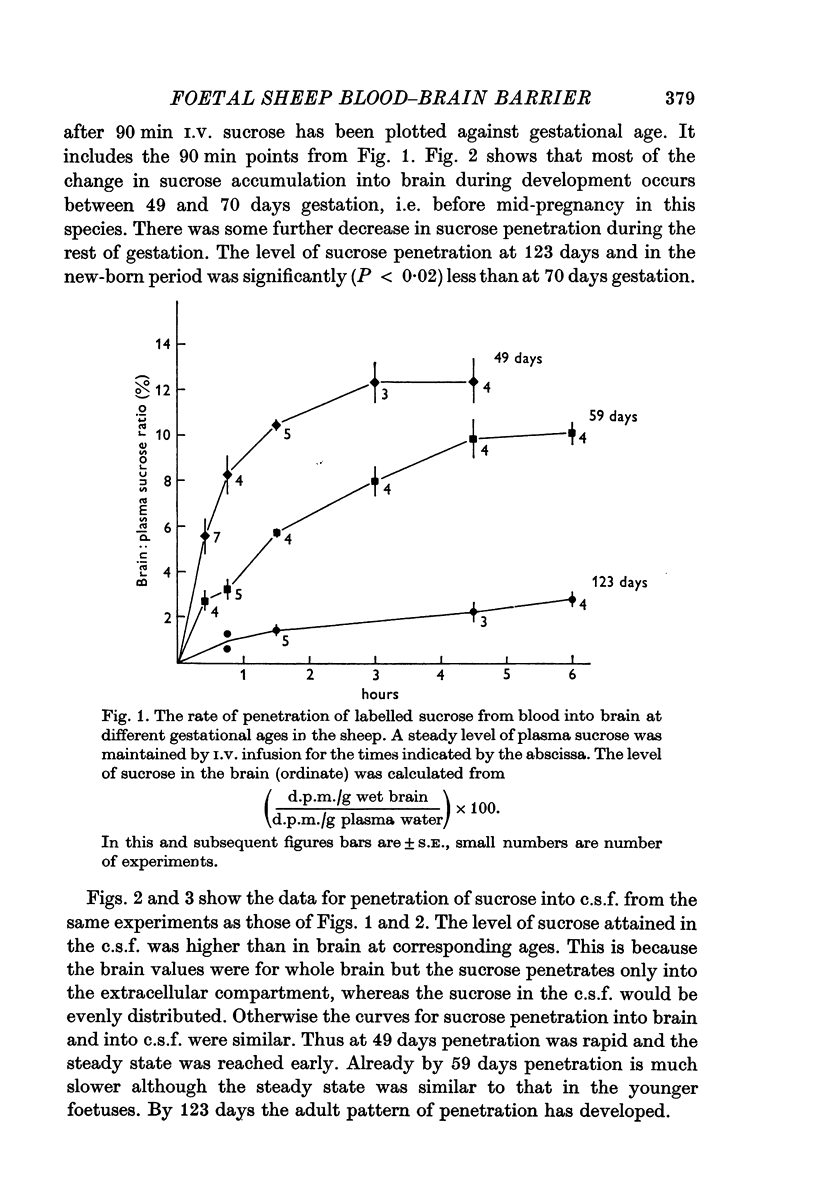
Abstract
1. The penetration of a metabolically inert, small molecular radius lipid insoluble substance ([13C] and [4H]sucrose), from blood into brain and c.s.f., has been studied in developing sheep from 50 days gestation (term, 150 days) through to the new-born stage. Around 50 days gestation sucrose accumulated rapidly into brain and c.s.f., and reached a steady-state level in brain of about 12% of the plasma level by 3 hr. By 60 days sucrose penetrated less freely into brain and c.s.f.; the brain steady-state level was 10% by 4½ hr. A large decrease in sucrose penetration occurred by 70 days gestation, and by 123 days (just before the time when a foetal lamb becomes viable) both the rate of penetration and the brain steady-state level of sucrose were similar to those of the adult of other species.
