Abstract
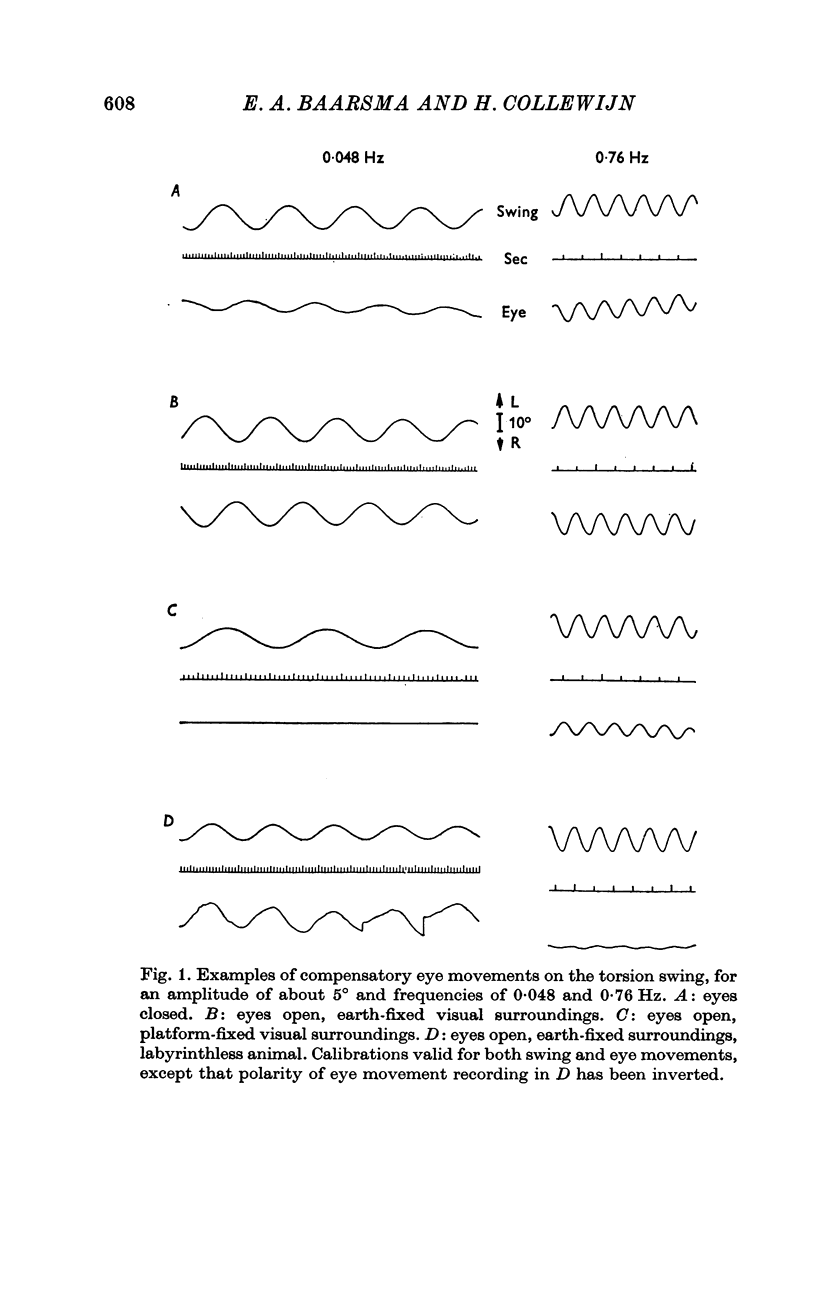
1. Compensatory eye movements due to sinusoidal yaw movements on a torsion swing were measured in alert rabbits. A range of combinations of frequencies (0·048-1·8 Hz) and amplitudes (1-25°) were used. Gain (cumulative slow phase eye movement amplitude/swing amplitude) and phase (eye position vs. swing position — 180°) were calculated from averaged records.
2. Eyes were either closed (canal-ocular reactions only), open in earth-fixed visual surroundings (natural interaction of vestibular and optokinetic reactions), or looking at platform-fixed surroundings, which rotated with the animal (conflict situation). In some rabbits, the same stimulus programme was applied a month after bilateral destruction of the labyrinths (optokinetic reactions only).
