Abstract
The ability of interleukin-10 (IL-10) to suppress accessory cell functions required for optimal T-cell activation makes it an important inhibitor of cell-mediated immunity. Thus, after infection with the protozoan parasite Toxoplasma gondii, IL-10 knockout (KO) mice develop a CD4+-T-cell-dependent shock-like reaction with high levels of IL-12 and gamma interferon (IFN-γ) in serum, leading to death of mice during the acute phase of infection. Previous studies from this laboratory have shown that simultaneous blockade of CD28 and CD40 can prevent this lethal reaction by inhibiting the production of IFN-γ. However, the blockade of costimulation did not affect systemic levels of IL-12. To better understand the relationship between IL-12 and the CD28 and CD40 pathways in mediating immune hyperactivity, antagonists of these factors were used to determine their effects on the development of a pathological T-cell response in IL-10 KO mice. Blockade of IL-12 or the CD28/B7 interaction alone did not affect survival; however, the combined blockade of both pathways resulted in decreased production of IFN-γ and the survival of IL-10 KO mice. To assess the role of the two ligands for CD28, B7.1 and B7.2, IL-10 KO mice were treated with αIL-12 plus αB7.1 or αB7.2 or the combination of all three antibodies. These studies revealed that blockade of both B7 molecules is required for decreased production of IFN-γ and survival of infected IL-10 KO mice, suggesting that B7.1 and B7.2 can contribute to the lethal shock-like reaction in IL-10 KO mice. In contrast, neutralization of IL-12 and blockade of the CD40/CD40 ligand (CD40L) interaction in vivo did not alter the production of IFN-γ and only resulted in a small delay in time to death of mice. Together, these data suggest that the CD28/B7 interaction has a central role in the development of a pathological T-cell response in IL-10 KO mice, which is distinct from the role of the CD40/CD40L and IL-12 pathways.
Interleukin-10 (IL-10), produced by many hemopoietic cells, including macrophages (16), dendritic cells (DCs) (51), and CD4+ T cells (29, 44), plays a key role in the inhibition of inflammatory responses and cell-mediated immunity (for a review, see reference 45). The anti-inflammatory effects of IL-10 are primarily attributed to its ability to inhibit accessory cell functions required for optimal T-cell responses. Thus, IL-10 can inhibit accessory cell production of cytokines such as tumor necrosis factor alpha (TNF-α), IL-1 and IL-12, which are required for the optimal production of gamma interferon (IFN-γ) (12, 21, 22, 32, 45, 61). In addition, IL-10 affects costimulation by decreasing the expression of B7.1 (CD80) and B7.2 (CD86) on monocytes, macrophages, and DCs (8, 18, 65) and inhibits CD40-mediated protein-tyrosine kinase activity (47, 57). Furthermore, IL-10 inhibits major histocompatibility complex class I and class II expression, thereby interfering with antigen presentation and priming of antigen-specific T cells (17, 31, 37).
The importance of IL-10 as an inhibitor of inflammatory responses is illustrated by the development of spontaneous enterocolitis in IL-10 knockout (KO) mice (38). In these mice, the lack of IL-10 leads to abnormal levels of IL-12 and IFN-γ in the intestine, and it has been shown that these cytokines are involved in the development of colitis (3, 15, 50). Another example that highlights the importance of IL-10 in balancing production of IL-12 and IFN-γ is provided by studies using Toxoplasma gondii. Resistance to this pathogen is dependent on IL-12-mediated production of IFN-γ by T cells (5, 42, 58-60). However, in the absence of IL-10, infection with T. gondii results in the systemic overproduction of IL-12 and IFN-γ, the development of severe liver pathology, and death of the mice (19, 27). Treatment of infected IL-10 KO mice with monoclonal antibodies to IL-12 or IFN-γ leads to a modest delay in time to death, suggesting that the overproduction of inflammatory cytokines contributes to this lethal reaction (19). In addition, studies by Gazzinelli et al. showed that the shock-like reaction in these mice is dependent on CD4+ T cells (27).
Since costimulation through CD28/B7 and CD40/CD40 ligand (CD40L) is important for optimal T-cell activation and both signaling pathways are regulated by IL-10 (8, 18, 47, 57, 65), experiments were performed to determine how costimulation affects infection-induced pathology in IL-10 KO mice. Recent studies from this laboratory demonstrated that in the absence of IL-10, inhibition of the CD40/CD40L or CD28/B7 pathways does not affect survival; however, simultaneous blockade of these pathways resulted in decreased production of IFN-γ and survival of IL-10 KO mice (63). These findings suggested that the CD28/B7 and CD40/CD40L interactions are parallel pathways that contribute to the CD4+-T-cell-mediated pathology in IL-10 KO mice. In addition, these studies raised fundamental questions about whether B7.1 or B7.2 played a preferential role in the regulation of T-cell activation in this model and whether the CD40/CD40L interaction directly regulates IL-12 production or T-cell activation. To address these questions, a series of studies were performed in which infected IL-10 KO mice were treated with various combinations of antibodies to antagonize IL-12, as well as the CD28/B7 and CD40/CD40L interactions, and the effects on survival, cytokine production, and pathology were monitored. Together with the findings of our previous studies, these data reveal a complex hierarchy between the costimulatory pathways and show that the CD28/B7 interaction has a unique contribution to the development of a pathological T-cell response, which is distinct from the role of the CD40/CD40L and IL-12 pathways.
MATERIALS AND METHODS
Mice.
Female CBA/CaJ and Swiss Webster mice were obtained from The Jackson Laboratories (Bar Habor, Maine). IL-10 KO mice, originally provided by DNAX (38), were generated by backcrossing C57BL/6-129/OLA IL-10 KO mice onto the C57BL/6 background for seven generations. IL-10 KO mice were genotyped by PCR (protocol was provided by D. Rennick, DNAX) and bred and maintained in Thoren Unit cages in the Gene Therapy Animal Facility at the University of Pennsylvania. Experiments were performed with 4- to 8-week-old female IL-10 KO mice.
Parasites.
The Me49 strain of T. gondii was maintained in infected Swiss Webster and CBA/CaJ mice. Me49 cysts were prepared from brains of donor mice as previously described (6, 7). Mice were infected with 20 cysts by intraperitoneal (i.p.) injection in a volume of 200 μl.
Reagents.