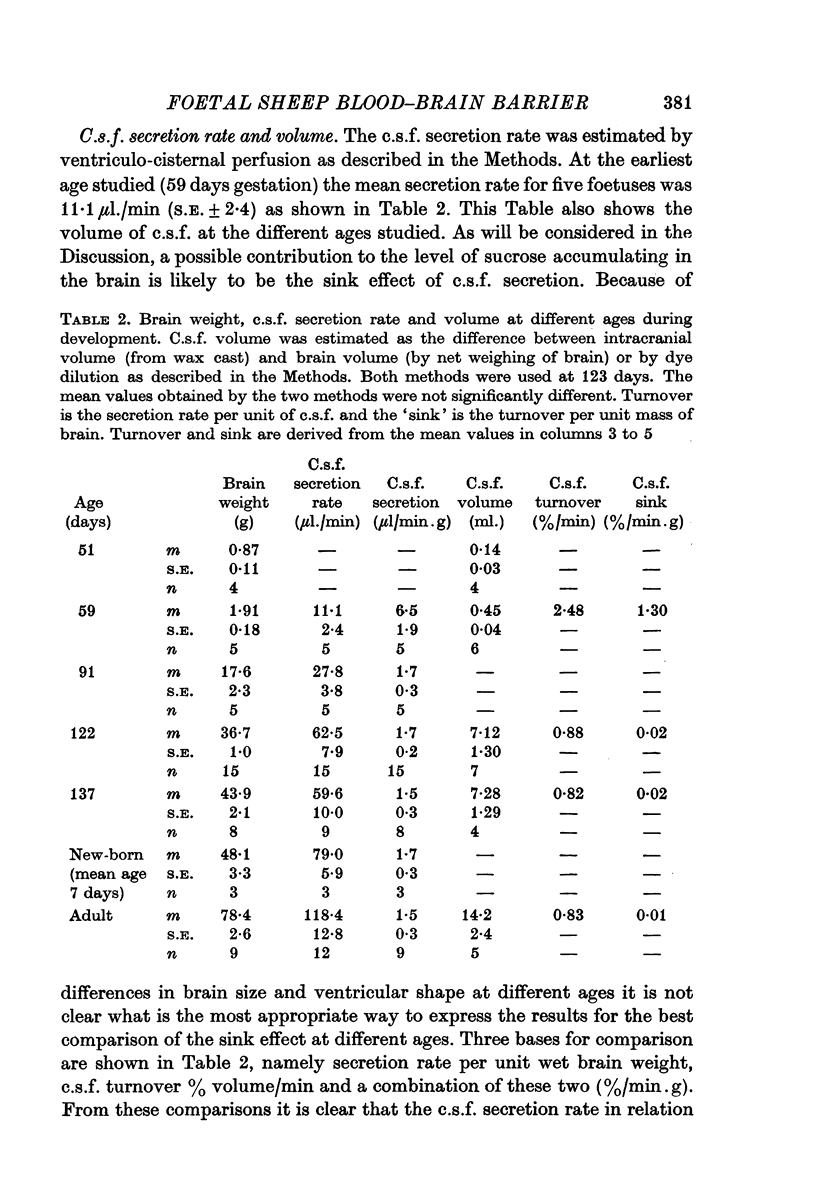
2. The rate of c.s.f. secretion at different ages has been estimated by dye dilution during ventriculo-cisternal perfusion. The turnover of c.s.f. in 60 day foetuses was high (1·36%/min.g wet weight brain). From 123 days gestation to the adult stage the turnover was much lower, 0·02%/min.g at 123 and 137 days gestation and 0·01%/min.g in the adult ewe.
3. A simple new method for measuring c.s.f. volume is described. The volume at 51 days was estimated to be 0·14 ml., S.E. ± 0·03, n = 4 (brain weight = 0·87 g ± 0·11), at 59 days it was 0·45 ml., S.E. ± 0·04, n = 6 (brain weight = 2·0 g ± 0·1) and near term it was 7·28 ml S.E. ± 1·29, n = 4 (brain weight 42·0 g ± 0·5).
