Abstract
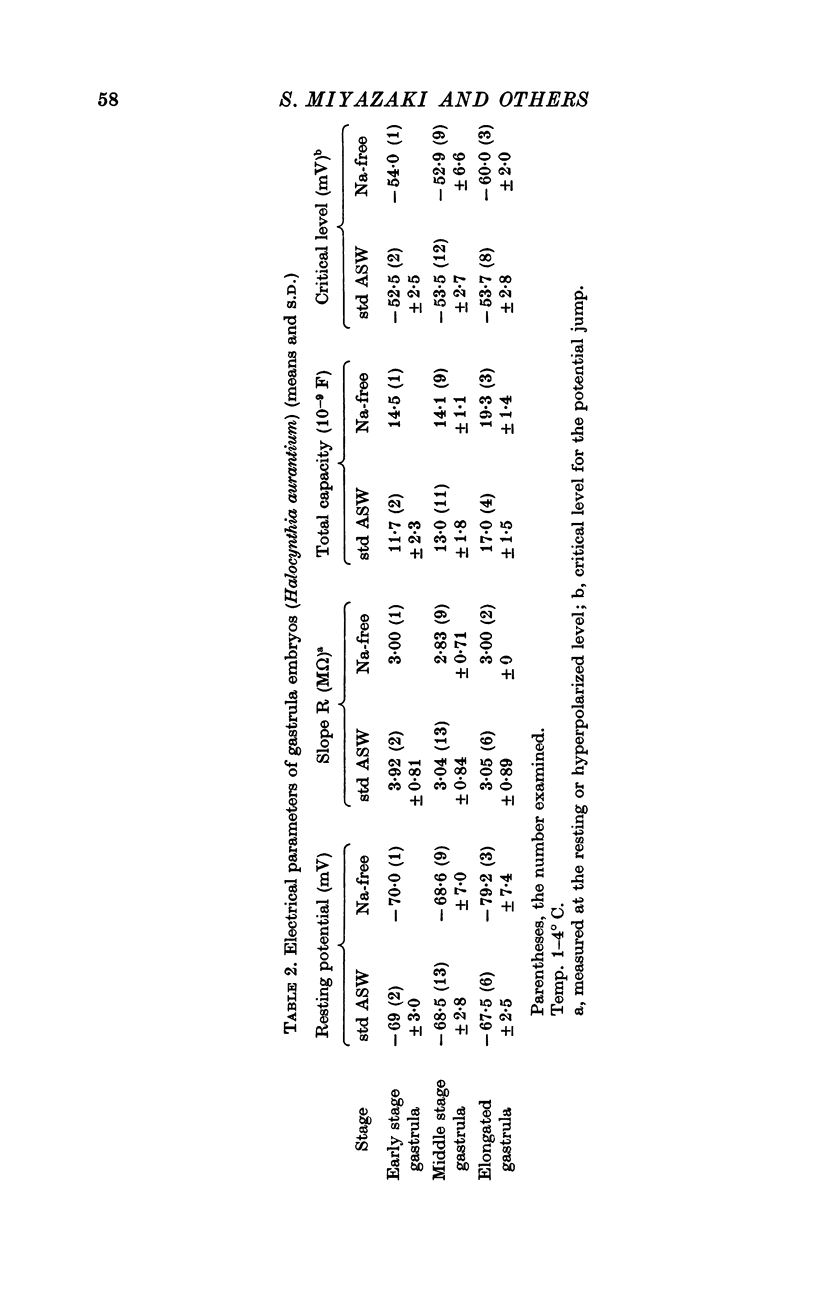
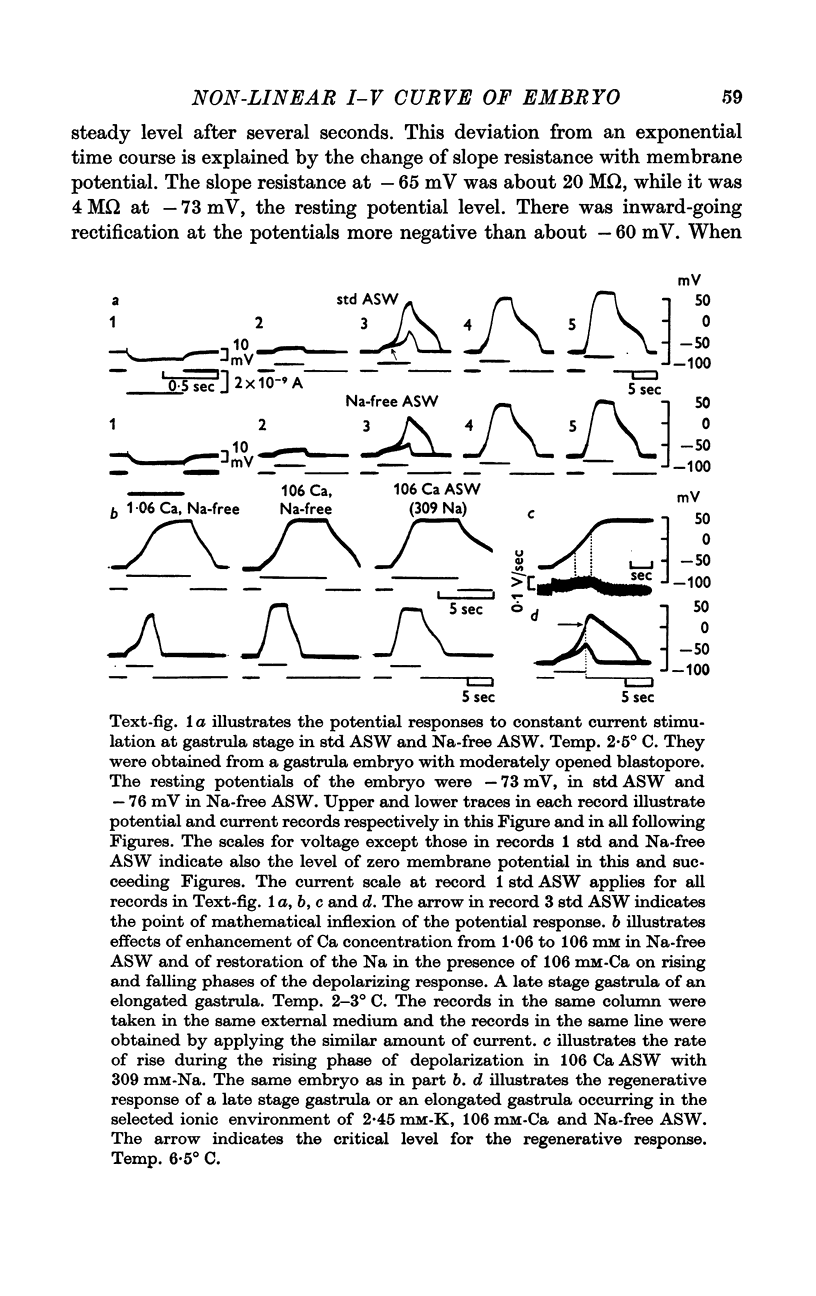
1. In the gastrula of the tunicate (Halocynthia aurantium) the resting potential of the embryonic membrane was about -70 mV in both std ASW and Na-free ASW.
2. The potential responses to depolarizing current in the early gastrula embryo showed distinct potential jumps from about -50 to about +40 mV during the application of current and from about 0 to resting level after its cessation thus forming