Complete RPMI 1640 (Life Technologies, Gaithersburg, Md.) medium was supplemented with 10% heat-inactivated fetal calf serum, 1% sodium pyruvate, 1% nonessential amino acids, 0.1% β-mercaptoethanol, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. HuCTLA4-Ig, a fusion protein comprised of the human CTLA4 extracellular domain and the Fc portion of human immunoglobulin G (IgG), was supplied by Bristol Myers Squibb Research Institute (Princeton, N.J.). αIL-12 was purified from ascites by ammonium sulfate precipitation, and αCD40L (MR1) was obtained from TSD Bioscience (Newark, Del.). αB7.1 and αB7.2 were provided by the Genetics Institute (Andover, Mass.). Human chimeric L6 (ChiL6; Bristol Myers Squibb), rat IgG, and hamIgG (Sigma, St. Louis, Mo.) were used as control antibodies. αIL-12 and αCD40L (or the control antibodies ratIgG and hamIgG, respectively) were used at a concentration of 200 μg per treatment per mouse, and αB7.1, αB7.2, and CTLA4-Ig (or the control antibodies ratIgG and ChiL6, respectively) were used at a concentration of 300 μg per treatment per mouse. IL-10 KO mice that had been infected with Me49 were given the blocking antibodies or the control antibodies at days 5 and 7 postinfection (p.i.). Sera from these mice were collected at day 8 p.i. and analyzed for cytokine production by enzyme-linked immunosorbent assay. For antigen-specific recall responses, antibodies were used at a concentration of 20 μg/ml.
Cytokine assays.
IL-12 p40 levels were measured by using monoclonal antibody C17.8 as a capture antibody and biotinylated C15.6 as a detecting antibody (hybridomas were provided by Giorgio Trinchieri, Wistar Institute, Philadelphia, Pa.). IFN-γ levels were measured by using R46A2 (capture antibody) and biotinylated AN18 (detecting antibody).
In vitro recall response.
Spleen cells from infected mice were harvested at day 8 p.i. and dissociated into a single-cell suspension. Erythrocytes were depleted by using 0.83% ammonium chloride (10 min, 4°C), cells were washed twice in complete RPMI medium and resuspended at a final concentration of 4 × 106/ml. A total of 4 × 105 cells were plated per well in a final volume of 200 μl in 96-well plates (Costar, Cambridge, Mass.) and were stimulated with soluble Toxoplasma lysate antigen (TLA). TLA was prepared from RH strain tachyzoites as previously described (52), titrated to determine the optimal concentration for induction of cytokines, and used at 30 μg/ml. Cells were cultured for 48 h at 37°C under CO2-saturating conditions. Supernatants were harvested and analyzed for cytokines by enzyme-linked immunosorbent assay and for reactive nitrogen intermediates by using the Greiss reaction as previously described (9).
Histology and immunohistochemistry.
At day 8 p.i. mice were sacrificed and spleen, liver, lungs, heart, and brain were removed from each mouse. Organs were prepared for hematoxylin and eosin (H&E) staining or immunohistochemical staining as previously described (33). Briefly, tissues were fixed overnight in Accustin 10% formalin neutral buffered solution (Sigma) and further embedded in paraffin. Then, 5-μm paraffin sections were stained with H&E for visualization of pathological changes. TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) staining of paraffin sections (spleen) was done by using an in situ cell death detection kit (POD; Roche Molecular Biochemicals, Mannheim, Germany). Briefly, tissue sections were incubated with fluorescein-labeled DNA strand breaks, followed by an incubation using anti-fluorescein antibodies conjugated with horseradish peroxidase. Apoptotic cells were visualized by substrate reaction and analyzed under the light microscope. At a magnification of ×600, 10 different fields on each slide were counted. The data represent the means of two slides per group from three independent experiments (n = 60 fields per group).
FACS analysis.
Spleen cells were harvested at day 8 p.i., and single-cell suspensions were prepared as described above. Cells were washed twice with fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline, 0.2% bovine serum albumin, 4 mM sodium azide) and resuspended at a final concentration of 107 cells/ml. Then, 106 cells were preincubated for 10 min at 4°C with Fc blockTM and stained for 30 min at 4°C with fluorescein isothiocyanate-labeled antibodies against CD4, CD8, F4/80, and NK1.1 (PharMingen, San Diego, Calif.) or B220 (Caltag, South San Francisco, Calif.). After a 30-min incubation at 4°C, cells were washed once in FACS buffer, and a FACScalibur flow cytometer and CellQuest software (Becton Dickinson, San Francisco, Calif.) were used to analyze experimental data.
Statistical analysis.
Statistical analyses were performed by using the INSTAT software (GraphPad, San Diego, Calif.). Unpaired Mann-Whitney test was used to determine the significance of differences in cytokine levels. P values of <0.03 were considered significant.
RESULTS
Effect of αIL-12 and/or CTLA4-Ig on survival and cytokine production in IL-10 KO mice.
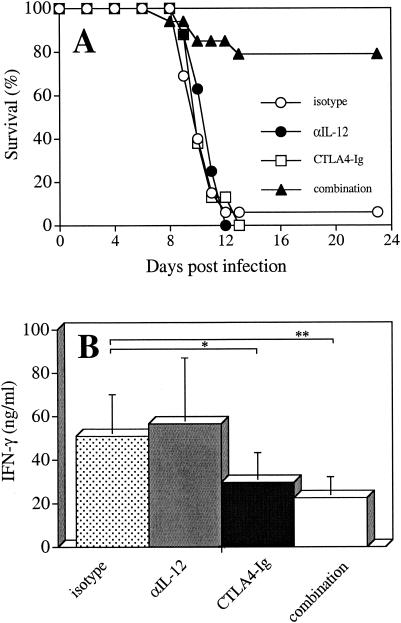
Infection of IL-10 KO mice with T. gondii results in the development of a CD4+-T-cell-mediated pathology (27) and is associated with elevated levels of IL-12 and IFN-γ and the death of mice within 10 to 12 days (19, 27). Since optimal activation of T cells to produce IFN-γ during toxoplasmosis requires stimulation through IL-12 and the costimulatory molecule CD28, studies were performed to assess the relationship between these pathways and the infection-induced pathology in IL-10 KO mice. αIL-12 was used to neutralize systemic IL-12, and CTLA4-Ig, which binds to the B7 molecules, was used to block the CD28/B7 interaction. Since previous studies have shown that the development of the immune response in IL-10 KO mice starts to diverge from that in wild-type mice between days 5 and 7 p.i. (19, 27), in vivo treatment with αIL-12 and/or CTLA4-Ig was performed at this time period. In these experiments, treatment with αIL-12 resulted in a reduction in serum levels of IL-12 by ca. 90% at day 8 p.i. (data not shown). As shown in Fig. 1, treatment with either αIL-12 or CTLA4-Ig alone did not affect survival of IL-10 KO mice, and these mice succumbed to infection at the same time as mice treated with isotype control antibodies (Fig. 1A). However, treatment of IL-10 KO mice with αIL-12 plus CTLA4-Ig resulted in increased survival (Fig. 1A) and analysis of cytokine levels in serum revealed a significant reduction of systemic IFN-γ (Fig. 1B). Surprisingly, although treatment with CTLA4-Ig alone did not result in the protection of IL-10 KO mice, levels of IFN-γ in serum were significantly reduced compared to mice treated with the control antibodies (Fig. 1B). In contrast, levels of IFN-γ in the sera in mice treated with αIL-12 alone were similar to those measured in the control mice (Fig. 1B), suggesting that, in the absence of IL-10, IL-12 is not required for production of IFN-γ. However, these studies further demonstrate that, although blockade of the CD28/B7 interaction alone is sufficient to reduce the production of IFN-γ, blockade of IL-12 plus CD28 is necessary for survival of T. gondii-infected IL-10 KO mice.
