Abstract
1. With the purpose of studying the differentiation of an excitable membrane, the electrical properties of the tunicate egg, a mosaic egg, was examined by intracellular recording techniques. The species used were Halocynthia aurantium Pallas and H. roretzi Drashe.
2. The membrane in the matured but unfertilized egg, the fertilized but uncleaved egg, and the cleaved egg showed the resting potential of +5 to -15 mV.
3. Hyperpolarization beyond -60 mV elicited a regenerative response in the form of an `off response' with a critical membrane potential of about -40 mV and with an over-shoot of 10-20 mV above the original resting potential.
4. The removal of Na from standard artificial sea water and the enhancement of Ca in Na-free ASW revealed both Na and Ca components in the `off response'.
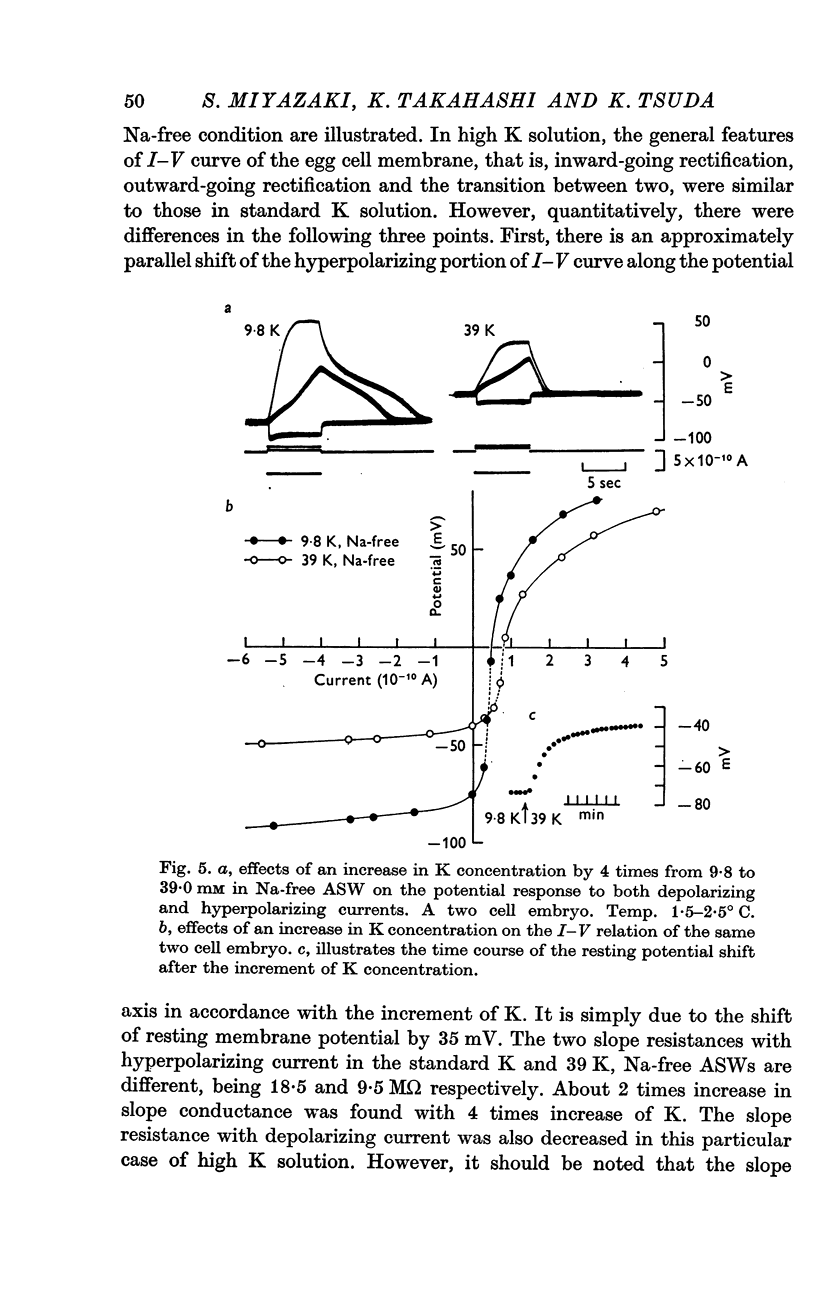
5. In both std ASW and Na-free ASW the I—V relation of the egg cell membrane showed marked non-linearity, forming an S-shaped curve. In the range of positive membrane potential, the embryonic membrane showed a moderate outward-going rectification; in the potential range below -60 mV there was a considerable inward-going rectification. So far as examined, the shape of the I—V relation was affected only by changes of K concentration in the ASW, but not by those of other cations.