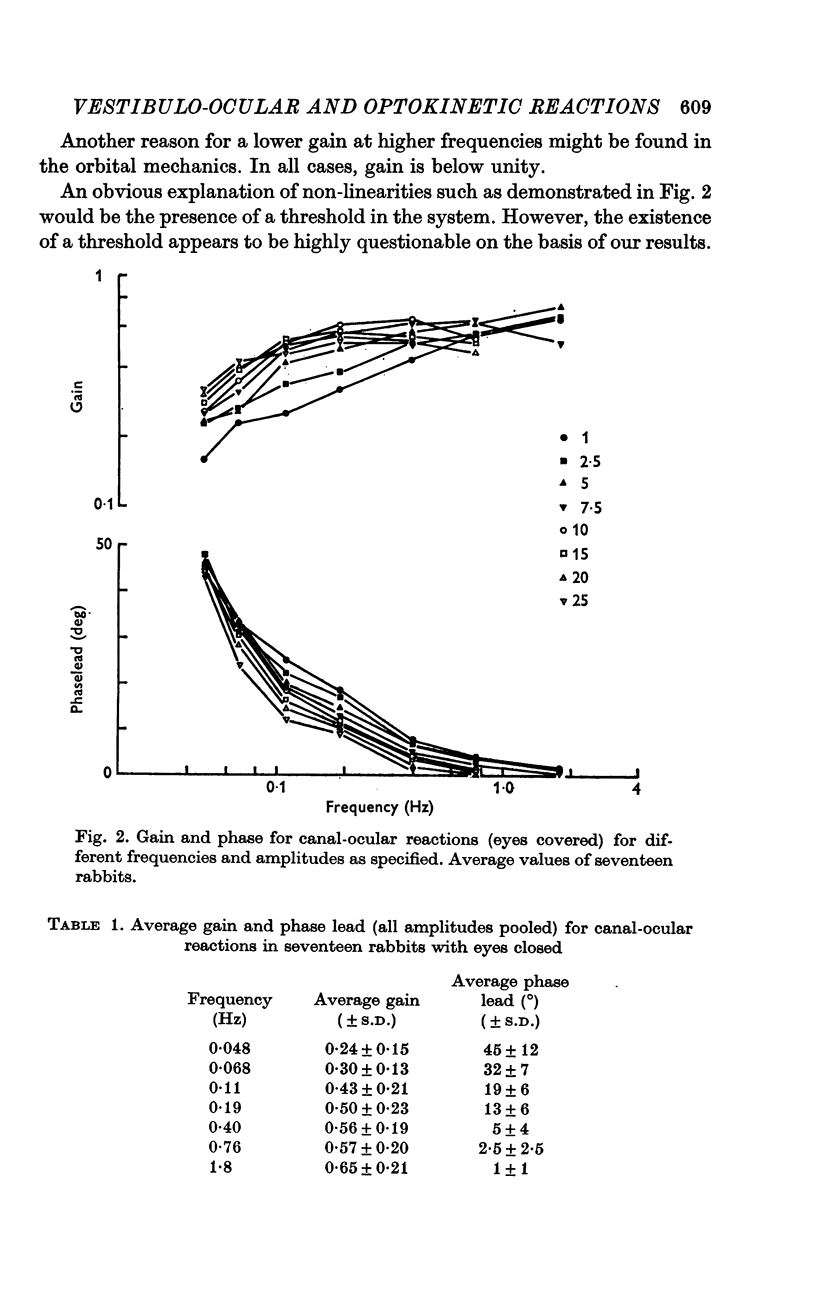
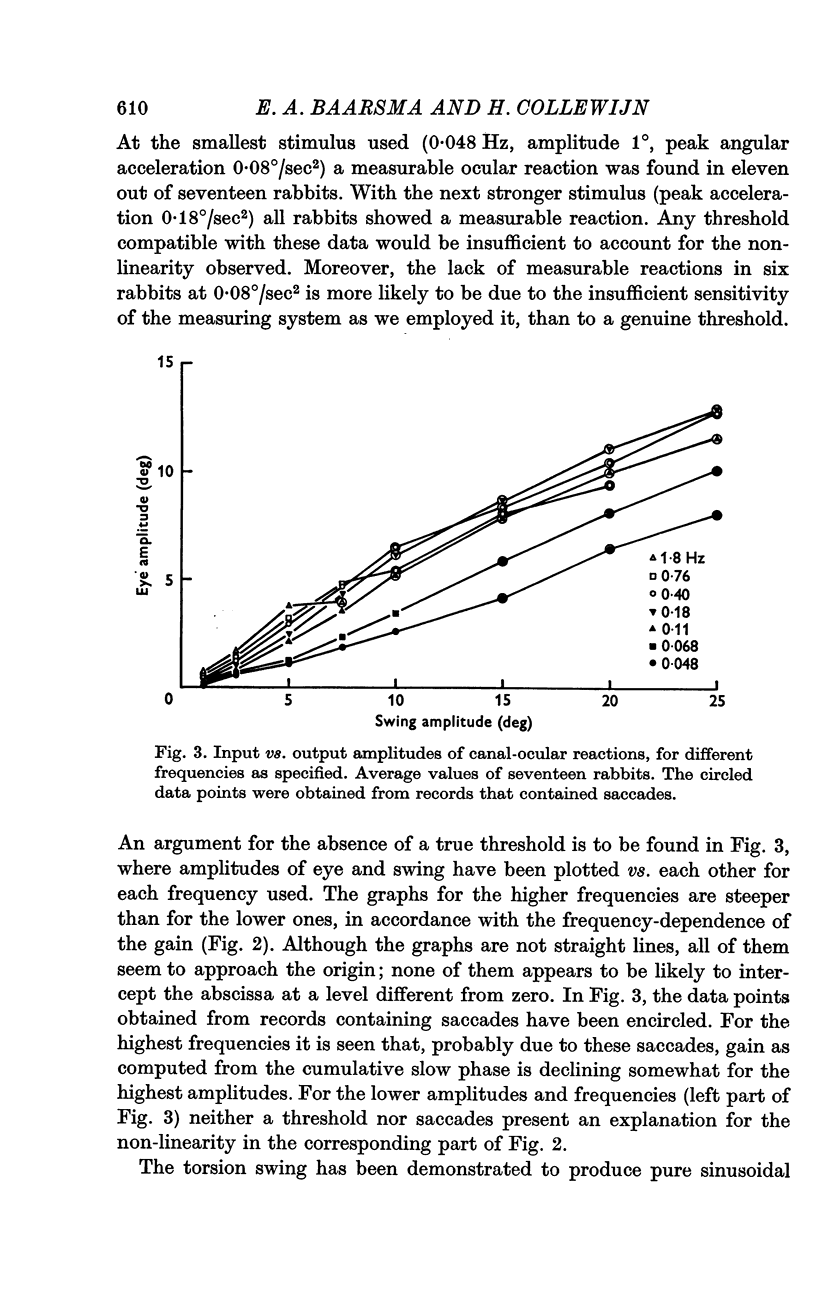
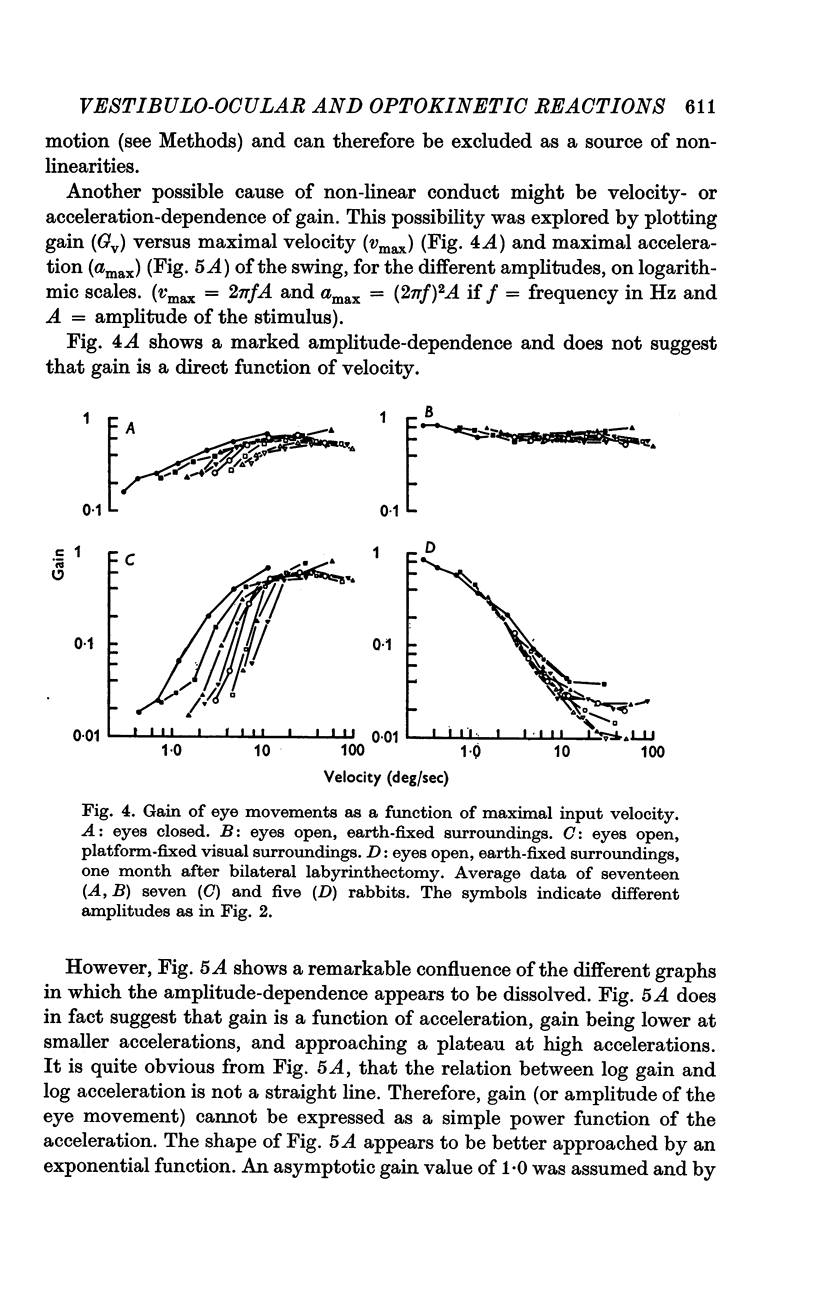
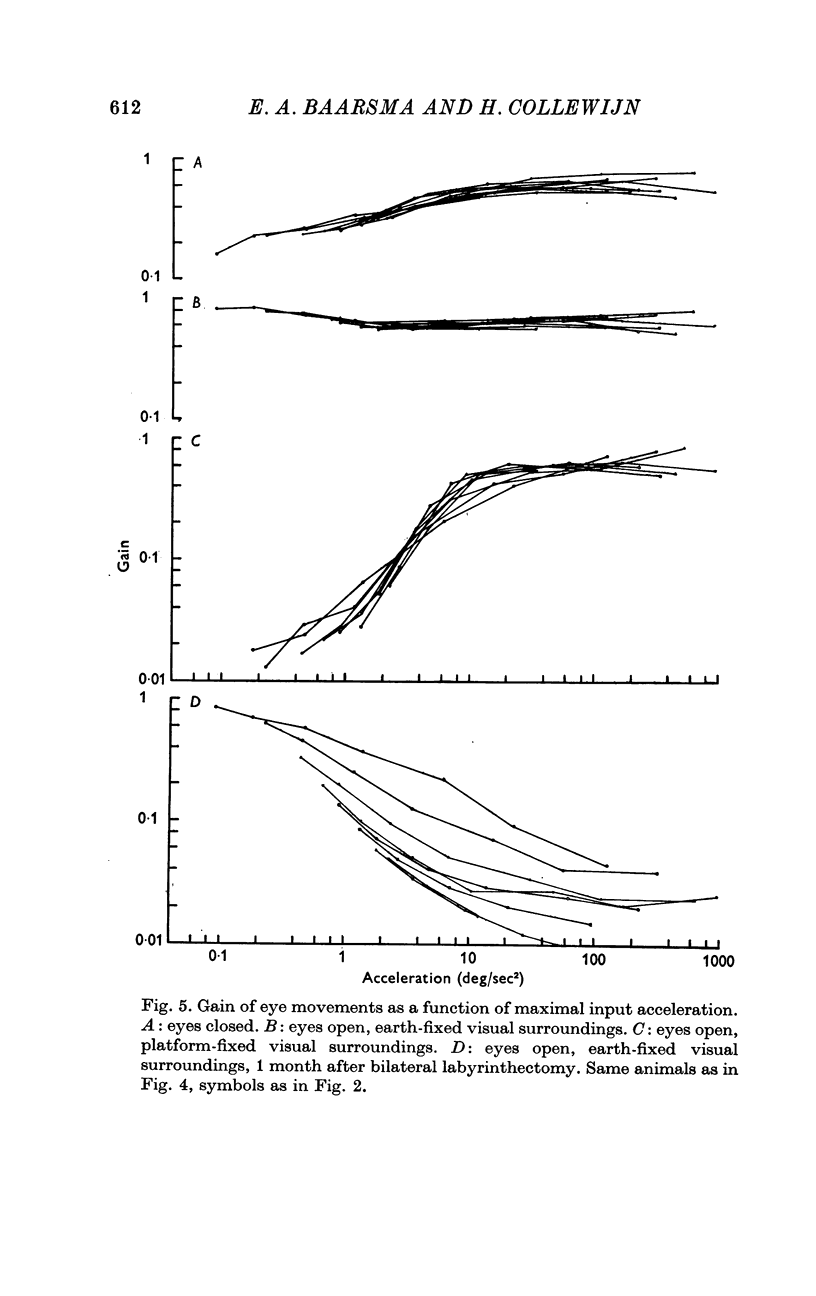
3. For canal-ocular reactions, no true threshold was found. Yet the system showed a small but