a plateau at about 0 mV. Those jumps were not altered significantly by the removal of both Na and Ca ions from ASW except for the duration of the plateau after the cessation of current.
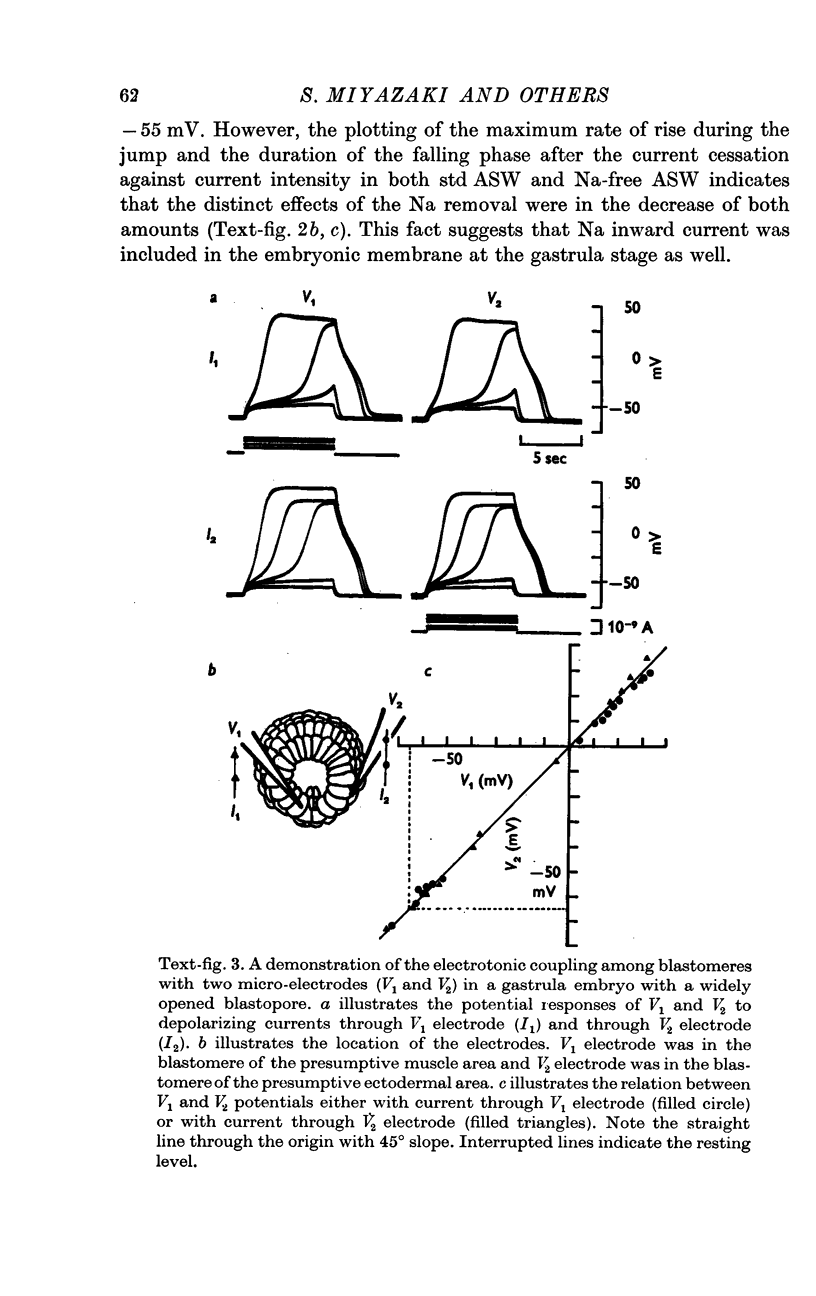
3. Blastomeres in an embryo were tightly coupled with each other electrotonically at the blastula and gastrula stages. The fact that the coupling ratio was nearly 1·0 made it reasonable to treat an embryo as one cell as far as electrical properties are concerned.