FIG. 1.
Effect of αIL-12 and CTLA4-Ig on survival and production of IFN-γ in IL-10 KO mice infected with T. gondii. IL-10 KO mice infected with T. gondii were treated i.p. with 200 μg of αIL-12 and 300 μg of CTLA4-Ig or the control antibodies (ratIgG or ChiL6) at days 5 and 7 p.i. (A) Survival of mice was monitored throughout the course of infection; (B) levels of IFN-γ in serum were determined at day 8 p.i. The data show the means ± the standard deviation (SD) of five independent experiments with n = 8 to 13 mice per group. ✽, P < 0.03; ✽✽, P < 0.001 (unpaired Mann-Whitney test). Treatment with αIL-12 resulted in a reduction in the levels of IL-12 in serum by ca. 90% at day 8 p.i. (data not shown). Mice that survived the acute phase of infection did not display increased susceptibility to infection, and at week 6 p.i. the experiments were terminated.
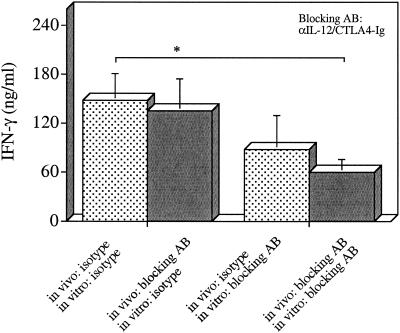
To further assess the effect of in vivo blockade of IL-12 and CD28/B7 costimulation on T-cell responses, parasite-specific recall responses were performed. In these experiments, IL-10 KO mice were infected with T. gondii and treated at days 5 and 7 p.i. with either the isotype control antibodies or αIL-12/CTLA4-Ig. At day 8 p.i., splenocytes from these mice were harvested and stimulated ex vivo with parasite-antigen (TLA). These experiments revealed that splenocytes from infected mice that were treated in vivo with αIL-12 and CTLA-Ig or isotype controls produced comparable levels of IFN-γ in response to TLA (Fig. 2A). Thus, in vivo treatment with αIL-12 plus CTLA4-Ig does not affect the ex vivo responses of T cells from IL-10 KO mice and, after removal of the blocking antibodies, cells are still able to respond to parasite-antigen with optimal production of IFN-γ. To further dissect the role of IL-12 and CD28/B7 costimulation in the regulation of IFN-γ production, the effect of αIL-12 plus CTLA4-Ig was assessed in vitro by using splenocytes from IL-10 KO mice treated in vivo with either the isotype controls or αIL-12/CTLA4-Ig. These studies revealed that the addition of αIL-12 plus CTLA4-Ig to these cultures only resulted in a significant reduction in the production of IFN-γ with splenocytes from mice that had been treated in vivo with αIL-12 plus CTLA4-Ig. Together, these data demonstrate that in vivo plus in vitro blockade of IL-12 and CD28/B7 is required for maximal reduction of IFN-γ produced in response to TLA by splenocytes from IL-10 KO mice. In contrast, production of reactive nitrogen intermediates, one of the mediators associated with parasite killing (1) was not affected by blockade of IL-12 and the CD28/B7 pathway (data not shown). FACS analysis revealed that blockade of costimulation and IL-12 in vivo did not alter the numbers of CD4+ T cells, CD8+ T cells, and NK cells in the spleens of IL-10 KO mice after (data not shown), suggesting that the reduced production of IFN-γ observed in these mice after treatment with αIL-12 and CTLA4-Ig was not associated with a loss of IFN-γ-producing cells. Together, these data indicate that the treatment with CTLA4-Ig plus αIL-12 did not affect development or expansion of parasite specific T-cell responses and that the protective effects of these blocking antibodies is due to their transient inhibition of T-cell responses.
FIG. 2.
Effects of αIL-12 and CTLA4-Ig on the production of IFN-γ by splenocytes in response to TLA. Splenocytes from IL-10 KO mice infected with T. gondii and treated with 200 μg of αIL-12 and 300 μg of CTLA4-Ig or the control antibodies (ratIgG or ChiL6) at days 5 and 7 p.i. were harvested at day 8 p.i., stimulated with TLA (30 μg/ml), and cocultured with αIL-12 and CTLA4-Ig or the respective isotype control antibodies (ratIgG or ChiL6) for 48 h. The data represent the means ± the SD of four independent experiments with n = 8 mice per group. ✽, P < 0.03 (unpaired Mann-Whitney test).