systematic non-linearity which is tentatively explained by an acceleration-dependence of gain. For the higher frequencies (0·40-1·8 Hz) used, gain was 0·55-0·75, with a decrease at the lower frequencies, down to 0·16-0·33 at 0·048 Hz. The response showed a phase-lead of about 45° at 0·048 Hz and was nearly in phase at 1-1·8 Hz. The long time constant of the cupula-endolymph system was estimated at about 3·3 sec.
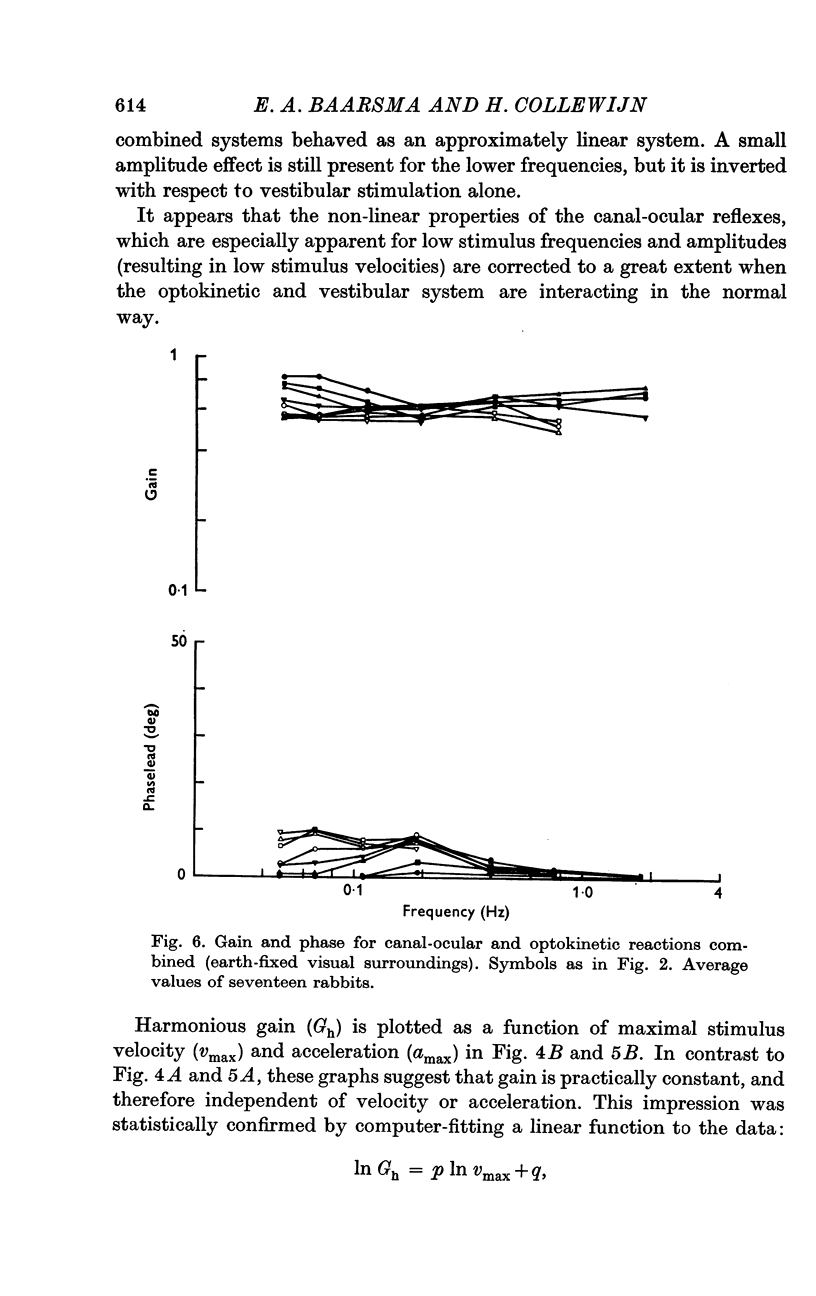
4. With earth-fixed visual surroundings a frequency-independent