Abstract
1. The sarcomere length (s) of ehick slow and fast muscles (anterior and posterior latissimus dorsi (ant. lat. dorsi and post. lat. dorsi)) was measured by the method of light diffraction. In resting ant. lat. dorsi, s changed from 1·76 to 2·30 μm during stretch from minimum to maximum muscle lengths in situ, and in resting post. lat. dorsi from 2·18 to 2·63 μm.
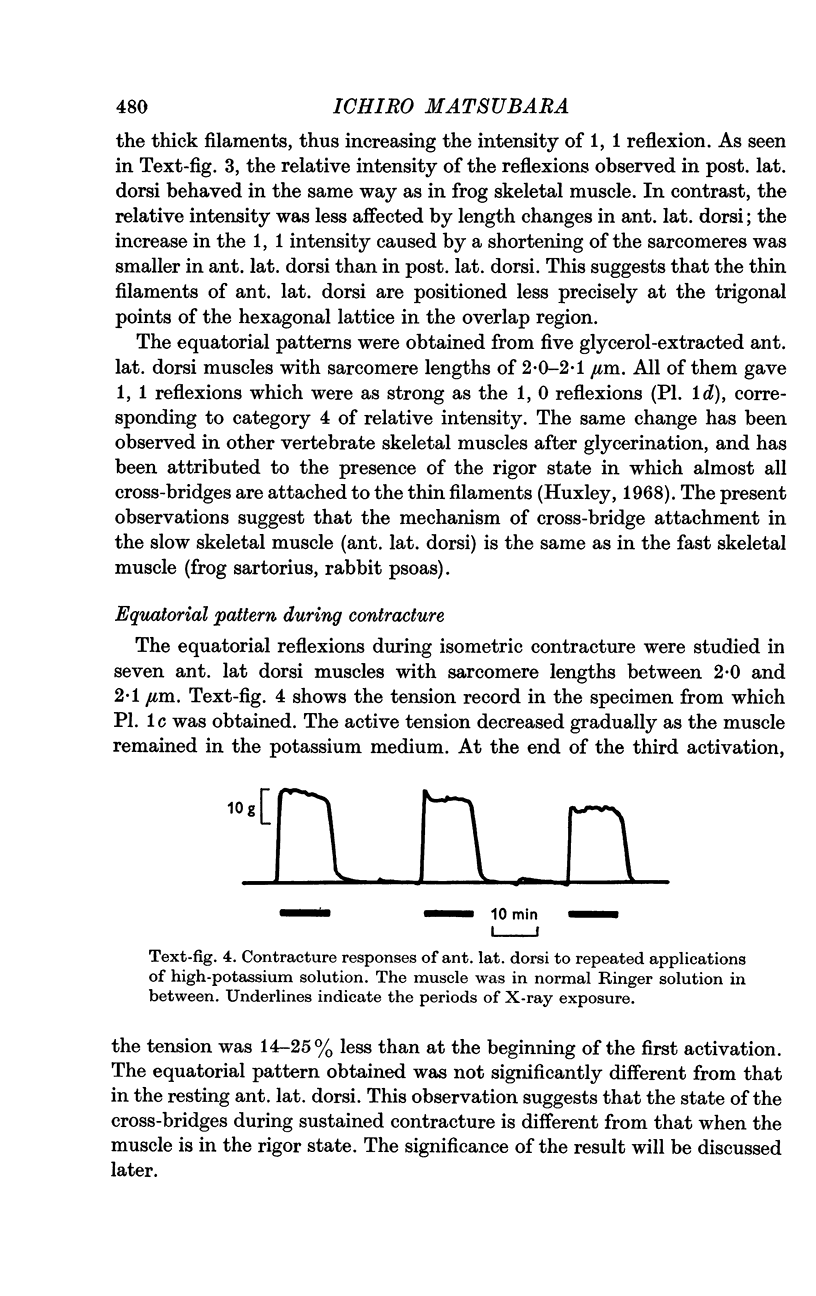
2. Resting tension started to rise in ant. lat. dorsi when s exceeded 1·7-1·8 μm, but in post. lat. dorsi not until s exceeded 2·6-2·7 μm.