6. Specific capacity of the egg cell membrane was 1·0 μF/cm2. The specific slope resistance below -70 mV was 130 kΩ cm2 for the unicellular egg and 50 kΩ cm2 for the two cell embryo.
Full text
PDF

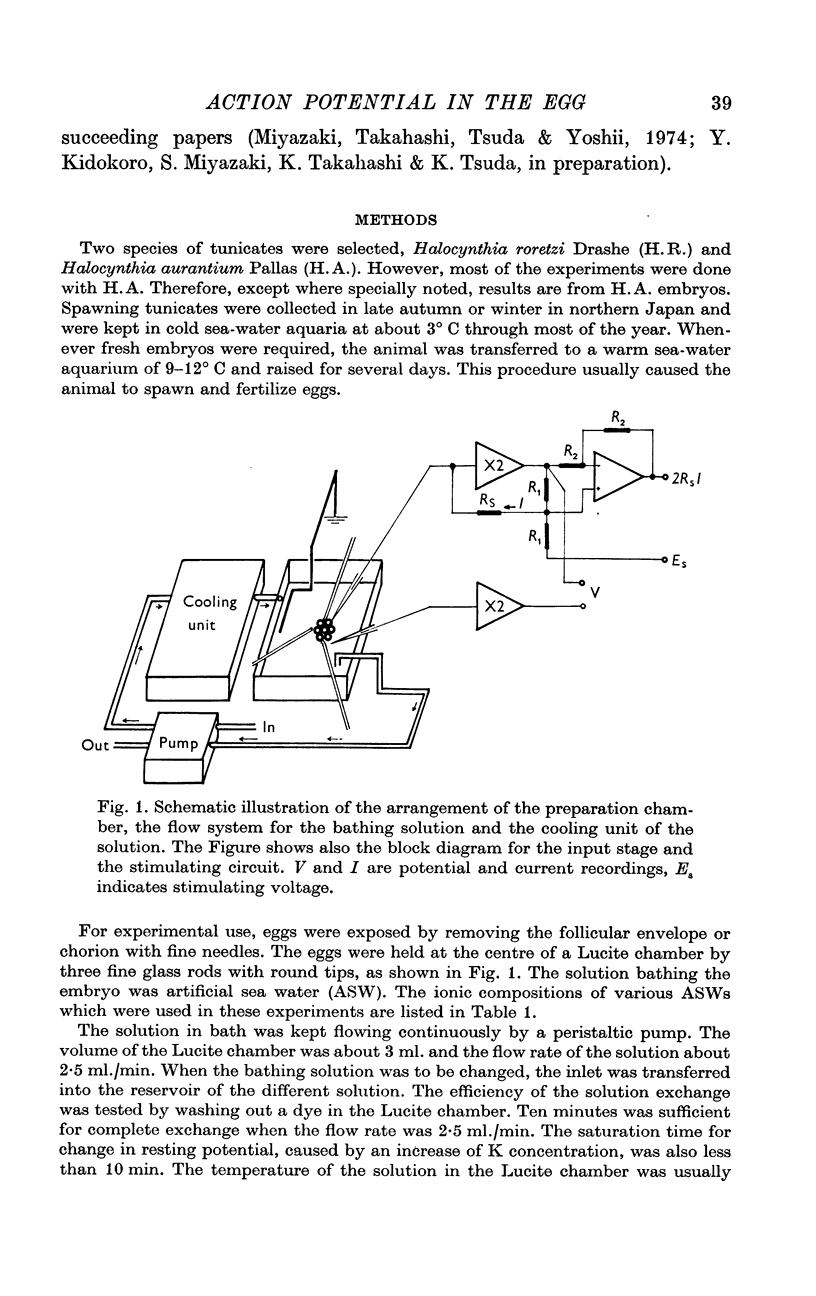

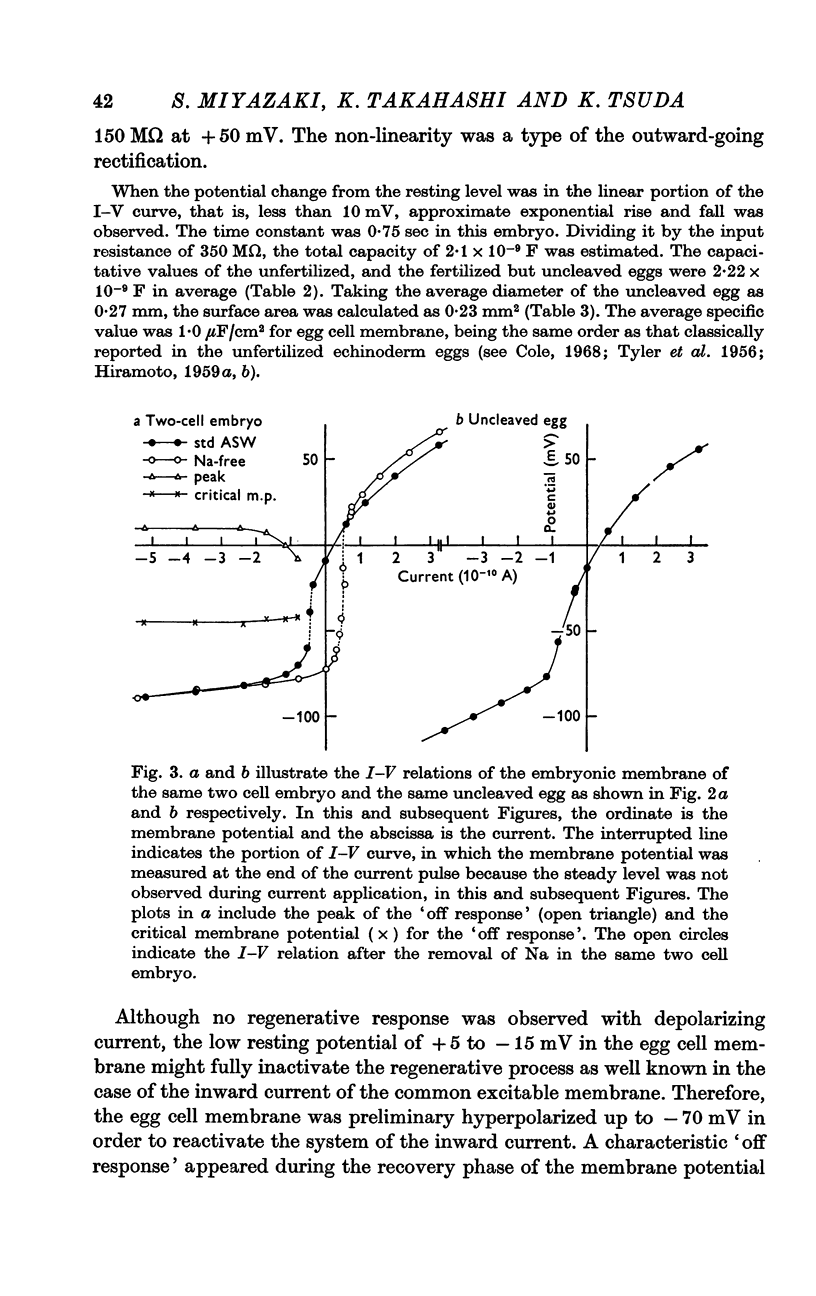
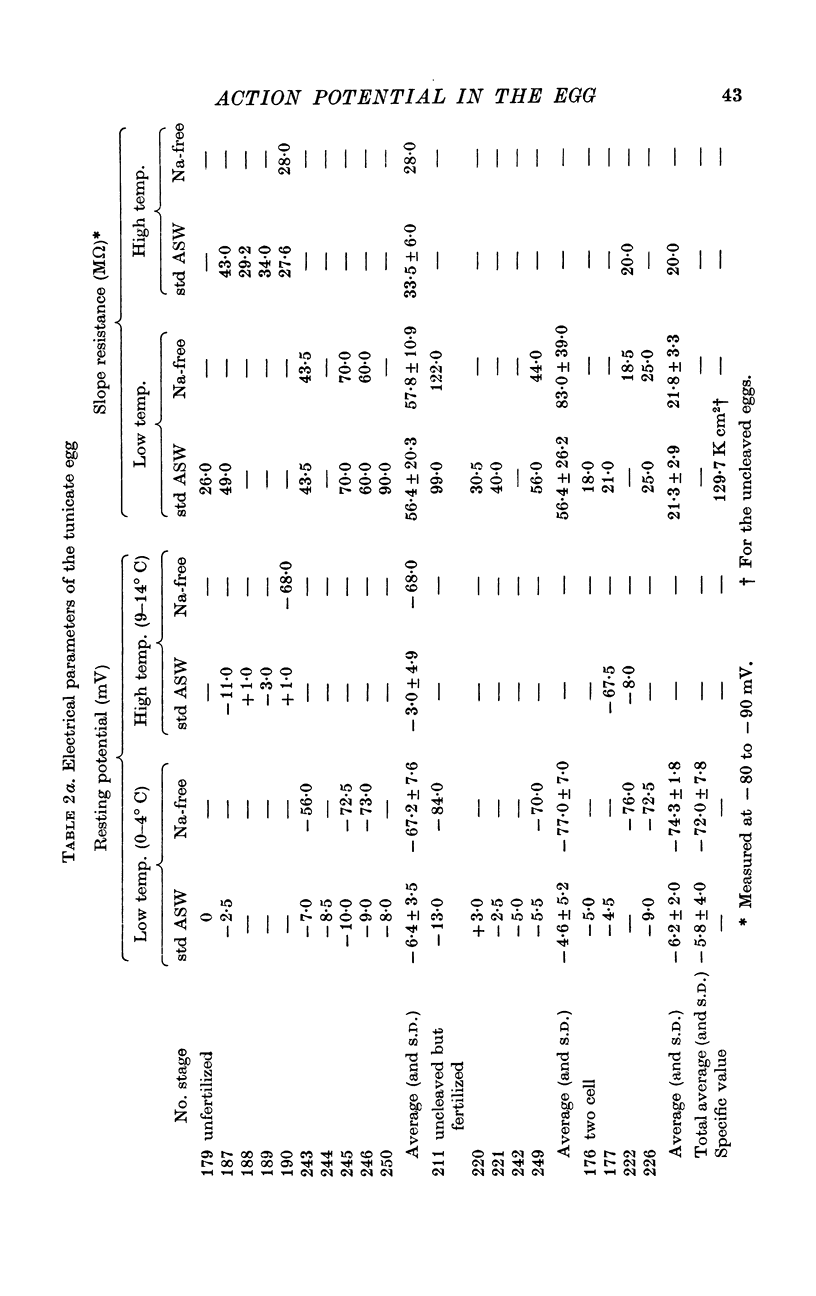










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DECK K. A., TRAUTWEIN W. IONIC CURRENTS IN CARDIAC EXCITATION. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jun 9;280:63–80. doi: 10.1007/BF00412616. [DOI] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig D., Junge D. Sodium and calcium components of action potentials in the Aplysia giant neurone. J Physiol. 1968 Dec;199(2):347–365. doi: 10.1113/jphysiol.1968.sp008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., NAKA K. I. THE INITIATION OF SPIKE POTENTIAL IN BARNACLE MUSCLE FIBERS UNDER LOW INTRACELLULAR CA++. J Gen Physiol. 1964 Sep;48:141–162. doi: 10.1085/jgp.48.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL A. E., HUTTER O. F., NOBLE D. Current-voltage relations of Purkinje fibres in sodium-deficient solutions. J Physiol. 1963 Apr;166:225–240. doi: 10.1113/jphysiol.1963.sp007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRAMOTO Y. Changes in electric properties upon fertilization in the sea urchin egg. Exp Cell Res. 1959 Feb;16(2):421–424. doi: 10.1016/0014-4827(59)90272-1. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L. Ionic movements and electrical activity in giant nerve fibres. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):1–37. doi: 10.1098/rspb.1958.0001. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Kidokoro Y. Na and Ca components of action potential in amphioxus muscle cells. J Physiol. 1971 Dec;219(1):217–232. doi: 10.1113/jphysiol.1971.sp009658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Hori N. Electrical characteristics of Triturus egg cells during cleavage. J Gen Physiol. 1966 May;49(5):1019–1027. doi: 10.1085/jgp.49.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Yoshioka K. Renal activation potential observed in sea urchin egg during fertilization. Exp Cell Res. 1972 Jun;72(2):547–551. doi: 10.1016/0014-4827(72)90026-2. [DOI] [PubMed] [Google Scholar]
- Iwasaki S., Satow Y. Sodium- and calcium-dependent spike potentials in the secretory neuron soma of the X-organ of the crayfish. J Gen Physiol. 1971 Feb;57(2):216–236. doi: 10.1085/jgp.57.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koketsu K., Nishi S. Calcium and action potentials of bullfrog sympathetic ganglion cells. J Gen Physiol. 1969 May;53(5):608–623. doi: 10.1085/jgp.53.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG A. Microelectrode experiments on unfertilized sea urchin eggs. Exp Cell Res. 1955 Dec;9(3):393–398. doi: 10.1016/0014-4827(55)90069-0. [DOI] [PubMed] [Google Scholar]
