Abstract
1. The effect of electrical stimulation of the motor nerve supplying the whiskers on the activity of single cells in the vibrissal region of the ventrobasal complex of the thalamus has been studied in rats under urethane anaesthesia.
2. The stimulation caused protraction of the ipsilateral whiskers. 60% of the cells which fired to mechanical movements of the whiskers were found to respond to this electrical stimulus with 1-2 impulses at short latency (average 7·7 msec), provided the stimulus was sufficient to move the whiskers.
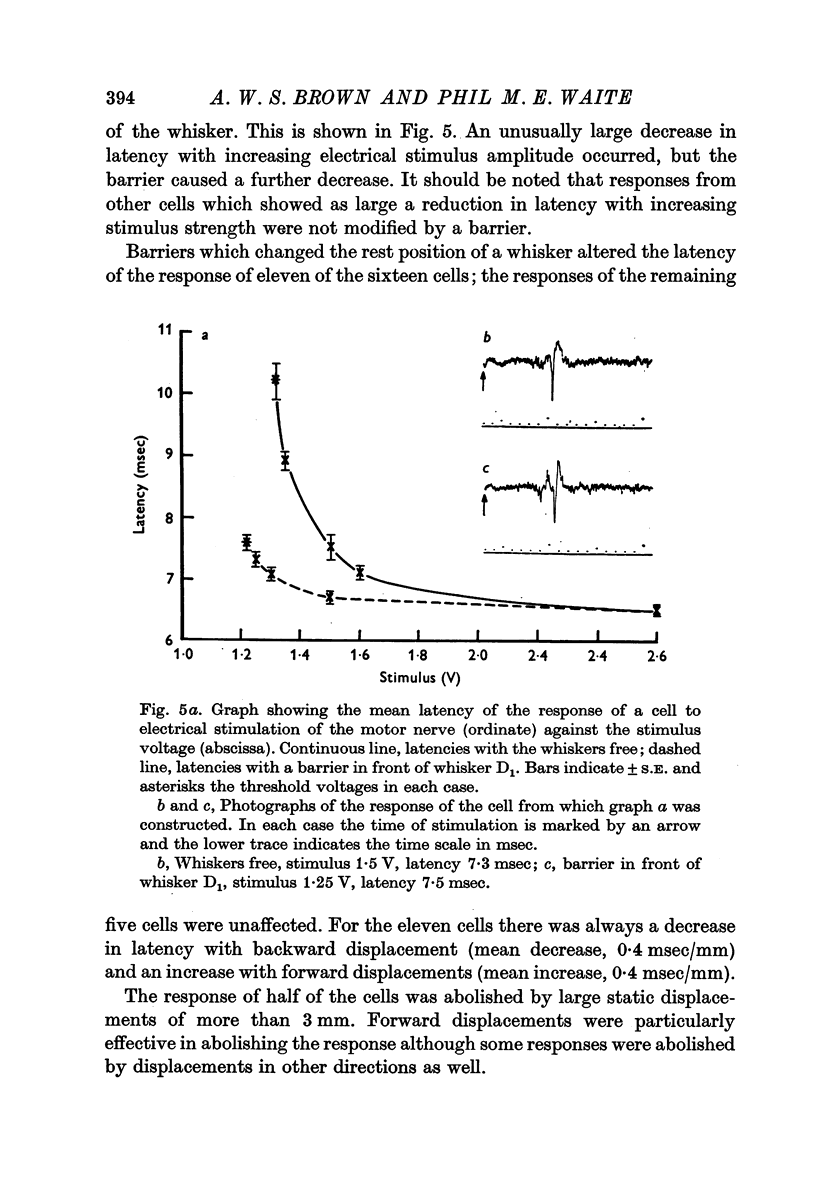
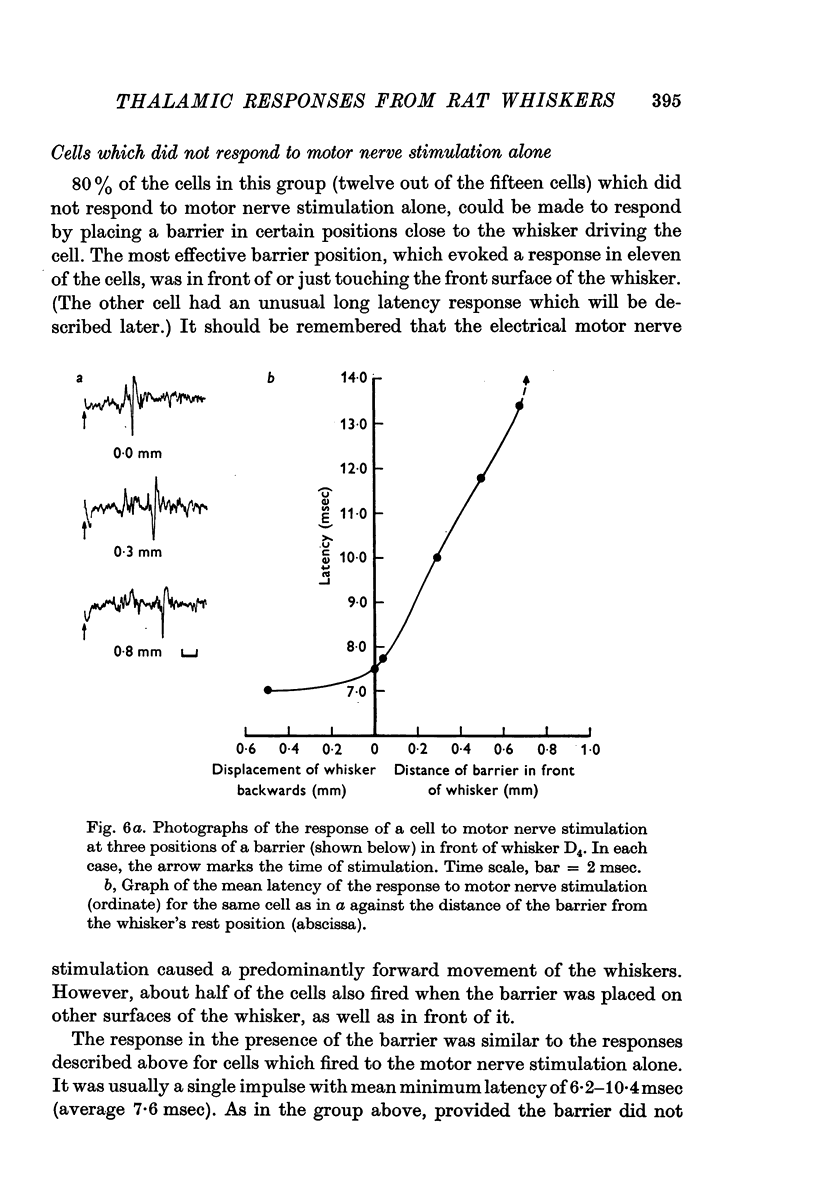