3. X-ray diffraction patterns showed that ant. lat. dorsi contains collagen filaments; collagen reflexions were not seen in patterns obtained from post. lat. dorsi with the same exposure time.
4. The relation between active tension and sarcomere length was similar for ant. and post. lat. dorsi. The maximum active tension was observed when s = 2·05-2·15 μm in ant. lat. dorsi, and when s = 2·10-2·25 μm in post. lat. dorsi.
5. X-ray diffraction patterns from both muscles showed that the periodic structures of the thick and thin filaments are similar to those in frog and rabbit skeletal muscles.
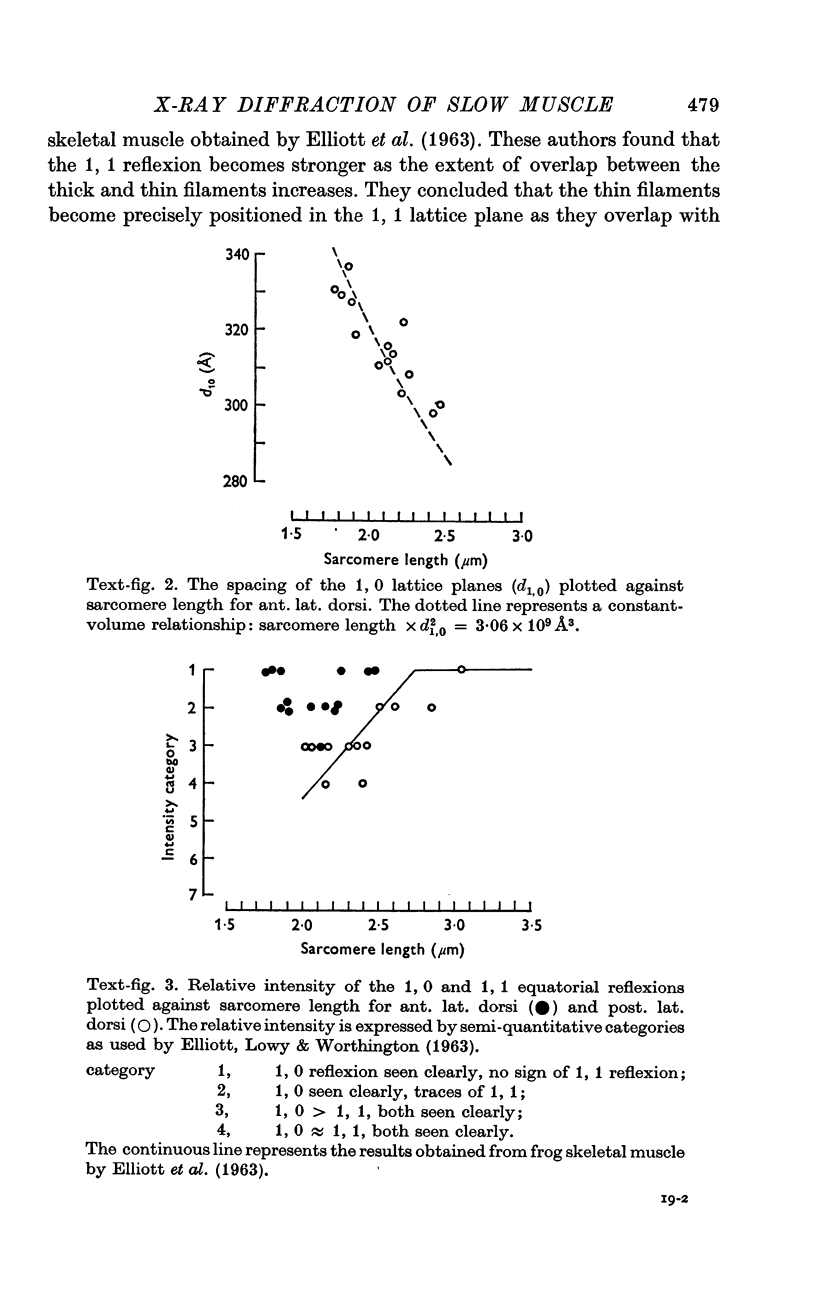
6. The volume of the myofilament lattice in resting ant. lat. dorsi was 3·06 (± 0·14) × 109 Å3, in resting post. lat. dorsi 2·98 (± 0·09) × 109 Å3. These values are close to that of frog skeletal muscle. The lattice volume remained constant in ant. lat. dorsi and post. lat. dorsi over the range of sarcomere lengths found in situ.