4. The results are discussed in relation to possible changes in permeability of the cerebral capillary endothelium, the sink effect of c.s.f., and changes in extracellular space of the brain during its development. It is concluded that the high rate of penetration and raised brain steady-state level of sucrose in immature sheep foetuses is probably due to immaturity of a permeability barrier at the level of the cerebral capillary endothelium or its associated glial processes. Some clinical implications of these findings are considered briefly.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bito L. Z., Myers R. E. The ontogenesis of haematoencephalic cation transport processes in the rhesus monkey. J Physiol. 1970 May;208(1):153–170. doi: 10.1113/jphysiol.1970.sp009111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M. W., Crowder J., Desai S., Reynolds J. M., Reynolds M., Saunders N. R. Electrolytes and water in the brain and cerebrospinal fluid of the foetal sheep and guinea-pig. J Physiol. 1972 Dec;227(2):591–610. doi: 10.1113/jphysiol.1972.sp010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron I. R., Davson H., Segal M. B. The effect of hypercapnia on the blood-brain barrier to sucrose in the rabbit. Yale J Biol Med. 1969 Dec;42(3-4):241–247. [PMC free article] [PubMed] [Google Scholar]
- DAWES G. S., MOTT J. C., WIDDICOMBE J. G., WYATT D. G. Changes in the lungs of the new-born lamb. J Physiol. 1953 Jul;121(1):141–162. doi: 10.1113/jphysiol.1953.sp004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBBING J. The blood-brain barrier. Physiol Rev. 1961 Jan;41:130–188. doi: 10.1152/physrev.1961.41.1.130. [DOI] [PubMed] [Google Scholar]
- Davson H., Welch K. The permeation of several materials into the fluids of the rabbit's brain. J Physiol. 1971 Oct;218(2):337–351. doi: 10.1113/jphysiol.1971.sp009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson R. K., Woodbury D. M. Penetration of 14C-inulin and 14C-sucrose into brain, cerebrospinal fluid, and skeletal muscle of developing rats. Exp Brain Res. 1969;7(3):181–194. doi: 10.1007/BF00239028. [DOI] [PubMed] [Google Scholar]
- Heymann M. A., Rudolph A. M. Effect of exteriorization of the sheep fetus on its cardiovascular function. Circ Res. 1967 Nov;21(5):741–745. doi: 10.1161/01.res.21.5.741. [DOI] [PubMed] [Google Scholar]
- Holloway L. S., Jr, Cassin S. Cerebrospinal fluid dynamics in the newborn dog during normoxia and hypoxia. Am J Physiol. 1972 Sep;223(3):499–502. doi: 10.1152/ajplegacy.1972.223.3.499. [DOI] [PubMed] [Google Scholar]
- Hosain P., Hosain F., Iqbal Q. M., Carulli N., Wagner H. N., Jr Measurement of plasma volu using 99Tcm, and 113Inm labelled proteins. Br J Radiol. 1969 Aug;42(500):627–630. doi: 10.1259/0007-1285-42-500-627. [DOI] [PubMed] [Google Scholar]
- Humphreys P. W., Normand I. C., Reynolds E. O., Strang L. B. Pulmonary lymph flow and the uptake of liquid from the lungs of the lamb at the start of breathing. J Physiol. 1967 Nov;193(1):1–29. doi: 10.1113/jphysiol.1967.sp008340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisels M. J. Bilirubin; on understanding and influencing its metabolism in the newborn infant. Pediatr Clin North Am. 1972 May;19(2):447–501. [PubMed] [Google Scholar]
- Meschia G., Makowski E. L., Battaglia F. C. The use of indwelling catheters in the uterine and umbilical veins of sheep for a description of fetal acid-base balance and oxygenation. Yale J Biol Med. 1969 Dec;42(3-4):154–165. [PMC free article] [PubMed] [Google Scholar]
- Oldendorf W. H., Davson H. Brain extracellular space and the sink action of cerebrospinal fluid. Measurement of rabbit brain extracellular space using sucrose labeled with carbon 14. Arch Neurol. 1967 Aug;17(2):196–205. doi: 10.1001/archneur.1967.00470260086010. [DOI] [PubMed] [Google Scholar]
- SLOBODY L. B., YANG D. C., LENDING M., BORRELLI F. J., TYREE M. Effect of severe hypoxia on blood-cerebrospinal fluid barrier. Am J Physiol. 1957 Aug;190(2):365–370. doi: 10.1152/ajplegacy.1957.190.2.365. [DOI] [PubMed] [Google Scholar]
- Sisson W. B., Oldendorf W. H., Cassen B. Liquid scintillation counting of 113mIn conversion electrons in the presence of 3H and 14C. J Nucl Med. 1970 Dec;11(12):749–752. [PubMed] [Google Scholar]