gain (range 0·55-0·82) with negligible phase error was found for the entire stimulus range tested. This natural combination of canal-ocular and optokinetic systems appears to function very efficiently, with mutual correction of the defects of the systems apart.
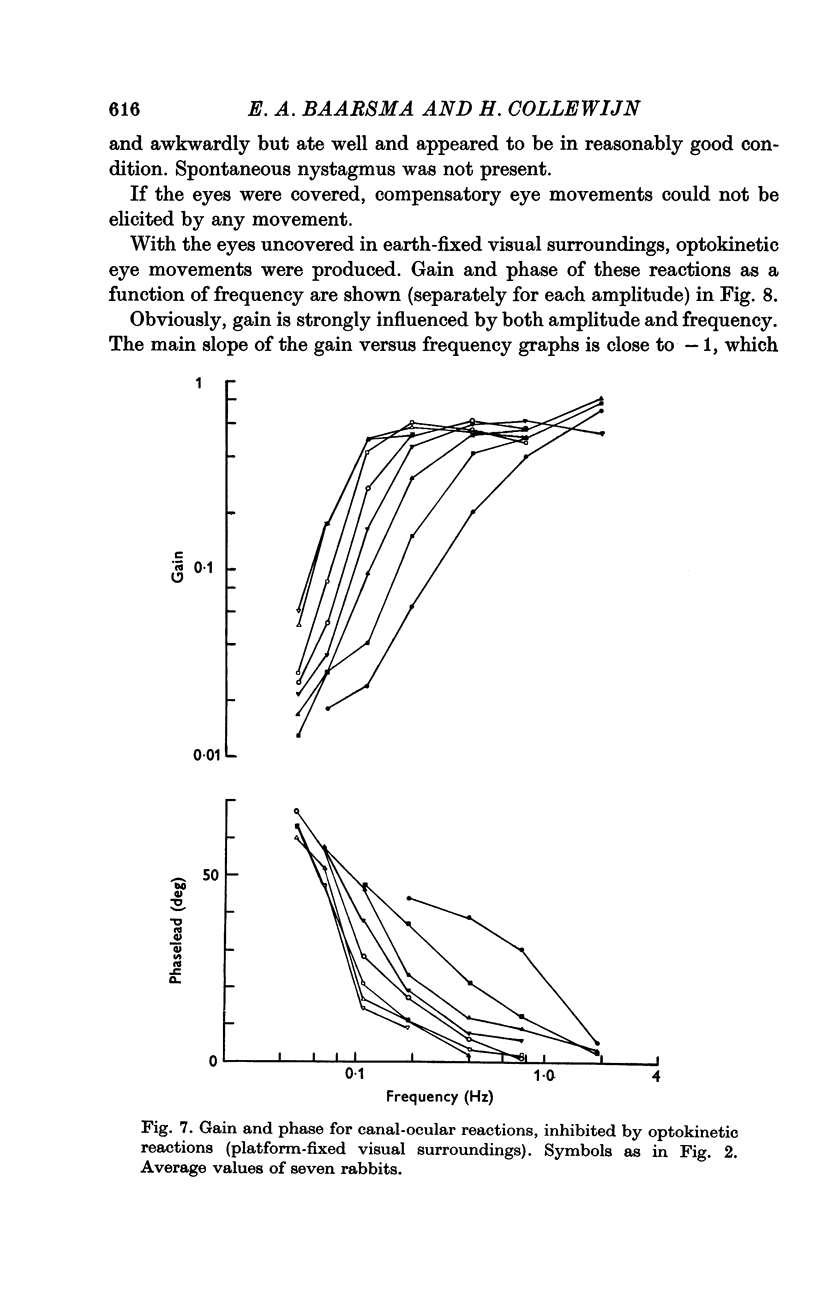
5. With platform-fixed visual surroundings the canal-ocular system was severely inhibited and its non-linearities were markedly enhanced by the optokinetic system, especially when the torsion swing moved slowly.
