is the signal for chromatolysis? Brain Res. 1970 Sep 29;23(1):1–21. doi: 10.1016/0006-8993(70)90345-8. [DOI] [PubMed] [Google Scholar]
- DOWNMAN C. B. B., ECCLES J. C., MCINTYRE A. K. Functional changes in chromatolysed motoneurones. J Comp Neurol. 1953 Feb;98(1):9–36. doi: 10.1002/cne.900980104. [DOI] [PubMed] [Google Scholar]
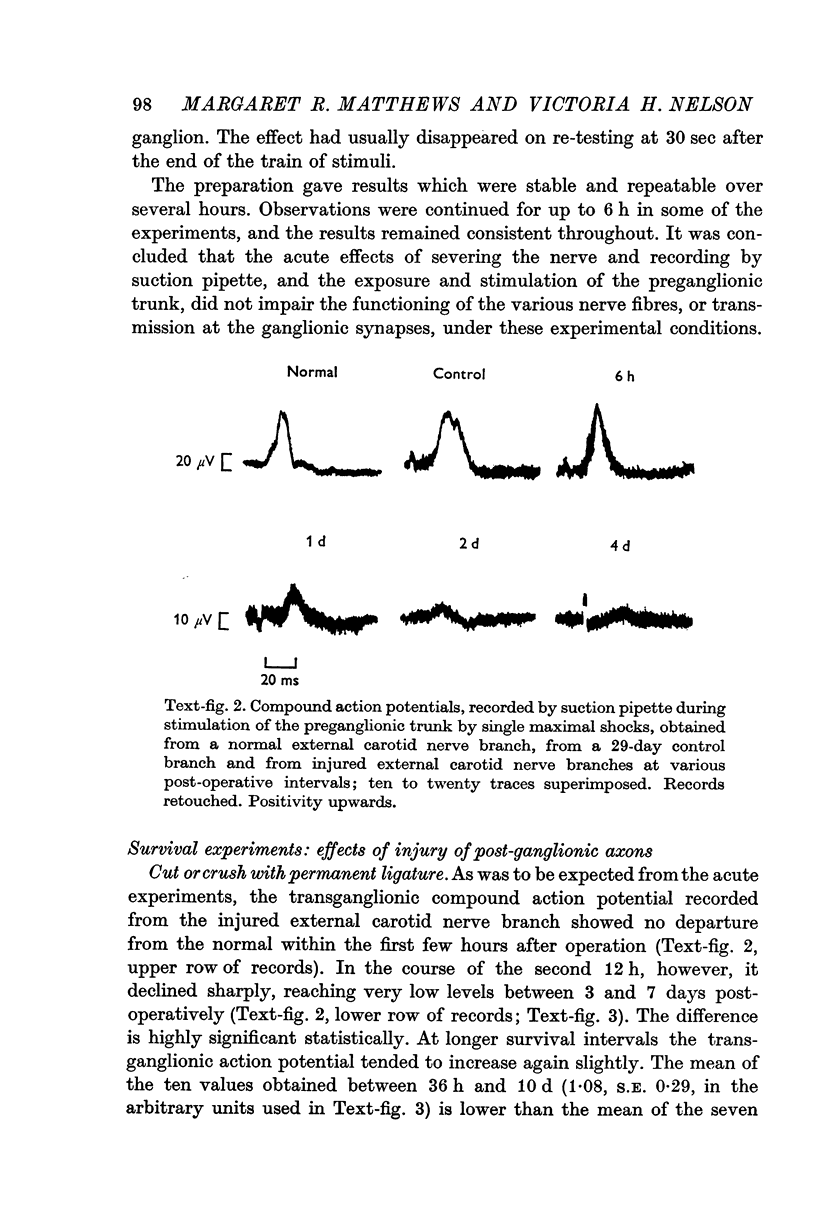
- Dunant Y. Organisation topographique et fonctionnelle du ganglion cervical supérieur chez le Rat. J Physiol (Paris) 1967 Jan-Feb;59(1):17–38. [PubMed] [Google Scholar]
- Dyck P. J., Hopkins A. P. Electron microscopic observations on degeneration and regeneration of unmyelinated fibres. Brain. 1972;95(2):233–234. doi: 10.1093/brain/95.2.223. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., LIBET B., YOUNG R. R. The behaviour of chromatolysed motoneurones studied by intracellular recording. J Physiol. 1958 Aug 29;143(1):11–40. doi: 10.1113/jphysiol.1958.sp006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfvin L. G. Ultrastructural studies on the synaptology of the inferior mesenteric ganglion of the cat. 3. The structure and distribution of the axodendritic and dendrodendritic contacts. J Ultrastruct Res. 1971 Nov;37(3):432–448. doi: 10.1016/s0022-5320(71)80137-5. [DOI] [PubMed] [Google Scholar]
- Elfvin L. G. Ultrastructural studies on the synaptology of the inferior mesenteric ganglion of the cat. I. Observations on the cell surface of the postganglionic perikarya. J Ultrastruct Res. 1971 Nov;37(3):411–425. doi: 10.1016/s0022-5320(71)80135-1. [DOI] [PubMed] [Google Scholar]
- Erulkar S. D., Woodward J. K. Intracellular recording from mammalian superior cervical ganglion in situ. J Physiol. 1968 Nov;199(1):189–203. doi: 10.1113/jphysiol.1968.sp008648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo M. A. Electron microscopy of sympathetic tissues. Pharmacol Rev. 1966 Mar;18(1):387–399. [PubMed] [Google Scholar]
- Hopkins A. P., Lambert E. H. Conduction in regenerating unmyelinated fibres. Brain. 1972;95(2):213–222. doi: 10.1093/brain/95.2.213. [DOI] [PubMed] [Google Scholar]
- Horoupian D. S., Ghetti B., Wiśniewski H. M. Retrograde transneuronal degeneration of optic fibers and their terminals in lateral geniculate nucleus of rhesus monkey. Brain Res. 1973 Jan 30;49(2):257–275. doi: 10.1016/0006-8993(73)90422-8. [DOI] [PubMed] [Google Scholar]
- Hunt C. C., Riker W. K. Properties of frog sympathetic neurons in normal ganglia and after axon section. J Neurophysiol. 1966 Nov;29(6):1096–1114. doi: 10.1152/jn.1966.29.6.1096. [DOI] [PubMed] [Google Scholar]
- Jacobowitz D., Woodward J. K. Adrenergic neurons in the cat superior cervical ganglion and cervical sympathetic nerve trunk. A histochemical study. J Pharmacol Exp Ther. 1968 Aug;162(2):213–226. [PubMed] [Google Scholar]
- Kerns J. M., Hinsman E. J. Neuroglial response to sciatic neurectomy. II. Electron microscopy. J Comp Neurol. 1973 Oct 1;151(3):255–280. doi: 10.1002/cne.901510304. [DOI] [PubMed] [Google Scholar]
- Kirpatrick J. B. Chromatolysis in the hypoglossal nucleus of the rat: an electron microscopic analysis. J Comp Neurol. 1968 Jan;132(1):189–212. doi: 10.1002/cne.901320110. [DOI] [PubMed] [Google Scholar]