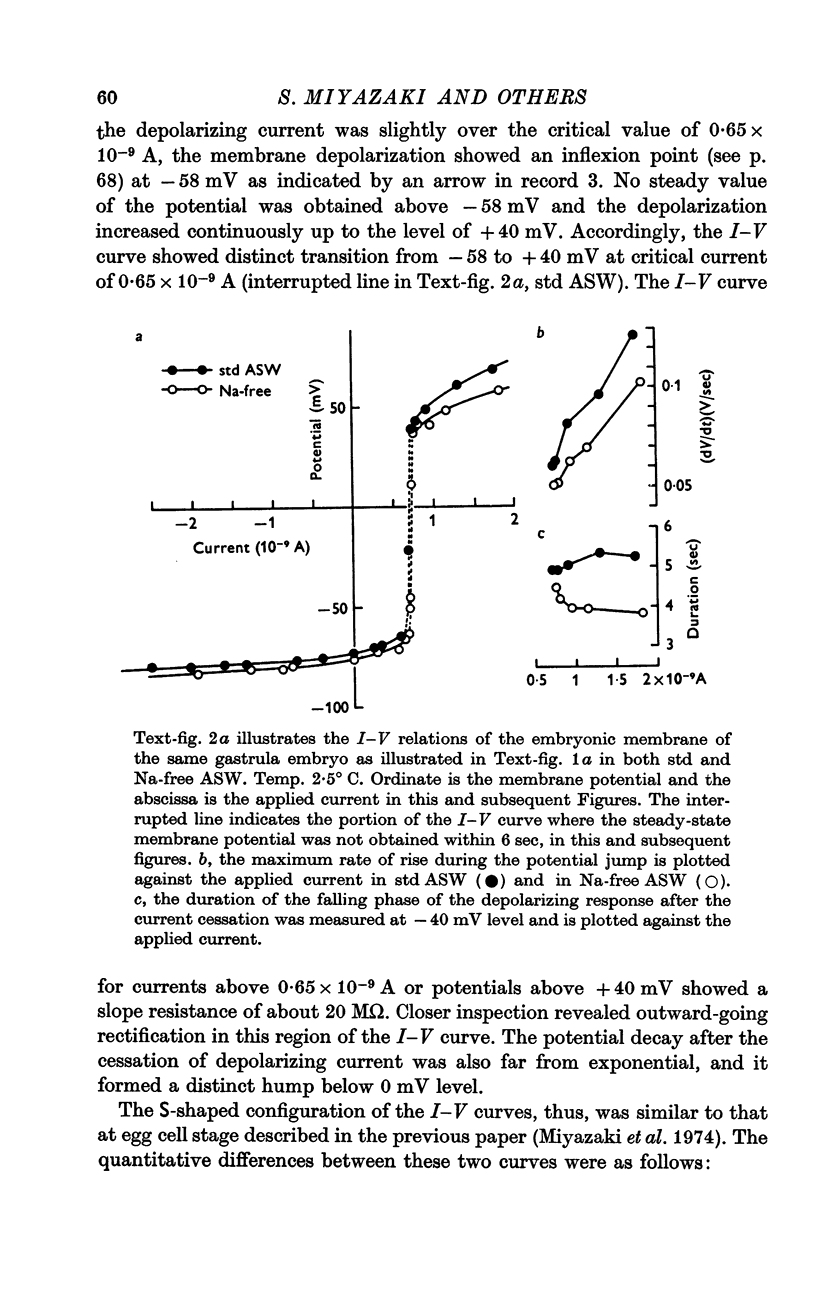
4. The I—V relation of the embryonic membrane consisted of an inward rectifying region, outward rectifying region and transitional zone between the two, resulting in a S-shaped curve as in the egg cell membrane. But in the gastrula embryo, the transitional zone actually included the discontinuous part of the I—V curve (from about -50 to about +40 mV) due to the potential jump.