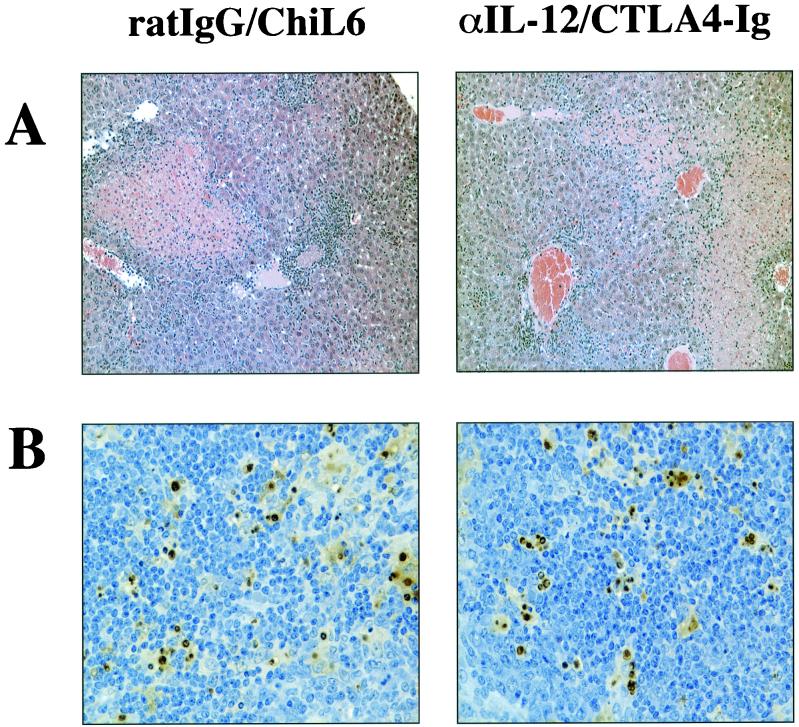
Previous studies have shown that infection of IL-10 KO mice with T. gondii results in the development of severe coagulative necrosis in the liver (27, 63). Since treatment of infected IL-10 KO mice with αIL-12 plus CTLA4-Ig resulted in increased survival (Fig. 1A), the effects of this treatment on the pathology in the livers, spleens, and lungs of infected mice were assessed. IL-10 KO mice were infected with T. gondii and treated at days 5 and 7 p.i. with αIL-12 plus CTLA4-Ig or the respective control antibodies (ratIgG and ChiL6) and were sacrificed on day 8 p.i. Livers, spleens, and lungs were removed and used to prepare sections stained with H&E. Microscopic examination of these tissues revealed that livers from both groups showed necrotic areas and strong inflammation and thrombi in the vessels (Fig. 3A) and that spleens from treated and control mice showed lymphoid necrosis (data not shown). However, there were no apparent differences between both groups of mice. Furthermore, the lungs did not show differences in the infection-induced pathology either (data not shown). Since lymphoid necrosis, mainly located in the germinal centers, was a prominent pathological feature in the spleen, a TUNEL assay was performed to determine whether treatment with αIL-12 plus CTLA4-Ig affected numbers of apoptotic cells. This analysis revealed that IL-10 KO mice treated with αIL-12 and CTLA4-Ig showed no difference in the levels of apoptosis compared to control mice (Fig. 3B) and numbers of apoptotic cells were similar in both groups of mice (isotype control treatment [20.9 ± 11.5] versus αIL-12/CTLA4-Ig treatment [20.7 ± 11.9]). Thus, although neutralization of IL-12 plus blockade of CD28/B7 resulted in decreased levels of IFN-γ in serum and of survival, protection of the IL-10 KO mice was not associated with reduced pathology or numbers of apoptotic cells.
FIG. 3.
Effects of αIL-12 and CTLA4-Ig on liver and spleen pathology in IL-10 KO mice infected with T. gondii. IL-10 KO mice infected with T. gondii were treated i.p. with 200 μg of αIL-12 and 300 μg of CTLA4-Ig or the control antibodies (ratIgG or ChiL6) at days 5 and 7 p.i. On day 8 p.i., livers and spleens were harvested. Livers were stained with H&E (A) and, to determine the numbers of apoptotic cells, a TUNEL assay was performed on spleen sections (B). The sections shown are representative of three independent experiments with n = 6 mice per group.
B7.1 and B7.2 contribute to the CD28-mediated pathology in IL-10 KO mice.
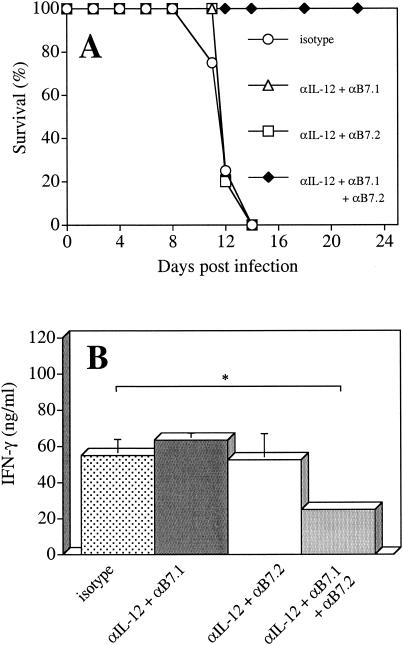
Stimulation of CD28 occurs through the ligands, B7.1 (CD80) and B7.2 (CD86) (2, 25, 40); however, whether B7.1 and B7.2 serve different functions remains unclear and may be dependent on the model system used (20, 24, 26, 39, 41, 46, 62). Since both B7 molecules are upregulated after infection with T. gondii (23, 55, 63, 64), studies were performed to determine whether B7.1 or B7.2 played different roles in the CD28-dependent infection-induced pathology in IL-10 KO mice. Therefore, IL-10 KO mice were infected with T. gondii and treated with αIL-12 plus either αB7.1 or αB7.2 or the combination of all three antibodies at days 5 and 7 p.i. Although blockade of either B7.1 or B7.2 together with neutralization of IL-12 did not affect mortality of mice, the administration of αB7.1 plus αB7.2 together with αIL-12 resulted in the survival of mice (Fig. 4A). Further analysis of these mice revealed that the level of systemic IFN-γ was not affected unless mice were treated with αIL-12 plus αB7.1 plus αB7.2 (Fig. 4B). Thus, only simultaneous blockade of B7.1 and B7.2 together with neutralization of IL-12 led to a survival of IL-10 KO mice and a significant decrease in production of IFN-γ. These findings suggest that B7.1 or B7.2 alone are sufficient to mediate the CD28-dependent component of the infection-induced pathology in IL-10 KO mice.
FIG. 4.
Effects of αB7.1, αB7.2, and αIL-12 on IL-10 KO mice infected with T. gondii. IL-10 KO mice infected with T. gondii were treated i.p. with 200 μg of αIL-12, 300 μg of αB7.1, and/or 300 μg of αB7.2 or the control antibody (ratIgG) at days 5 and 7 p.i. (A) Survival of IL-10 KO mice was monitored; (B) levels of IFN-γ in serum were determined at day 8.p.i. The data represent the means ± the SD of four independent experiments with n = 6 to 16 mice per group. ✽, P < 0.003 (unpaired Mann-Whitney test).
CD40/CD40L costimulation and IL-12 do not affect systemic production of IFN-γ.