- Kuno M., Llinás R. Alterations of synaptic action in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):823–838. doi: 10.1113/jphysiol.1970.sp009244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Llinás R. Enhancement of synaptic transmission by dendritic potentials in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):807–821. doi: 10.1113/jphysiol.1970.sp009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman A. R. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- Mark R. F., Marotte L. R., Mart P. E. The mechanism of selective reinnervation of fish eye muscles. IV. Identification of repressed synapses. Brain Res. 1972 Nov 13;46:149–157. doi: 10.1016/0006-8993(72)90012-1. [DOI] [PubMed] [Google Scholar]
- Marotte L. R., Mark R. P. The mechanism of selective reinnervation of fish eye muscle. II. Evidence from electronmicroscopy of nerve endings. Brain Res. 1970 Apr 1;19(1):53–62. doi: 10.1016/0006-8993(70)90236-2. [DOI] [PubMed] [Google Scholar]
- Matthews M. R. An ultrastructural study of axonal changes following constriction of postganglionic branches of the superior cervical ganglion in the rat. Philos Trans R Soc Lond B Biol Sci. 1973;264(866):479–505. doi: 10.1098/rstb.1973.0002. [DOI] [PubMed] [Google Scholar]
- Matthews M. R., Ostberg A. Effects of preganglionic nerve section upon the afferent innervation of the small granule-containing cells in the rat superior cervical ganglion. Acta Physiol Pol. 1973 Jan-Feb;24(1):215–223. [PubMed] [Google Scholar]
- Matthews M. R., Raisman G. A light and electron microscopic study of the cellular response to axonal injury in the superior cervical ganglion of the rat. Proc R Soc Lond B Biol Sci. 1972 Apr 18;181(1062):43–79. doi: 10.1098/rspb.1972.0040. [DOI] [PubMed] [Google Scholar]
- McINTYRE A. K., BRADLEY K., BROCK L. G. Responses of motoneurons undergoing chromatolysis. J Gen Physiol. 1959 May 20;42(5):931–958. doi: 10.1085/jgp.42.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinoff P. B., Axelrod J. Biochemistry of catecholamines. Annu Rev Biochem. 1971;40:465–500. doi: 10.1146/annurev.bi.40.070171.002341. [DOI] [PubMed] [Google Scholar]
- Perri V., Sacchi O., Caella C. Electrical properties and synaptic connections of the sympathetic neurons in the rat and guinea-pig superior cervical ganglion. Pflugers Arch. 1970;314(1):40–54. doi: 10.1007/BF00587045. [DOI] [PubMed] [Google Scholar]
- Pfenninger K. H. The cytochemistry of synaptic densities. II. Proteinaceous components and mechanism of synaptic connectivity. J Ultrastruct Res. 1971 Jun;35(5):451–475. doi: 10.1016/s0022-5320(71)80005-9. [DOI] [PubMed] [Google Scholar]
- Price D. L. The response of amphibian glial cells to axonal transection. J Neuropathol Exp Neurol. 1972 Apr;31(2):267–277. doi: 10.1097/00005072-197204000-00004. [DOI] [PubMed] [Google Scholar]
- Raisman G., Field P. M. A quantitative investigation of the development of collateral reinnervation after partial deafferentation of the septal nuclei. Brain Res. 1973 Feb 28;50(2):241–264. doi: 10.1016/0006-8993(73)90729-4. [DOI] [PubMed] [Google Scholar]
- Rouiller C., Nicolescu P., Orci L., Rufener C. The effect of anoxia on the ultrastructure of the superior cervical ganglion of the rat in vitro. Virchows Arch B Cell Pathol. 1971;7(4):269–292. doi: 10.1007/BF02892098. [DOI] [PubMed] [Google Scholar]