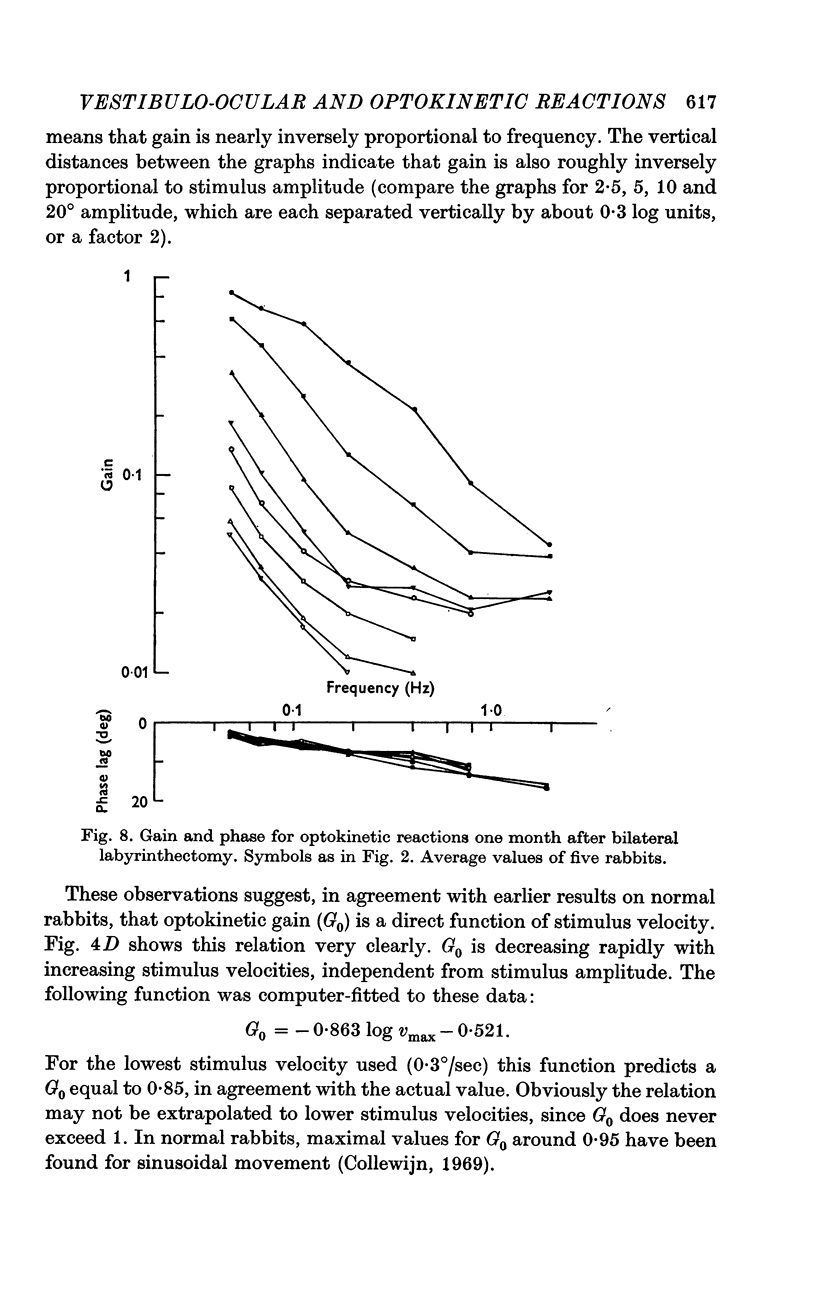
6. The general shape of input—output relations of optokinetic reactions after labyrinthectomy were similar to those found earlier in normal animals, but gain was subnormal for the entire stimulus range tested.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen B., Komatsuzaki A. Eye movements induced by stimulation of the pontine reticular formation: evidence for integration in oculomotor pathways. Exp Neurol. 1972 Jul;36(1):101–117. doi: 10.1016/0014-4886(72)90139-2. [DOI] [PubMed] [Google Scholar]
- Collewijn H. An analog model of the rabbit's optokinetic system. Brain Res. 1972 Jan 14;36(1):71–88. doi: 10.1016/0006-8993(72)90767-6. [DOI] [PubMed] [Google Scholar]
- Collewijn H. Latency and gain of the rabbit's optokinetic reactions to small movements. Brain Res. 1972 Jan 14;36(1):59–70. doi: 10.1016/0006-8993(72)90766-4. [DOI] [PubMed] [Google Scholar]
- Collewijn H. Optokinetic eye movements in the rabbit: input-output relations. Vision Res. 1969 Jan;9(1):117–132. doi: 10.1016/0042-6989(69)90035-2. [DOI] [PubMed] [Google Scholar]
- Fernandez C., Goldberg J. M. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971 Jul;34(4):661–675. doi: 10.1152/jn.1971.34.4.661. [DOI] [PubMed] [Google Scholar]
- GUTMAN J., ZELIG S., BERGMANN F. OPTOKINETIC NYSTAGMUS IN THE LABYRINTHECTOMISED RABBIT. Confin Neurol. 1964;24:158–162. doi: 10.1159/000104110. [DOI] [PubMed] [Google Scholar]
- Goldberg J. M., Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J Neurophysiol. 1971 Jul;34(4):635–660. doi: 10.1152/jn.1971.34.4.635. [DOI] [PubMed] [Google Scholar]