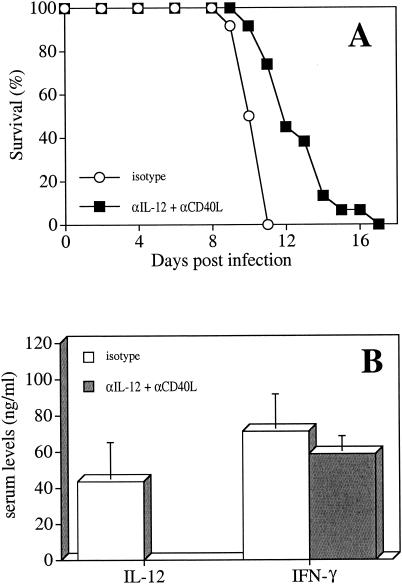
CD40 ligation on accessory cells contributes to optimal T-cell activation by enhancing the secretion of cytokines such as IL-12, IL-1, and TNF-α and by increasing the expression of B7.1 and B7.2 (10, 11, 34, 36, 53, 66). Signaling through CD40 has been shown to be required for IL-12-mediated production of IFN-γ after infection with T. gondii (56), and the CD40/CD40L interaction is therefore critical for optimal T-cell-mediated protection against this pathogen (48, 56). However, previous studies from this laboratory have shown that IL-10 KO mice produce high systemic levels of IL-12, although the CD40/CD40L interaction is blocked (63), suggesting that in this system the IL-12 and CD40/CD40L pathways are regulated independently. To test whether blockade of IL-12 and the CD40/CD40L pathway together would affect the development of the infection-induced pathology, IL-10 KO mice were infected with T. gondii and treated with αCD40L plus αIL-12 at days 5 and 7 p.i. As shown in Fig. 5, coadministration of both antibodies did not affect survival but resulted in a small delay in death compared to mice that were treated with the isotype control antibodies (Fig. 5A). Surprisingly, this treatment did not affect the levels of IFN-γ in serum (Fig. 5B), suggesting that the inhibition of CD40/CD40L costimulation, together with the neutralization of IL-12, is not sufficient to alter the production of IFN-γ. Thus, in IL-10 KO mice infected with T. gondii, once the immune response has been initiated, the production of IFN-γ does not require CD40/CD40L signaling nor IL-12, and the high levels of IFN-γ detected in IL-10 KO mice are not affected by blockade of these pathways.
FIG. 5.
Effects of αCD40L and αIL-12 on IL-10 KO mice infected with T. gondii. IL-10 KO mice infected with T. gondii were treated i.p. with 200 μg of αIL-12 and 200 μg of αCD40L or the control antibodies (ratIgG or hamIgG) at days 5 and 7 p.i. (A) Survival of IL-10 KO mice was monitored; (B) levels of IL-12 and IFN-γ in serum were determined at day 8 p.i. The data represent the means ± the SD of three independent experiments with n = 9 to 11 mice per group.
DISCUSSION
Protective immunity against many intracellular pathogens requires a strong cell-mediated immune response that is associated with the production of IFN-γ by CD4+ and CD8+ T cells. IL-12 is a central regulator of these responses, and its ability to activate T cells to produce IFN-γ plays a critical role in resistance to the protozoan parasite T. gondii. However, in the absence of IL-10, infection with T. gondii results in the systemic overproduction of IL-12 and IFN-γ and mice succumb to a lethal CD4+-T-cell-mediated-pathology (19, 27). Recent studies have shown that simultaneous blockade of CD28/B7 and CD40/CD40L costimulation decreases production of IFN-γ in these mice and prevents the lethal shock-like reaction (63). However, these studies also demonstrated that blockade of costimulation did not affect the systemic levels of IL-12 in IL-10 KO mice (63). Since IL-12 has been implicated in the production of IFN-γ required for the development of immune-mediated pathology, we were interested in its relationship with the CD28/B7 and CD40/CD40L pathways. The results presented here demonstrate that the development of immunopathology in IL-10 KO mice after infection is largely independent of IL-12 and CD40/CD40L costimulation; however, when signaling through CD28/B7 is blocked, IL-12 and CD40 contribute to immune hyperactivity and the lethal shock-like reaction observed in IL-10 KO mice. These findings place CD28 at an important point in the development of immunopathology, and further studies to address whether B7.1 and B7.2 may play different roles in this process indicate that both B7 molecules contribute to the CD28-mediated pathology.
Although early IL-12 is critical for the generation of T. gondii-specific T cells to produce IFN-γ, the administration of αIL-12 to infected IL-10 KO mice at days 5 and 7 p.i. did not affect the production of IFN-γ or survival, suggesting that, as in previous studies with wild-type mice (28, 35), the production of IFN-γ is largely independent of IL-12 after the T-cell response is initiated. These results, however, contrast with studies on inflammatory bowel disease, which develops spontaneously in IL-10 KO mice and is, similar to our model, characterized by abnormal levels of IL-12 and IFN-γ (49). Treatment with αIL-12 can completely prevent the development of colitis in young mice, and IL-12 has been suggested to be the major mediator during chronic disease (13, 14). Thus, the importance of IL-12 in the development of pathology in the absence of IL-10 appears to vary with different models. Interestingly, our data suggest that during infection with T. gondii, IL-12 may contribute to the development of the lethal shock-like reaction in IL-10 KO mice by synergizing with CD28/B7 costimulation, since the administration of αIL-12 plus CTLA4-Ig at days 5 and 7 p.i. decreased production of IFN-γ and resulted in survival of mice, whereas CTLA4-Ig alone did not prevent immunopathology. Blockade of IL-12 plus CD28 in wild-type mice also resulted in decreased levels of IFN-γ (data not shown) in serum but did not result in increased susceptibility of treated mice that survived for 4 weeks before the experiment was terminated.
Previous studies indicated a role for the CD40/CD40L interaction in the production of maximal levels of IL-12 in response to infection with T. gondii (48, 54, 56); however, in IL-10 KO mice the blockade of CD40/CD40L costimulation does not alter systemic levels of IL-12 (63). These studies suggested that in the absence of IL-10, production of IL-12 is largely independent of the CD40/CD40L pathway and led us to determine how the simultaneous blockade of the two independent pathways may affect the outcome of infection. Our data show that administration of αIL-12 plus αCD40L to infected IL-10 KO mice did not alter the production of IFN-γ or the survival of mice and resulted in a delay in time to death only. These findings indicate that, in this model system, immunopathology develops independently of both IL-12 and CD40L and that signaling through CD28 may provide a signal that is strong enough to promote optimal production of IFN-γ independently of IL-12 and the CD40/CD40L interaction.