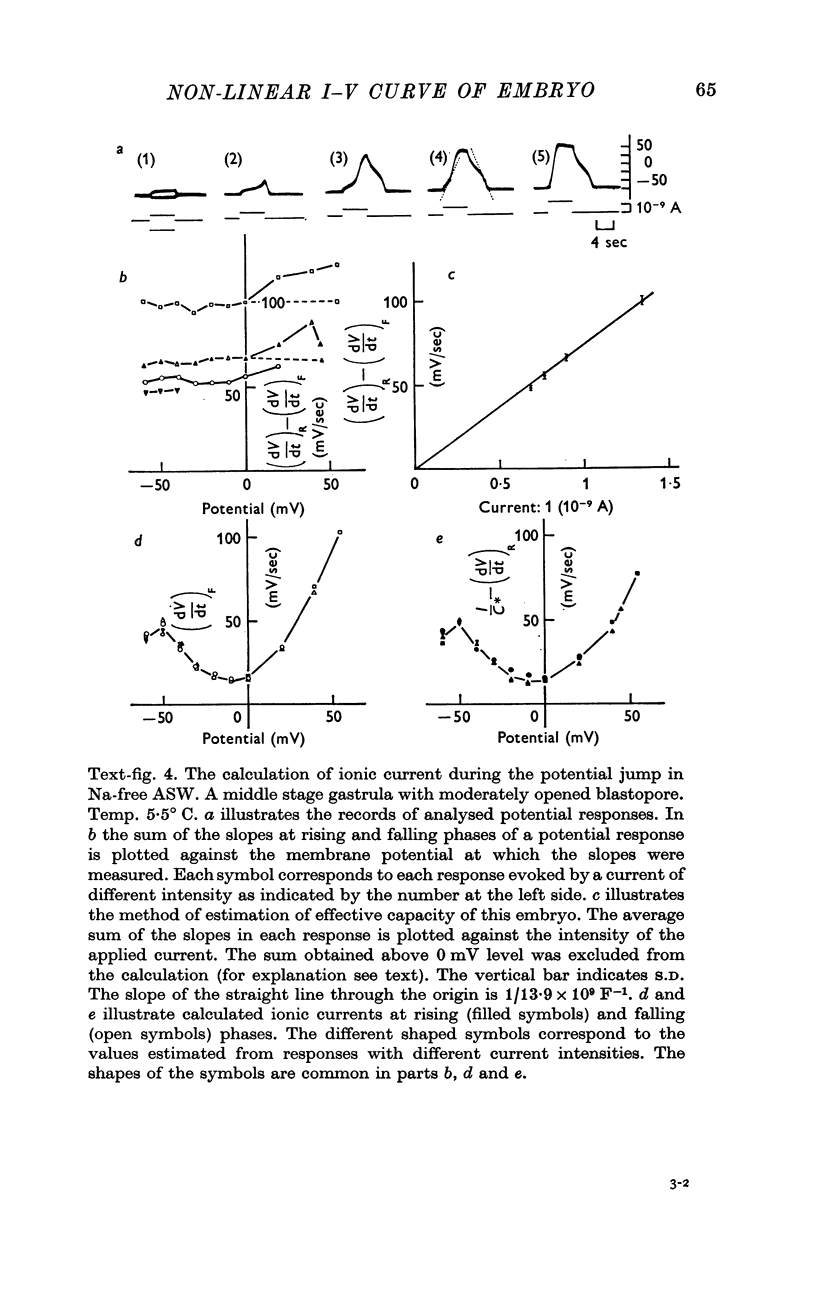
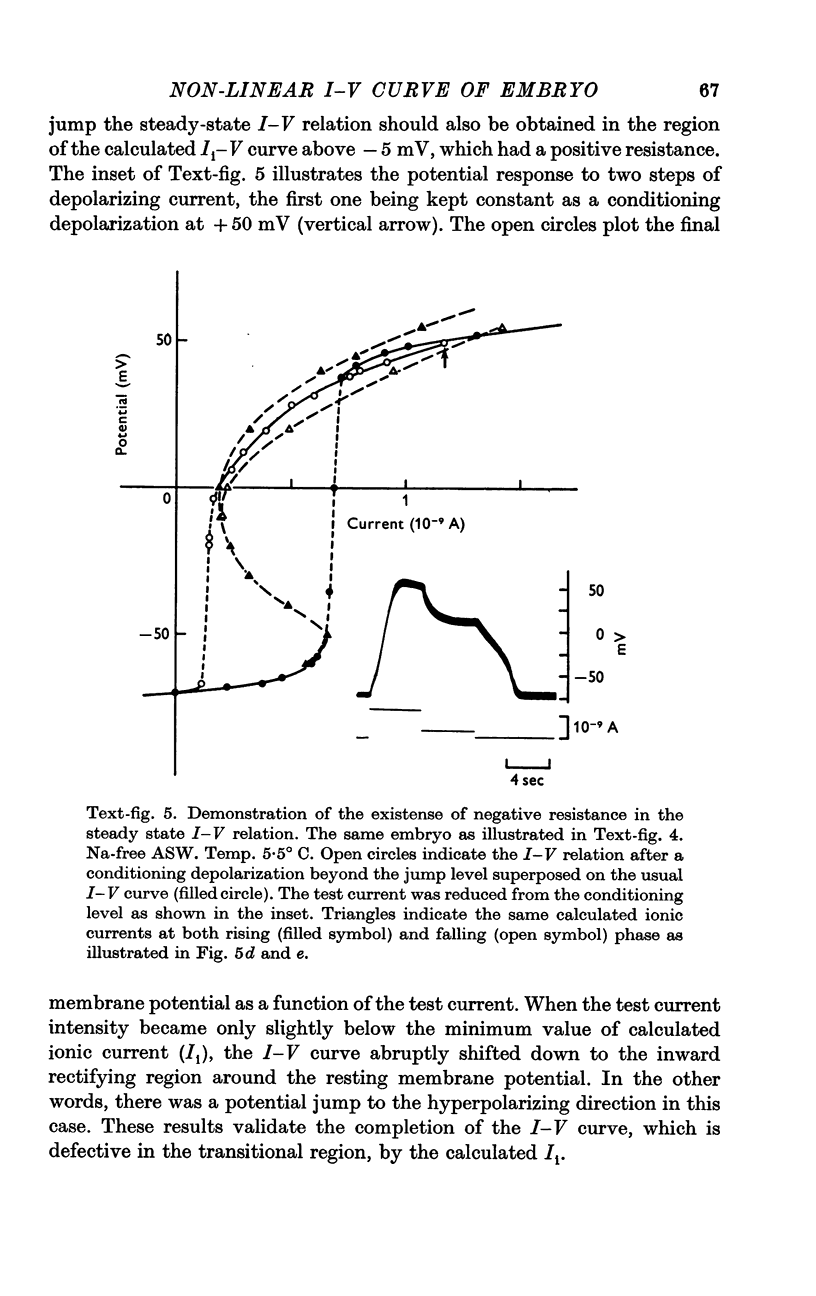
5. The ionic current (Ii) during the potential jump in Na-free ASW was estimated from the slope of the potential response based on the assumption of constant capacitance during the membrane potential changes. The calculated Ii—V curve complemented the interrupted I—V curve smoothly and showed the existence of a negative resistance region from about -50 to about 0 mV in the steady-state I—V relation.