3. When the moving whiskers hit a barrier, 92% of the cells responded to the stimulus. The most effective position of the barrier was in front of the whiskers, although other positions often produced a response as well. Static displacement of the whiskers, particularly in the forward direction, could abolish the response or increase its latency.
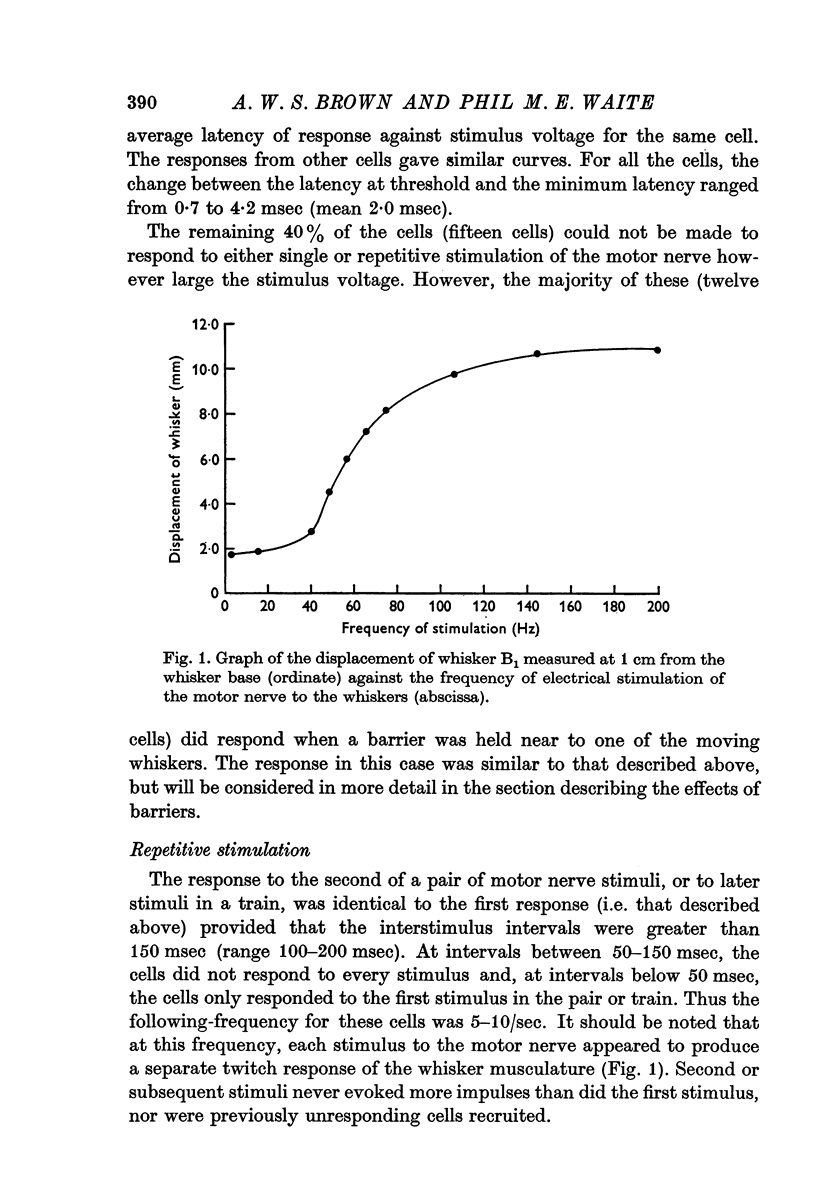
4. The following-frequencies for these cells were 5-10 stimuli/sec. Combinations of electrical stimuli with mechanical ramp movements of the whiskers showed that similar recovery times followed both types of stimuli.