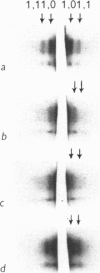
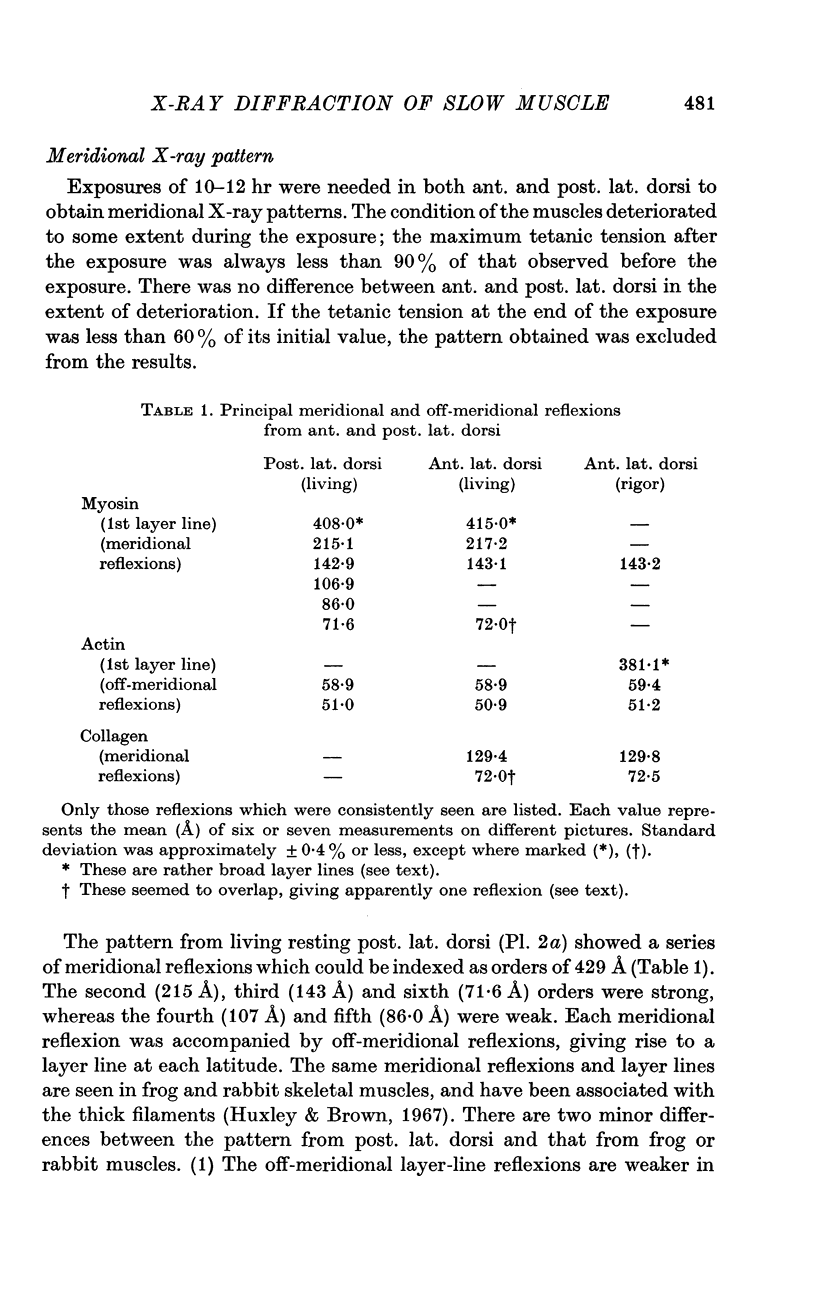
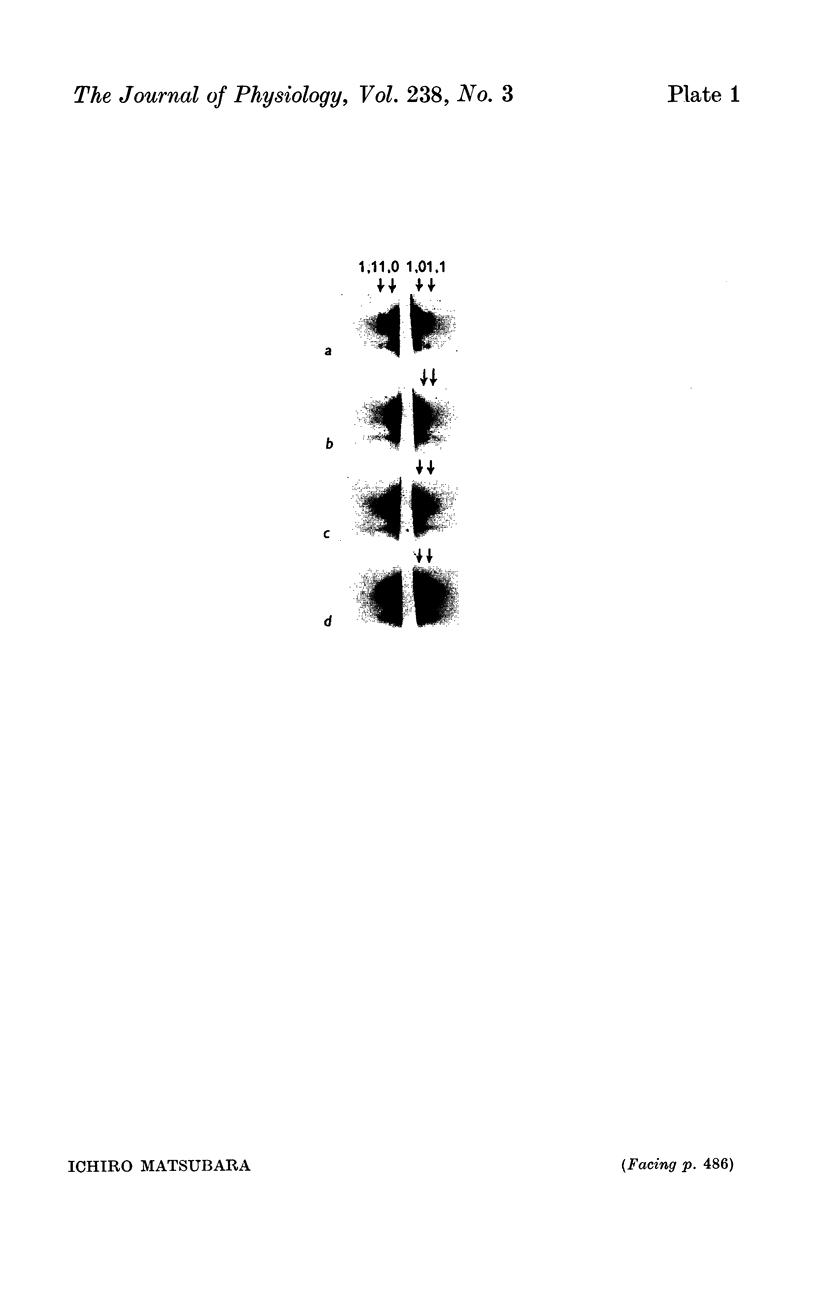
7. The equatorial diffraction patterns from the ant. lat. dorsi in rigor (glycerol extracted) were different from that of the resting muscle, and suggested that a large number of cross-bridges were attached to the thin filaments during rigor. During potassium contracture, however, the diffraction pattern remained similar to that from the resting ant. lat. dorsi.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown L. M., Gonzalez-Serratos H., Huxley A. F. Electron microscopy of frog muscle fibres in extreme passive shortening. J Physiol. 1970 Jun;208(2):86P–88P. [PubMed] [Google Scholar]
- Cambier E. Propriétés mécaniques et fonctions d'un muscle tonique d'oiseau: le latissimus dorsi anterior. Arch Int Physiol Biochim. 1969 Aug;77(3):416–426. doi: 10.3109/13813456909069822. [DOI] [PubMed] [Google Scholar]
- Canfield S. P. The mechanical properties and heat production of chicken latissimus dorsi muscles during tetanic contractions. J Physiol. 1971 Dec;219(2):281–302. doi: 10.1113/jphysiol.1971.sp009662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT G. F., WORTHINGTON C. R. A SMALL-ANGLE OPTICALLY FOCUSING X-RAY DIFFRACTION CAMERA IN BIOLOGICAL RESEARCH. I. J Ultrastruct Res. 1963 Aug;49:166–170. doi: 10.1016/s0022-5320(63)80044-1. [DOI] [PubMed] [Google Scholar]