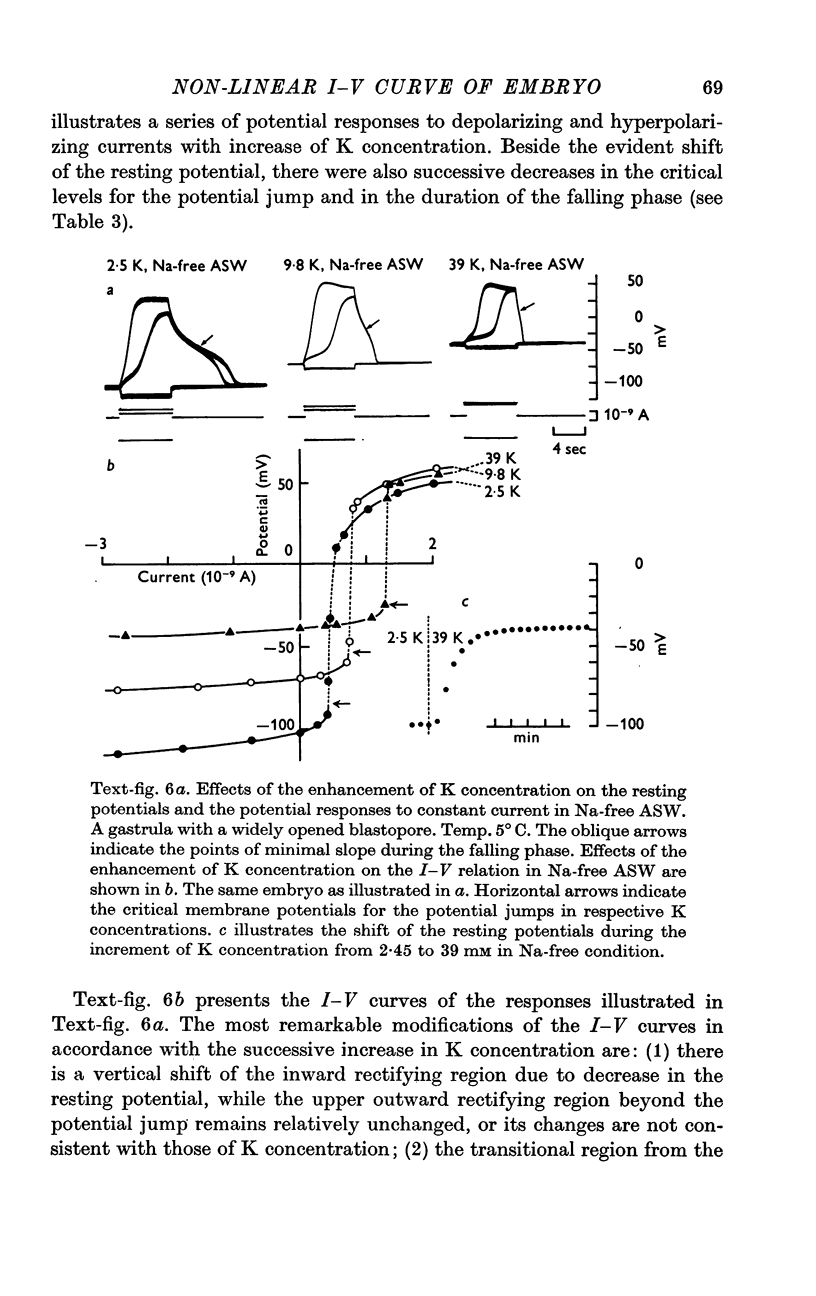
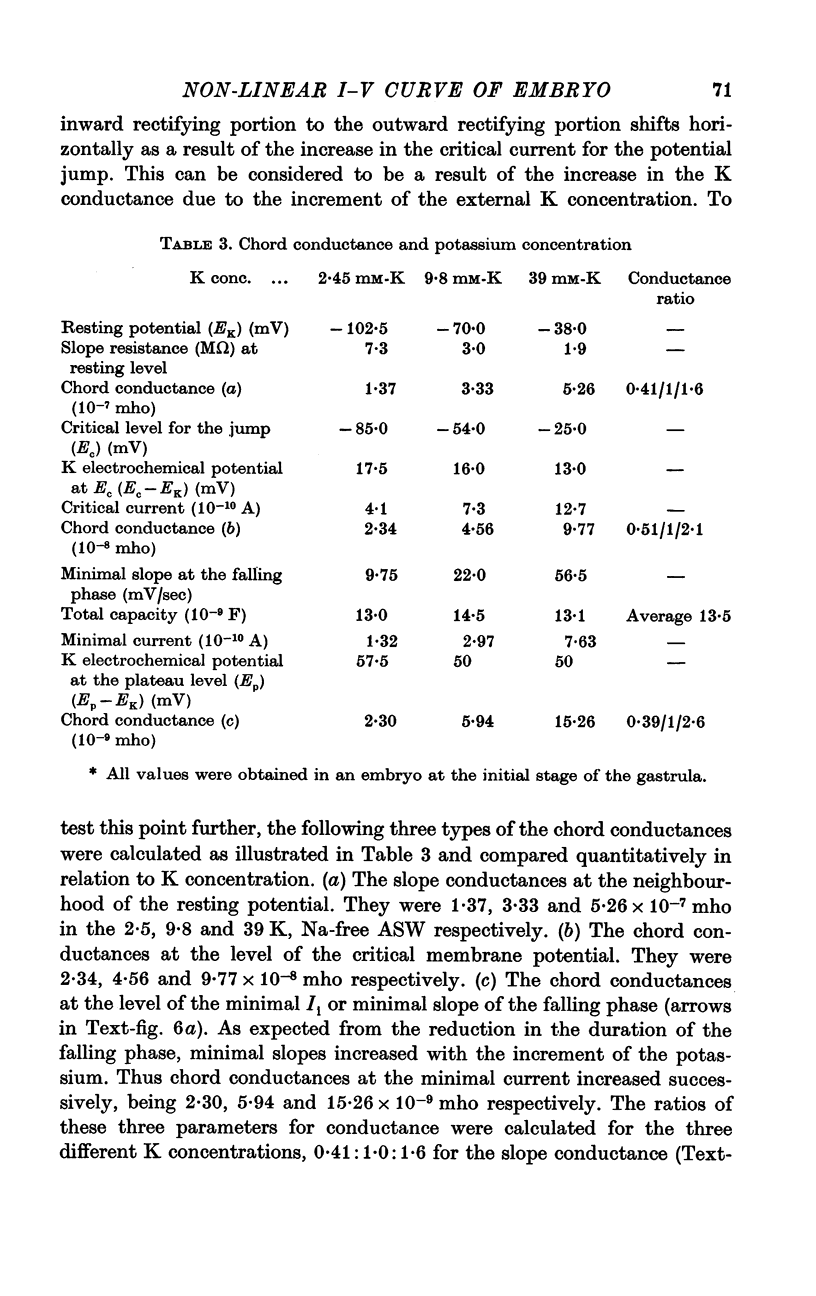
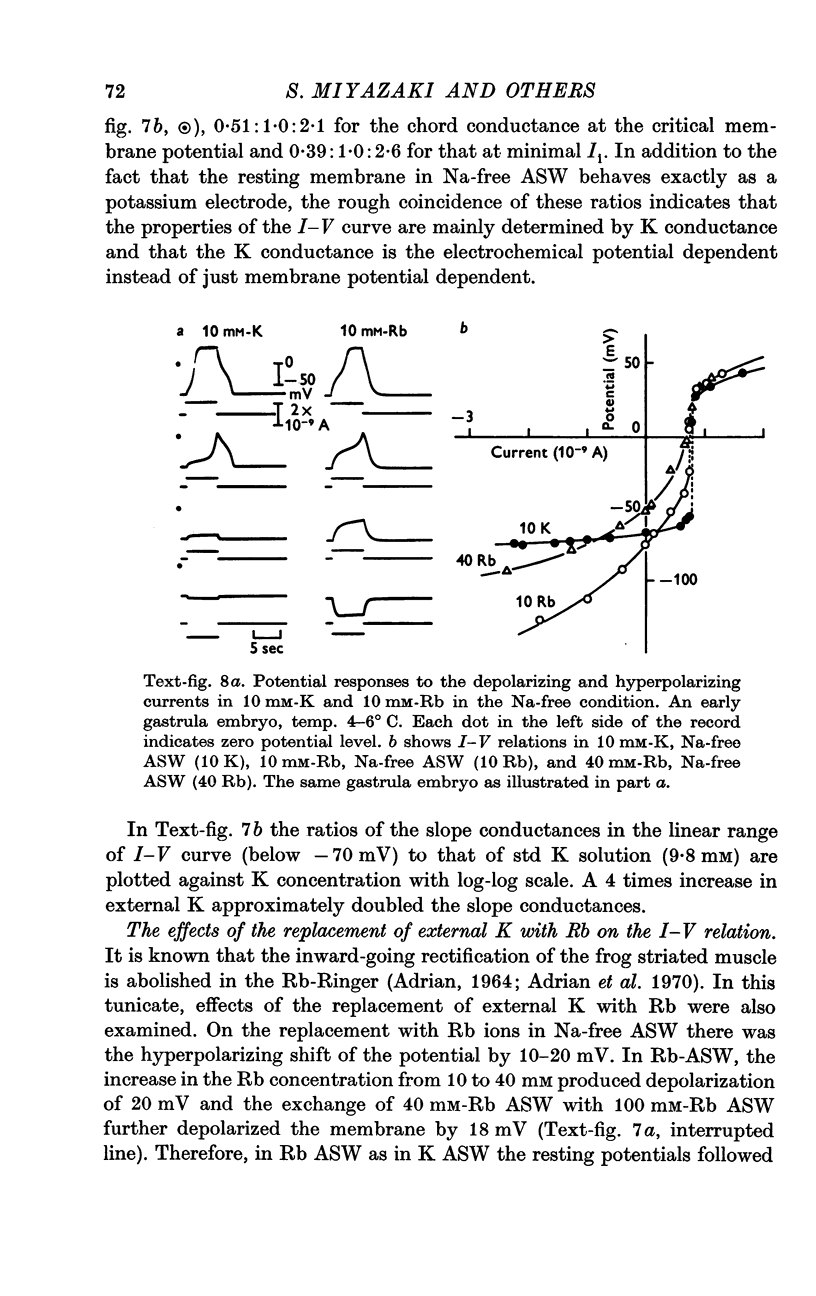
6. The steady-state I—V relation was altered significantly by changing the K concentration in ASW, and the existence of the negative resistance can be reasonably explained by the marked inward-going rectification or anomalous rectification of K conductance.