5. These results are compared with those reported from studies in the afferent nerve fibres after electrical stimulation of the motor nerve and also with responses in the thalamus following mechanical movements of the whiskers. The possible importance of the latency of these sensory responses is considered.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitkin L. M., Anderson D. J., Brugge J. F. Tonotopic organization and discharge characteristics of single neurons in nuclei of the lateral lemniscus of the cat. J Neurophysiol. 1970 May;33(3):421–440. doi: 10.1152/jn.1970.33.3.421. [DOI] [PubMed] [Google Scholar]
- Andres K. H. Uber die Feinstruktur der Rezeptoren an Sinushaaren. Z Zellforsch Mikrosk Anat. 1966;75(1):339–365. [PubMed] [Google Scholar]
- Davidson N. The projection of afferent pathways on the thalamus of the rat. J Comp Neurol. 1965 Jun;124(3):377–390. doi: 10.1002/cne.901240308. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B., DAVIES P. W., BERMAN A. L. Response properties of neurons of cat's somatic sensory cortex to peripheral stimuli. J Neurophysiol. 1957 Jul;20(4):374–407. doi: 10.1152/jn.1957.20.4.374. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B., POWELL T. P. Neural mechanisms subserving cutaneous sensibility, with special reference to the role of afferent inhibition in sensory perception and discrimination. Bull Johns Hopkins Hosp. 1959 Oct;105:201–232. [PubMed] [Google Scholar]
- Nord S. G. Receptor field characteristics of single cells in the rat spinal trigeminal complex. Exp Neurol. 1968 Jun;21(2):236–243. doi: 10.1016/0014-4886(68)90142-8. [DOI] [PubMed] [Google Scholar]
- POGGIO G. F., MOUNTCASTLE V. B. THE FUNCTIONAL PROPERTIES OF VENTROBASAL THALAMIC NEURONSSTUDIED IN UNANESTHETIZED MONKEYS. J Neurophysiol. 1963 Sep;26:775–806. doi: 10.1152/jn.1963.26.5.775. [DOI] [PubMed] [Google Scholar]
- Patrizi G., Munger B. L. The ultrastructure and innervation of rat vibrissae. J Comp Neurol. 1966 Mar;126(3):423–435. doi: 10.1002/cne.901260305. [DOI] [PubMed] [Google Scholar]
- ROSE J. E., MOUNTCASTLE V. B. Activity of single neurons in the tactile thalamic region of the cat in response to a transient peripheral stimulus. Bull Johns Hopkins Hosp. 1954 May;94(5):238–282. [PubMed] [Google Scholar]
- TOWE A. L., KENNEDY T. T. Response of cortical neurons to variation of stimulus intensity and locus. Exp Neurol. 1961 Jun;3:570–587. doi: 10.1016/s0014-4886(61)80006-x. [DOI] [PubMed] [Google Scholar]
- Waite P. M. Somatotopic organization of vibrissal responses in the ventro-basal complex of the rat thalamus. J Physiol. 1973 Jan;228(2):527–540. doi: 10.1113/jphysiol.1973.sp010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite P. M. The responses of cells in the rat thalamus to mechanical movements of the whiskers. J Physiol. 1973 Jan;228(2):541–561. doi: 10.1113/jphysiol.1973.sp010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 1971 Mar 5;26(2):259–275. [PubMed] [Google Scholar]
- Zucker E., Welker W. I. Coding of somatic sensory input by vibrissae neurons in the rat's trigeminal ganglion. Brain Res. 1969 Jan;12(1):138–156. doi: 10.1016/0006-8993(69)90061-4. [DOI] [PubMed] [Google Scholar]