Although this study has shown that blockade of costimulatory pathways or cytokines can prevent the development of immune hyperactivity, it remains unclear how these interventions actually affect the outcome of infection. A major question that still remains is how CD4+ T cells and the overproduction of cytokines actually leads to the death of the IL-10 KO mice. The presence of severe pathology in the liver provided a likely basis for the infection-induced mortality in IL-10 KO mice (27). However, our studies demonstrate that the treatment of IL-10 KO mice with αIL-12 plus CTLA4-Ig led to survival, although these mice had liver, spleen, and lung pathologies similar to those of IL-10 KO mice treated with isotype control antibodies. Moreover, treatment with CTLA4-Ig alone led to a reduced production of IFN-γ similar to that observed with CTLA4-Ig plus αIL-12 but did not affect survival. Thus, it appears that other factors besides the severe organ pathology and the high levels of IFN-γ observed in these mice are involved in the lethal shock-like reaction. What these factors are remains unclear, but it may be related to systemic changes in the vascular system similar to the changes observed during sepsis (4).
Our results reveal a complex hierarchy for IL-12 and the CD28/B7 and CD40/CD40L interactions in the regulation of IFN-γ production in this model. The transient neutralization of IL-12 or blockade of the CD40/CD40L interaction does not affect production of IFN-γ, as long as costimulation through CD28/B7 is provided. These results suggest that the cross talk between CD40/CD40L and CD28/B7 costimulation, proposed for other systems (30, 43, 54, 66), is not required for optimal production of IFN-γ in IL-10 KO mice infected with T. gondii. This conclusion is supported by studies on human peripheral blood mononuclear cells, which show that CD28 stimulation may be a critical mechanism for IL-12-independent production of IFN-γ (43). However, these in vitro studies also demonstrated that maximal CD28-mediated production of IFN-γ depends on either CD40 or IL-12 (43). In addition, Subauste and Wessendarp (54), in a study of human DCs infected with T. gondii, indicated that CD28/B7 and, to a lesser extent, CD40/CD40L costimulation controls IL-12-independent production of IFN-γ. Nevertheless, the studies presented here demonstrate that in IL-10 KO mice the CD28/B7 interaction plays a central role in T-cell activation and that it bypasses the requirement for CD40 and IL-12 for optimal production of IFN-γ. However, since treatment with CTLA4-Ig alone fails to protect IL-10 KO mice from infection-induced pathology, it is likely that there are CD28-independent costimulatory pathways that are involved in the development of the pathological CD4+-T-cell response observed in IL-10 KO mice.
Acknowledgments
This work was supported by NIH grant AI42334 and the State of Pennsylvania. U.W. was supported by a fellowship from the Deutsche Forschungsgemeinschaft, and E.N.V. was supported by an NIH Predoctoral Fellowship Award (AI09562).
We thank Joe Sypek (Genetics Institute) for the generous gift of the αB7 antibodies.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Adams, L. B., J. B. Hibbs, Jr., R. R. Taintor, and J. L. Krahenbuhl. 1990. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii: role for synthesis of inorganic nitrogen oxides from l-arginine. J. Immunol. 144:2725-2729. [PubMed] [Google Scholar]
- 2.Azuma, M., D. Ito, H. Yagita, K. Okumura, J. H. Phillips, L. L. Lanier, and C. Somoza. 1993. B70 antigen is a second ligand for CTLA-4 and CD28. Nature 366:76-79. [DOI] [PubMed] [Google Scholar]
- 3.Berg, D. J., N. Davidson, R. Kuhn, W. Muller, S. Menon, G. Holland, L. Thompson-Snipes, M. W. Leach, and D. Rennick. 1996. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ Th1-like responses. J. Clin. Investig. 98:1010-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard, G. R., J. L. Vincent, P. F. Laterre, S. P. LaRosa, J. F. Dhainaut, A. Lopez-Rodriguez, J. S. Steingrub, G. E. Garber, J. D. Helterbrand, E. W. Ely, and C. J. Fisher, Jr. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344:699-709. [DOI] [PubMed] [Google Scholar]
- 5.Black, C. M., J. R. Catterall, and J. S. Remington. 1987. In vivo and in vitro activation of alveolar macrophages by recombinant IFN-γ. J. Immunol. 138:491-495. [PubMed] [Google Scholar]
- 6.Blewett, D. A., J. K. Miller, and J. Harding. 1983. Simple technique for the direct isolation of Toxoplasma tissue cysts from fetal ovine brain. Vet. Rec. 112:98-100. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann, V., S. D. Sharma, and J. S. Remington. 1986. Different regulation of the L3T4-T cell subset by B cells in different mouse strains bearing the H-2k haplotype. J. Immunol. 137:2991-2997. [PubMed] [Google Scholar]
- 8.Buelens, C., F. Willems, A. Delvaux, G. Pierard, J. P. Delville, T. Velu, and M. Goldman. 1995. Interleukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur. J. Immunol. 25:2668-2672. [DOI] [PubMed] [Google Scholar]
- 9.Cai, G., R. Kastelein, and C. A. Hunter. 2000. Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii. Infect. Immun. 68:6932-6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caux, C., C. Massacrier, B. Vanbervliet, B. Dubois, C. Van Kooten, I. Durand, and J. Banchereau. 1994. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 180:1263-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T-cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Andrea, A., M. Aste-Amezaga, N. M. Valiante, X. Ma, M. Kubin, and G. Trinchieri. 1993. Interleukin 10 (IL-10) inhibits human lymphocyte IFN-γ production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 178:1041-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson, N. J., M. M. Fort, W. Muller, M. W. Leach, and D. M. Rennick. 2000. Chronic colitis in IL-10−/− mice: insufficient counter regulation of a Th1 response. Int. Rev. Immunol. 19:91-121. [DOI] [PubMed] [Google Scholar]
- 14.Davidson, N. J., S. A. Hudak, R. E. Lesley, S. Menon, M. W. Leach, and D. M. Rennick. 1998. IL-12, but not IFN-γ, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J. Immunol. 161:3143-3149. [PubMed] [Google Scholar]