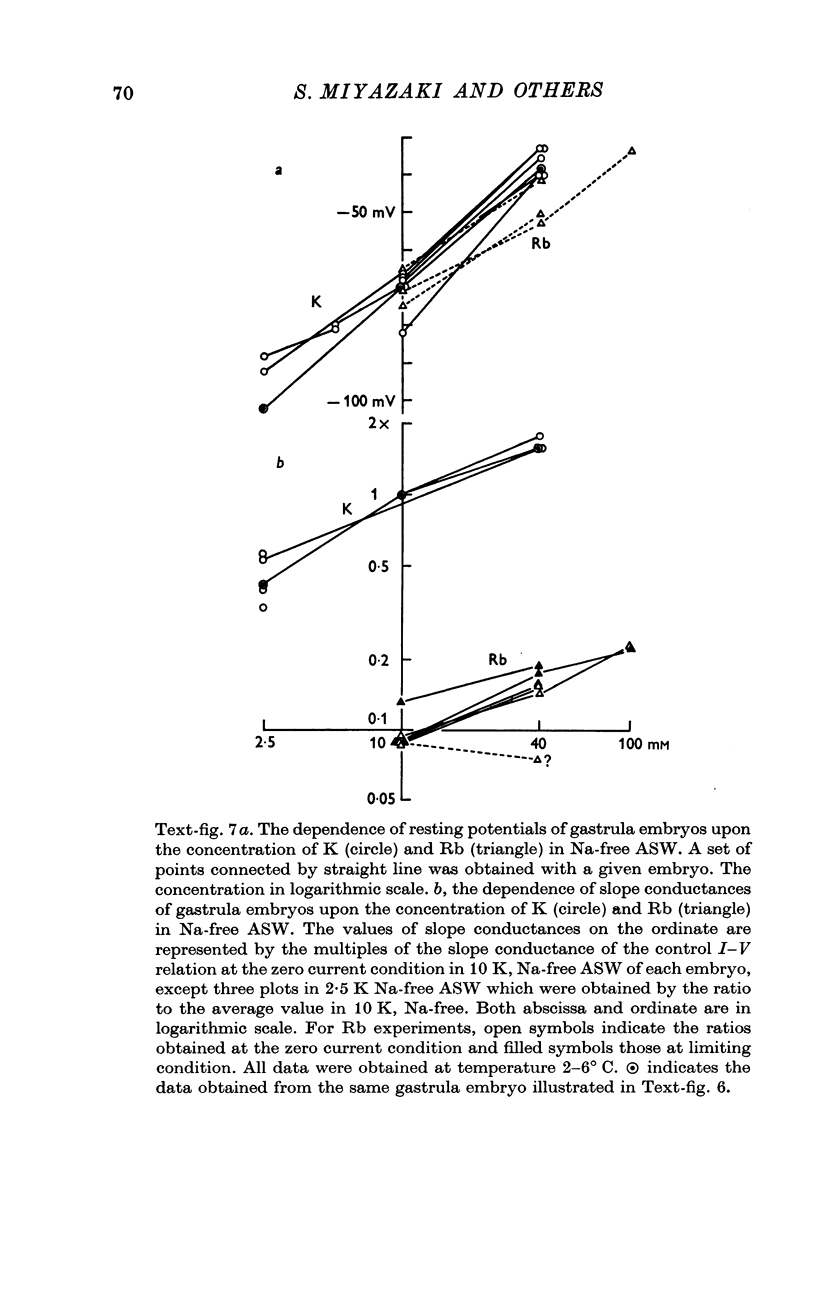
7. The replacement of K with Rb in ASW produced the marked suppression of the inward-going rectification of the embryonic membrane.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H., FREYGANG W. H. Potassium conductance of frog muscle membrane under controlled voltage. J Physiol. 1962 Aug;163:104–114. doi: 10.1113/jphysiol.1962.sp006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADRIAN R. H. THE RUBIDIUM AND POTASSIUM PERMEABILITY OF FROG MUSCLE MEMBRANE. J Physiol. 1964 Dec;175:134–159. doi: 10.1113/jphysiol.1964.sp007508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHMAN R. F., KANNO Y., LOEWENSTEIN W. R. INTERCELLULAR ELECTRICAL COUPLING AT A FORMING MEMBRANE JUNCTION IN A DIVIDING CELL. Science. 1964 Aug 7;145(3632):604–605. doi: 10.1126/science.145.3632.604. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Slow changes in potassium permeability in skeletal muscle. J Physiol. 1970 Jul;208(3):645–668. doi: 10.1113/jphysiol.1970.sp009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H. Rectification in muscle membrane. Prog Biophys Mol Biol. 1969;19(2):339–369. [PubMed] [Google Scholar]
- Bennett M. V., Grundfest H. Analysis of depolarizing and hyperpolarizing inactivation responses in gymnotid electroplaques. J Gen Physiol. 1966 Sep;50(1):141–169. doi: 10.1085/jgp.50.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Trinkaus J. P. Electrical coupling between embryonic cells by way of extracellular space and specialized junctions. J Cell Biol. 1970 Mar;44(3):592–610. doi: 10.1083/jcb.44.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARMELIET E. E. Chloride ions and the membrane potential of Purkinje fibres. J Physiol. 1961 Apr;156:375–388. doi: 10.1113/jphysiol.1961.sp006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DECK K. A., TRAUTWEIN W. IONIC CURRENTS IN CARDIAC EXCITATION. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jun 9;280:63–80. doi: 10.1007/BF00412616. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S., Gage P. W. Ionic conductances of the surface and transverse tubular membranes of frog sartorius fibers. J Gen Physiol. 1969 Mar;53(3):279–297. doi: 10.1085/jgp.53.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNDFEST H. Ionic mechanisms in electrogenesis. Ann N Y Acad Sci. 1961 Sep 6;94:405–457. doi: 10.1111/j.1749-6632.1961.tb35554.x. [DOI] [PubMed] [Google Scholar]
- Grundfest H. Heterogeneity of excitable membrane: electrophysiological and pharmacological evidence and some consequences. Ann N Y Acad Sci. 1966 Jul 14;137(2):901–949. doi: 10.1111/j.1749-6632.1966.tb50208.x. [DOI] [PubMed] [Google Scholar]
- HALL A. E., HUTTER O. F., NOBLE D. Current-voltage relations of Purkinje fibres in sodium-deficient solutions. J Physiol. 1963 Apr;166:225–240. doi: 10.1113/jphysiol.1963.sp007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. Rectifying properties of heart muscle. Nature. 1960 Nov 5;188:495–495. doi: 10.1038/188495a0. [DOI] [PubMed] [Google Scholar]