- Honrubia V., Jenkins H. A., Downey W. L., Ward P. H. Experimental studies in optokinetic nystagmus. 3. Modifications produced by labyrinthectomy and cerebral hemidecortication in cats. Ann Otol Rhinol Laryngol. 1971 Feb;80(1):97–110. doi: 10.1177/000348947108000114. [DOI] [PubMed] [Google Scholar]
- JONES G. M., SPELLS K. E. A theoretical and comparative study of the functional dependence of the semicircular canal upon its physical dimensions. Proc R Soc Lond B Biol Sci. 1963 Mar 26;157:403–419. doi: 10.1098/rspb.1963.0019. [DOI] [PubMed] [Google Scholar]
- Jones G. M., Milsum J. H. Characteristics of neural transmission from the semicircular canal to the vestibular nuclei of cats. J Physiol. 1970 Aug;209(2):295–316. doi: 10.1113/jphysiol.1970.sp009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. M., Milsum J. H. Frequency-response analysis of central vestibular unit activity resulting from rotational stimulation of the semicircular canals. J Physiol. 1971 Dec;219(1):191–215. doi: 10.1113/jphysiol.1971.sp009657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON D. A. A METHOD OF MEASURING EYE MOVEMENT USING A SCLERAL SEARCH COIL IN A MAGNETIC FIELD. IEEE Trans Biomed Eng. 1963 Oct;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Skavenski A. A., Robinson D. A. Role of abducens neurons in vestibuloocular reflex. J Neurophysiol. 1973 Jul;36(4):724–738. doi: 10.1152/jn.1973.36.4.724. [DOI] [PubMed] [Google Scholar]
- VAN EGMOND A. A. J., GROEN J. J., JONGKEES L. B. W. The mechanics of the semicircular canal. J Physiol. 1949 Dec 15;110(1-2):1–17. doi: 10.1113/jphysiol.1949.sp004416. [DOI] [PMC free article] [PubMed] [Google Scholar]