- 15.Davidson, N. J., M. W. Leach, M. M. Fort, L. Thompson-Snipes, R. Kuhn, W. Muller, D. J. Berg, and D. M. Rennick. 1996. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J. Exp. Med. 184:241-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Waal Malefyt, R., J. Abrams, B. Bennett, C. G. Figdor, and J. E. de Vries. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Waal Malefyt, R., J. Haanen, H. Spits, M. G. Roncarolo, A. te Velde, C. Figdor, K. Johnson, R. Kastelein, H. Yssel, and J. E. de Vries. 1991. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T-cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 174:915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding, L., P. S. Linsley, L. Y. Huang, R. N. Germain, and E. M. Shevach. 1993. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the upregulation of B7 expression. J. Immunol. 151:1224-1234. [PubMed] [Google Scholar]
- 19.Ellis-Neyer, L., G. Grunig, M. Fort, J. S. Remington, D. Rennick, and C. A. Hunter. 1997. Role of IL-10 in regulation of T-cell-dependent and T-cell-independent mechanism of resistance. Infect. Immun. 65:1675-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields, P. E., R. J. Finch, G. S. Gray, R. Zollner, J. L. Thomas, K. Sturmhoefel, K. Lee, S. Wolf, T. F. Gajewski, and F. W. Fitch. 1998. B7.1 is a quantitatively stronger costimulus than B7.2 in the activation of naive CD8+ TCR-transgenic T cells. J. Immunol. 161:5268-5275. [PubMed] [Google Scholar]
- 21.Fiorentino, D. F., A. Zlotnik, T. R. Mosmann, M. Howard, and A. O'Garra. 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147:3815-3822. [PubMed] [Google Scholar]
- 22.Fiorentino, D. F., M. W. Bond, and T. R. Mosmann. 1989. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 170:2081-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer, H. G., R. Dorfler, B. Schade, and U. Hadding. 1999. Differential CD86/B7-2 expression and cytokine secretion induced by Toxoplasma gondii in macrophages from resistant or susceptible BALB H-2 congenic mice. Int. Immunol. 11:341-349. [DOI] [PubMed] [Google Scholar]
- 24.Freeman, G. J., V. A. Boussiotis, A. Anumanthan, G. M. Bernstein, X. Y. Ke, P. D. Rennert, G. S. Gray, J. G. Gribben, and L. M. Nadler. 1995. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity 2:523-532. [DOI] [PubMed] [Google Scholar]
- 25.Freeman, G. J., J. G. Gribben, V. A. Boussiotis, J. W. Ng, V. A. Restivo, Jr., L. A. Lombard, G. S. Gray, and L. M. Nadler. 1993. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T-cell proliferation. Science 262:909-911. [DOI] [PubMed] [Google Scholar]
- 26.Gajewski, T. F., F. Fallarino, C. Uyttenhove, and T. Boon. 1996. Tumor rejection requires a CTLA4 ligand provided by the host or expressed on the tumor: superiority of B7-1 over B7-2 for active tumor immunization. J. Immunol. 156:2909-2917. [PubMed] [Google Scholar]
- 27.Gazzinelli, R. T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J. Immunol. 157:798-805. [PubMed] [Google Scholar]
- 28.Gazzinelli, R. T., M. Wysocka, S. Hayashi, E. Y. Denkers, S. Hieny, P. Caspar, G. Trinchieri, and A. Sher. 1994. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 153:2533-2543. [PubMed] [Google Scholar]
- 29.Gerosa, F., C. Paganin, D. Peritt, F. Paiola, M. T. Scupoli, M. Aste-Amezaga, I. Frank, and G. Trinchieri. 1996. Interleukin-12 primes human CD4 and CD8 T-cell clones for high production of both IFN-γ and interleukin-10. J. Exp. Med. 183:2559-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grewal, I. S., H. G. Foellmer, K. D. Grewal, J. Xu, F. Hardardottir, J. L. Baron, C. A. Janeway, Jr., and R. A. Flavell. 1996. Requirement for CD40 ligand in costimulation induction, T-cell activation, and experimental allergic encephalomyelitis. Science 273:1864-1867. [DOI] [PubMed] [Google Scholar]
- 31.Groux, H., M. Bigler, J. E. de Vries, and M. G. Roncarolo. 1998. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J. Immunol. 160:3188-3193. [PubMed] [Google Scholar]
- 32.Hasko, G., L. Virag, G. Egnaczyk, A. L. Salzman, and C. Szabo. 1998. The crucial role of IL-10 in the suppression of the immunological response in mice exposed to staphylococcal enterotoxin B. Eur. J. Immunol. 28:1417-1425. [DOI] [PubMed] [Google Scholar]
- 33.Hunter, C. A., J. S. Abrams, M. H. Beaman, and J. S. Remington. 1993. Cytokine mRNA in the central nervous system of SCID mice infected with Toxoplasma gondii: importance of T-cell-independent regulation of resistance to T. gondii. Infect. Immun. 61:4038-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy, M. K., K. S. Picha, W. C. Fanslow, K. H. Grabstein, M. R. Alderson, K. N. Clifford, W. A. Chin, and K. M. Mohler. 1996. CD40/CD40 ligand interactions are required for T cell-dependent production of interleukin-12 by mouse macrophages. Eur. J. Immunol. 26:370-378. [DOI] [PubMed] [Google Scholar]
- 35.Khan, I. A., T. Matsuura, and L. H. Kasper. 1994. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect. Immun. 62:1639-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch, F., U. Stanzl, P. Jennewein, K. Janke, C. Heufler, E. Kampgen, N. Romani, and G. Schuler. 1996. High-level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 184:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koppelman, B., J. J. Neefjes, J. E. de Vries, and R. de Waal Malefyt. 1997. Interleukin-10 downregulates MHC class II αβ peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity 7:861-871. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn, R., J. Lohler, D. Rennick, K. Rajewsky, and W. Muller. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263-274. [DOI] [PubMed] [Google Scholar]
- 39.Lanier, L. L., S. O'Fallon, C. Somoza, J. H. Phillips, P. S. Linsley, K. Okumura, D. Ito, and M. Azuma. 1995. CD80(B7.1) and CD86(B7.2) provide similar costimulatory signals for T-cell proliferation, cytokine production, and generation of CTL. J. Immunol. 154:97-105. [PubMed] [Google Scholar]