- Ito S., Hori N. Electrical characteristics of Triturus egg cells during cleavage. J Gen Physiol. 1966 May;49(5):1019–1027. doi: 10.1085/jgp.49.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Loewenstein W. R. Ionic communication between early embryonic cells. Dev Biol. 1969 Mar;19(3):228–243. doi: 10.1016/0012-1606(69)90062-1. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R., Nakas M., Socolar S. J. Junctional membrane uncoupling. Permeability transformations at a cell membrane junction. J Gen Physiol. 1967 Aug;50(7):1865–1891. doi: 10.1085/jgp.50.7.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Takahashi K., Tsuda K. Electrical excitability in the egg cell membrane of the tunicate. J Physiol. 1974 Apr;238(1):37–54. doi: 10.1113/jphysiol.1974.sp010509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Takahashi K., Tsuda K. Calcium and sodium contributions to regenerative responses in the embryonic excitable cell membrane. Science. 1972 Jun 30;176(4042):1441–1443. doi: 10.1126/science.176.4042.1441. [DOI] [PubMed] [Google Scholar]
- NAKAJIMA S., IWASAKI S., OBATA K. Delayed rectification and anomalous rectification in frog's skeletal muscle membrane. J Gen Physiol. 1962 Sep;46:97–115. doi: 10.1085/jgp.46.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S. Analysis of K inactivation and TEA action in the supramedullary cells of puffer. J Gen Physiol. 1966 Mar;49(4):629–640. doi: 10.1085/jgp.49.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Kusano K. Behavior of delayed current under voltage clamp in the supramedullary neurons of puffer. J Gen Physiol. 1966 Mar;49(4):613–628. doi: 10.1085/jgp.49.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Nakajima S., Grundfest H. Analysis of Spike Electrogenesis and Depolarizing K Inactivation in Electroplaques of Electrophorus electricus, L. J Gen Physiol. 1965 Nov 1;49(2):321–349. doi: 10.1085/jgp.49.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. F., Slack C. Some bio-electric parameters of early Xenopus embryos. J Embryol Exp Morphol. 1970 Nov;24(3):535–553. [PubMed] [Google Scholar]
- Potter D. D., Furshpan E. J., Lennox E. S. Connections between cells of the developing squid as revealed by electrophysiological methods. Proc Natl Acad Sci U S A. 1966 Feb;55(2):328–336. doi: 10.1073/pnas.55.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J. D. Dye movement and low-resistance junctions between reaggregated embryonic cells. Dev Biol. 1971 Dec;26(4):627–636. doi: 10.1016/0012-1606(71)90145-x. [DOI] [PubMed] [Google Scholar]
- Sheridan J. D. Electrophysiological evidence for low-resistance intercellular junctions in the early chick embryo. J Cell Biol. 1968 Jun;37(3):650–659. doi: 10.1083/jcb.37.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C., Palmer J. F. The permeability of intercellular junctions in the early embryo of Xenopus laevis, studied with a fluorescent tracer. Exp Cell Res. 1969 Jun;55(3):416–419. doi: 10.1016/0014-4827(69)90577-1. [DOI] [PubMed] [Google Scholar]
- TRAUTWEIN W., KASSEBAUM D. G. On the mechanism of spontaneous impulse generation in the pacemaker of the heart. J Gen Physiol. 1961 Nov;45:317–330. doi: 10.1085/jgp.45.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Miyazaki S. I., Kidokoro Y. Development of excitability in embryonic muscle cell membranes in certain tunicates. Science. 1971 Jan 29;171(3969):415–418. doi: 10.1126/science.171.3969.415. [DOI] [PubMed] [Google Scholar]
- Tupper J., Saunders J. W., Jr, Edwards C. The onset of electrical communication between cells in the developing starfish embryo. J Cell Biol. 1970 Jul;46(1):187–191. doi: 10.1083/jcb.46.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner A. E. Electrical connexions between cells at neural stages of the axolotl. J Physiol. 1970 Sep;210(2):150P–151P. [PubMed] [Google Scholar]