- GINSBORG B. L. Spontaneous activity in muscle fibres of the chick. J Physiol. 1960 Mar;150:707–717. doi: 10.1113/jphysiol.1960.sp006413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink G., Larson R. E., Davies R. E. Fluctuations in sarcomere length in the chick anterior and posterior latissimus dorsi muscles during isometric contraction. Experientia. 1970 Jan 15;26(1):16–18. doi: 10.1007/BF01900361. [DOI] [PubMed] [Google Scholar]
- HESS A. Structural differences of fast and slow extrafusal muscle fibres and their nerve endings in chickens. J Physiol. 1961 Jul;157:221–231. doi: 10.1113/jphysiol.1961.sp006717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., GORDON A. M. Striation patterns in active and passive shortening of muscle. Nature. 1962 Jan 20;193:280–281. doi: 10.1038/193280b0. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F., PEACHEY L. D. The maximum length for contraction in vertebrate straiated muscle. J Physiol. 1961 Apr;156:150–165. doi: 10.1113/jphysiol.1961.sp006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY H. E. X-ray analysis and the problem of muscle. Proc R Soc Lond B Biol Sci. 1953 Mar 11;141(902):59–62. doi: 10.1098/rspb.1953.0017. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. Structural difference between resting and rigor muscle; evidence from intensity changes in the lowangle equatorial x-ray diagram. J Mol Biol. 1968 Nov 14;37(3):507–520. doi: 10.1016/0022-2836(68)90118-6. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Elliott G. F. X-ray diffraction studies on skinned single fibres of frog skeletal muscle. J Mol Biol. 1972 Dec 30;72(3):657–669. doi: 10.1016/0022-2836(72)90183-0. [DOI] [PubMed] [Google Scholar]
- Page S. G. Fine structure of tortoise skeletal muscle. J Physiol. 1968 Aug;197(3):709–715. doi: 10.1113/jphysiol.1968.sp008583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S. G. Structure and some contractile properties of fast and slow muscles of the chicken. J Physiol. 1969 Nov;205(1):131–145. doi: 10.1113/jphysiol.1969.sp008956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome E. Light and X-ray diffraction studies of the filament lattice of glycerol-extracted rabbit psoas muscle. J Mol Biol. 1967 Aug 14;27(3):591–602. doi: 10.1016/0022-2836(67)90061-7. [DOI] [PubMed] [Google Scholar]
- SELBY C. C., BEAR R. S. The structure of actin-rich filaments of muscles according to x-ray diffraction. J Biophys Biochem Cytol. 1956 Jan 25;2(1):71–85. doi: 10.1083/jcb.2.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons R. M. Resistance to shortening at the I-filament length in frog muscle fibres. J Physiol. 1971 Jan;212(2):20P–22P. [PubMed] [Google Scholar]