- 40.Linsley, P. S., W. Brady, M. Urnes, L. S. Grosmaire, N. K. Damle, and J. A. Ledbetter. 1991. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 174:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matulonis, U., C. Dosiou, G. Freeman, C. Lamont, P. Mauch, L. M. Nadler, and J. D. Griffin. 1996. B7-1 is superior to B7-2 costimulation in the induction and maintenance of T cell-mediated antileukemia immunity. Further evidence that B7-1 and B7-2 are functionally distinct. J. Immunol. 156:1126-1131. [PubMed] [Google Scholar]
- 42.McCabe, R. E., B. J. Luft, and J. S. Remington. 1984. Effect of murine IFN-γ on murine toxoplasmosis. J. Infect. Dis. 150:961-962. [DOI] [PubMed] [Google Scholar]
- 43.McDyer, J. F., T. J. Goletz, E. Thomas, C. H. June, and R. A. Seder. 1998. CD40 ligand/CD40 stimulation regulates the production of IFN-γ from human peripheral blood mononuclear cells in an IL-12- and/or CD28-dependent manner. J. Immunol. 160:1701-1707. [PubMed] [Google Scholar]
- 44.Meyaard, L., E. Hovenkamp, S. A. Otto, and F. Miedema. 1996. IL-12-induced IL-10 production by human T cells as a negative feedback for IL-12-induced immune responses. J. Immunol. 156:2776-2782. [PubMed] [Google Scholar]
- 45.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 46.Natesan, M., Z. Razi-Wolf, and H. Reiser. 1996. Costimulation of IL-4 production by murine B7-1 and B7-2 molecules. J. Immunol. 156:2783-2791. [PubMed] [Google Scholar]
- 47.Poe, J. C., D. H. Wagner, Jr., R. W. Miller, R. D. Stout, and J. Suttles. 1997. IL-4 and IL-10 modulation of CD40-mediated signaling of monocyte IL-1β synthesis and rescue from apoptosis. J. Immunol. 159:846-852. [PubMed] [Google Scholar]
- 48.Reichmann, G., W. Walker, E. N. Villegas, L. Craig, G. Cai, J. Alexander, and C. A. Hunter. 2000. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect. Immun. 68:1312-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rennick, D. M., and M. M. Fort. 2000. Lessons from genetically engineered animal models. XII. IL-10-deficient mice and intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 278:G829-G833. [DOI] [PubMed] [Google Scholar]
- 50.Rennick, D. M., M. M. Fort, and N. J. Davidson. 1997. Studies with IL-10−/− mice: an overview. J. Leukoc. Biol. 61:389-396. [DOI] [PubMed] [Google Scholar]
- 51.Rissoan, M. C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, R. de Waal Malefyt, and Y. J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science 283:1183-1186. [DOI] [PubMed] [Google Scholar]
- 52.Sharma, S. D., J. Mullenax, F. G. Araujo, H. A. Erlich, and J. S. Remington. 1983. Western Blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J. Immunol. 131:977-983. [PubMed] [Google Scholar]
- 53.Shu, U., M. Kiniwa, C. Y. Wu, C. Maliszewski, N. Vezzio, J. Hakimi, M. Gately, and G. Delespesse. 1995. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur. J. Immunol. 25:1125-1128. [DOI] [PubMed] [Google Scholar]
- 54.Subauste, C. S., and Wessendarp, M. 2000. Human dendritic cells discriminate between viable and killed Toxoplasma gondii tachyzoites: dendritic cell activation after infection with viable parasites results in CD28 and CD40 ligand signaling that controls IL-12-dependent and independent T-cell production of IFN-γ. J. Immunol. 165:1498-1505. [DOI] [PubMed] [Google Scholar]
- 55.Subauste, C. S., R. de Waal Malefyt, and F. Fuh. 1998. Role of CD80 (B7.1) and CD86 (B7.2) in the immune response to an intracellular pathogen. J. Immunol. 160:1831-1840. [PubMed] [Google Scholar]
- 56.Subauste, C. S., M. Wessendarp, R. U. Sorensen, and L. E. Leiva. 1999. CD40-CD40 ligand interaction is central to cell-mediated immunity against Toxoplasma gondii: patients with hyper IgM syndrome have a defective type 1 immune response that can be restored by soluble CD40 ligand trimer. J. Immunol. 162:6690-6700. [PubMed] [Google Scholar]
- 57.Suttles, J., D. M. Milhorn, R. W. Miller, J. C. Poe, L. M. Wahl, and R. D. Stout. 1999. CD40 signaling of monocyte inflammatory cytokine synthesis through an ERK1/2-dependent pathway: a target of interleukin IL-4 and IL-10 anti-inflammatory action. J. Biol. Chem. 274:5835-5842. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki, Y., F. K. Conley, and J. S. Remington. 1989. Importance of endogenous IFN-γ for prevention of toxoplasmic encephalitis in mice. J. Immunol. 143:2045-2050. [PubMed] [Google Scholar]
- 59.Suzuki, Y., M. A. Orellana, R. D. Schreiber, and J. S. Remington. 1988. IFN-γ: the major mediator of resistance against Toxoplasma gondii. Science 240:516-518. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki, Y., F. K. Conley, and J. S. Remington. 1990. Treatment of toxoplasmic encephalitis in mice with recombinant gamma interferon. Infect. Immun. 58:3050-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tripp, C. S., S. F. Wolf, and E. R. Unanue. 1993. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. USA 90:3725-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Dijk, A. M., H. G. Otten, S. M. Vercauteren, F. L. Kessler, M. de Boer, L. F. Verdonck, and G. C. de Gast. 1996. Human B7-1 is more efficient than B7-2 in providing co-stimulation for alloantigen-specific T cells. Eur. J. Immunol. 26:2275-2278. [DOI] [PubMed] [Google Scholar]
- 63.Villegas, E. N., U. Wille, L. Craig, P. S. Linsley, D. M. Rennick, R. Peach, and C. A. Hunter. 2000. Blockade of costimulation prevents infection-induced immunopathology in interleukin-10-deficient mice. Infect. Immun. 68:2837-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wille, U., E. N. Villegas, B. Striepen, D. S. Roos, and C. A. Hunter. 2001. Interleukin-10 does not contribute to the pathogenesis of a virulent strain of Toxoplasma gondii. Parasite Immunol. 23:291-296. [DOI] [PubMed] [Google Scholar]
- 65.Willems, F., A. Marchant, J. P. Delville, C. Gerard, A. Delvaux, T. Velu, M. de Boer, and M. Goldman. 1994. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur. J. Immunol. 24:1007-1009. [DOI] [PubMed] [Google Scholar]
- 66.Yang, Y., and J. M. Wilson. 1996. CD40 ligand-dependent T-cell activation: requirement of B7-CD28 signaling through CD40. Science 273:1862-1864. [DOI] [PubMed] [Google Scholar]