- MAENO T. Electrical characteristics and activation potential of Bufo eggs. J Gen Physiol. 1959 Sep;43:139–157. doi: 10.1085/jgp.43.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. The ionic requirements for the production of action potentials in helix pomatia neurones. Pflugers Arch. 1968;304(3):215–241. doi: 10.1007/BF00592126. [DOI] [PubMed] [Google Scholar]
- Miyazaki S. I., Takahashi K., Tsuda K., Yoshii M. Analysis of non-linearity observed in the current-voltage relation of the tunicate embryo. J Physiol. 1974 Apr;238(1):55–77. doi: 10.1113/jphysiol.1974.sp010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Takahashi K., Tsuda K. Calcium and sodium contributions to regenerative responses in the embryonic excitable cell membrane. Science. 1972 Jun 30;176(4042):1441–1443. doi: 10.1126/science.176.4042.1441. [DOI] [PubMed] [Google Scholar]
- Morrill G. A., Rosenthal J., Watson D. E. Membrane permeability changes in amphibian eggs at ovulation. J Cell Physiol. 1966 Jun;67(3):375–381. doi: 10.1002/jcp.1040670303. [DOI] [PubMed] [Google Scholar]
- Morrill G. A., Watson D. E. Transmembrane electropotential changes in amphibian eggs at ovulation, activation and first cleavage. J Cell Physiol. 1966 Feb;67(1):85–92. doi: 10.1002/jcp.1040670110. [DOI] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEER B. T., MONROY A., SANTANGELO M., RICCOBONO G. Action potentials in sea urchin eggs at fertilization. Exp Cell Res. 1954 Aug;7(1):284–287. doi: 10.1016/0014-4827(54)90065-8. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Lundin L., Mazia D. Bioelectric responses of the echinoderm egg to fertilization. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2426–2430. doi: 10.1073/pnas.68.10.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R. A., Shen S., Mazia D. Membrane potential, membrane resistance and an energy requirement for the development of potassium conductance in the fertilization reaction of echinoderm eggs. Exp Cell Res. 1972 May;72(1):195–203. doi: 10.1016/0014-4827(72)90581-2. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Miyazaki S. I., Kidokoro Y. Development of excitability in embryonic muscle cell membranes in certain tunicates. Science. 1971 Jan 29;171(3969):415–418. doi: 10.1126/science.171.3969.415. [DOI] [PubMed] [Google Scholar]
- WERMAN R., GRUNDFEST H. Graded and all-or-none electrogenesis in arthropod muscle. II. The effects of alkali-earth and onium ions on lobster muscle fibers. J Gen Physiol. 1961 May;44:997–1027. doi: 10.1085/jgp.44.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward D. J. Electrical signs of new membrane production during cleavage of Rana pipiens eggs. J Gen Physiol. 1968 Sep;52(3):509–531. doi: 10.1085/jgp.52.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]