- Sotelo C. Permanence of postsynaptic specializations in the frog sympathetic ganglion cells after denervation. Exp Brain Res. 1968;6(4):294–305. doi: 10.1007/BF00233181. [DOI] [PubMed] [Google Scholar]
- Streit P., Akert K., Sandri C., Livingston R. B., Moor H. Dynamic ultrastructure of presynaptic membranes at nerve terminals in the spinal cord of rats. Anesthetized and unanesthetized preparations compared. Brain Res. 1972 Dec 24;48:11–26. doi: 10.1016/0006-8993(72)90168-0. [DOI] [PubMed] [Google Scholar]
- Sumner B. E., Sutherland F. I. Quantitative electron microscopy on the injured hypoglossal nucleus in the rat. J Neurocytol. 1973 Sep;2(3):315–328. doi: 10.1007/BF01104033. [DOI] [PubMed] [Google Scholar]
- Sumner B. E., Watson W. E. Retraction and expansion of the dendritic tree of motor neurones of adult rats induced in vivo. Nature. 1971 Sep 24;233(5317):273–275. doi: 10.1038/233273a0. [DOI] [PubMed] [Google Scholar]
- TAXI J. ETUDE DE CERTAINES SYNAPSES INTERNEURONALES DU SYST'EME NERVEUX AUTONOME. Acta Neuroveg (Wien) 1964 Jan 27;26:360–372. doi: 10.1007/BF01234602. [DOI] [PubMed] [Google Scholar]
- TAXI J. [Study of the ultrastkucture of the synaptic zones in the sympathetic ganglia of the frog]. C R Hebd Seances Acad Sci. 1961 Jan 4;252:174–176. [PubMed] [Google Scholar]
- Taxi J., Gautron J., L'Hermite P. Données ultrastructurales sur une éventuelle modulation adrénergique de l'activité du ganglion cervical supérieur du rat. C R Acad Sci Hebd Seances Acad Sci D. 1969 Oct;269(14):1281–1284. [PubMed] [Google Scholar]
- Thoenen H. Induction of tyrosine hydroxylase in peripheral and central adrenergic neurones by cold-exposure of rats. Nature. 1970 Nov 28;228(5274):861–862. doi: 10.1038/228861a0. [DOI] [PubMed] [Google Scholar]
- Torvik A., Heding A. Effect of actinomycin D on retrograde nerve cell reaction. Further observations. Acta Neuropathol. 1969 Sep 9;14(1):62–71. doi: 10.1007/BF00687703. [DOI] [PubMed] [Google Scholar]
- Torvik A., Skjörten F. Electron microscopic observations on nerve cell regeneration and degeneration after axon lesions. I. Changes in the nerve cell cytoplasm. Acta Neuropathol. 1971;17(3):248–264. doi: 10.1007/BF00685058. [DOI] [PubMed] [Google Scholar]
- Torvik A., Skjörten F. Electron microscopic observations on nerve cell regeneration and degeneration after axon lesions. II. Changes in the glial cells. Acta Neuropathol. 1971;17(3):265–282. doi: 10.1007/BF00685059. [DOI] [PubMed] [Google Scholar]
- Watson W. E. Observations on the nucleolar and total cell body nucleic acid of injured nerve cells. J Physiol. 1968 Jun;196(3):655–676. doi: 10.1113/jphysiol.1968.sp008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. Some quantitative observations upon the responses of neuroglial cells which follow axotomy of adjacent neurones. J Physiol. 1972 Sep;225(2):415–435. doi: 10.1113/jphysiol.1972.sp009947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrum L. E., Black R. G. Fine structural aspects of the synaptic organization of the spinal trigeminal nucleus (pars interpolaris) of the cat. Brain Res. 1971 Jan 22;25(2):265–287. doi: 10.1016/0006-8993(71)90438-0. [DOI] [PubMed] [Google Scholar]