Abstract
The hygiene hypothesis postulates that the prevalence of allergy has increased due to decreased microbial stimulation early in life, leading to delayed maturation of the immune system. The aim of this study was to examine the cytokine pattern produced from cord blood mononuclear cells relative to adult cells after stimulation with bacterial strains from the normal flora. Mononuclear cells from cord and adult blood samples were stimulated with the following bacteria: Bifidobacterium adolescentis, Enterococcus faecalis, Lactobacillus plantarum, Streptococcus mitis, Corynebacterium minutissimum, Clostridium perfringens, Bacteroides vulgatus, Escherichia coli, Pseudomonas aeruginosa, Veillonella parvula, and Neisseria sicca. The levels of interleukin 12 (IL-12), tumor necrosis factor alpha (TNF-α), IL-10, and IL-6 were measured by enzyme-linked immunosorbent assay. The TNF-α production was also analyzed after blocking CD14, Toll-like receptor 2 (TLR-2), and TLR-4 prior to stimulation with bacteria. The levels of IL-12 and TNF-α were similar in cord and adult cells. Gram-positive bacteria induced considerably higher levels of IL-12 and TNF-α than gram-negative bacteria in both cord and adult cells. The levels of IL-6 were significantly higher in newborns than in adults, whereas the levels of IL-10 were similar in newborns and adults. Gram-negative and gram-positive bacteria induced similar levels of IL-6 and IL-10 in cord cells. L. plantarum bound or signaled through CD14, TLR-2, and TLR-4, whereas E. coli acted mainly through CD14 and TLR-4. These results indicate that the innate immune response in newborns to commensal bacteria is strong and also suggest that different bacterial strains may have differential effects on the maturation of the immune system of infants.
The innate immune responses to bacteria might have a role in modulating the adaptive immunity to allergens as postulated by the hygiene hypothesis. An association between the normal flora and development of allergies has been based on findings of differences in composition of the gut flora between allergic and nonallergic children (3-5). The composition of the intestinal flora of children differs in Estonia and Sweden, which are two countries that have low and high prevalences of allergies, respectively (34). Allergic 2-year-old Swedish and Estonian children were less often colonized by lactobacilli and harbored higher counts of aerobic bacteria than did nonallergic children (3). A Finnish study showed that perinatal administration of a gram-positive probiotic bacterium, Lactobacillus rhamnosus, decreased the occurrence of eczema in infants at high risk (21). Other recent studies have shown that early colonization with bifidobacteria and low counts of Bacteroides and Clostridium difficile appear to be associated with protection against allergy (4, 20).
Bacteria can be divided into gram-positive and gram-negative species according to their different cell wall structures and compositions. The cell wall of the gram-positive bacterium is composed of a thick layer of peptidoglycan with chains of lipotechoic acid linked to the cytoplasmic membrane. Gram-negative bacteria have a thinner peptidoglycan layer and are arranged with an outer membrane containing lipopolysaccharide (LPS). It has previously been shown in adults that gram-positive bacterial species are strong interleukin 12 (IL-12) inducers in enriched monocytes, while gram-negative bacteria are more efficient IL-10 inducers (17). However, only a few studies have examined how newborns respond to bacterial stimulation. Some studies indicate that cord cells have a decreased ability to produce IL-12 after bacterial stimulation (14, 19, 24), whereas others have observed levels comparable to those for adult cells (6, 33). As this initial cytokine production may be significant for the polarization of T cells, it is important to examine how bacteria affect the antigen-presenting cells of neonates.
Encounter with microbial antigens is essential for the maturation of the immune systems of neonates. Lack of microbial stimulation early in life could lead to an increased differentiation of Th2 cells in genetically susceptible individuals. Monocytes and macrophages, together with dendritic cells, play a crucial role in the innate immune response against microbial antigens, which in turn leads to activation of the adaptive immune system (reviewed in reference 28). Antigen-presenting cells recognize conserved molecular patterns of bacterial components through Toll-like receptors (TLR). These receptors signal through pathways that lead to activation of a variety of transcription factors, which triggers the production of cytokines. Recent data indicate that TLR-2 is mainly involved in responses to cell wall components of gram-positive bacteria, while TLR-4 has a role in recognition of gram-negative bacterial compounds (36). Signaling through TLR-2 and TLR-4 is enhanced by CD14, but the role of the CD14 molecule during cell activation induced by gram-positive and gram-negative bacteria is still controversial (7, 22). Cytokines produced by antigen-presenting cells together with certain surface receptors are instrumental in the development of T-cell differentiation to Th1, Th2, or T regulatory cells. IL-12 is a major Th1-promoting factor (39), whereas IL-10 downregulates the production of gamma interferon (IFN-γ) and IL-12 (8). Proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and IL-6, are among the first cytokines produced in response to bacteria and have a role in the early induction of the immune response and in the clearance of pathogens.
Thus, since the normal flora is the main bacterial stimulus of the immune system in infants, we examined the innate immune responses of cord cells relative to adult cells to a panel of gram-positive and gram-negative bacteria.
MATERIALS AND METHODS
Bacteria.
Isolates of bacterial strains inhabiting human gastrointestinal or respiratory mucosa were obtained from the Culture Collection of the University of Göteborg (Göteborg, Sweden). A strain of Lactobacillus plantarum was isolated from rectal mucosa of a healthy volunteer (described in reference 1). The strains used in this study represent commensals and pathogens as well as aerobic and anaerobic strains (Table 1). Aerobic and facultative bacteria were cultured on blood agar plates aerobically in 37°C for 24 h, and anaerobic species were cultured anaerobically for 3 days. Thereafter, bacteria were harvested and washed three times in phosphate-buffered saline (PBS) (1,000 g, 10 min). The bacteria were then counted under a microscope, and the strains were suspended at a concentration of 109 cells per ml, which was verified by viable count. The bacteria were killed by exposure to UV light for 15 min, which was confirmed by negative viable count, and stored at −70°C.
TABLE 1.
Description of the bacterial strains
| Straina | CCUGb strain no. | Metabolism | Isolation site |
|---|---|---|---|
| Gram-positive bacteria | |||
| Bifidobacterium adolescentis | 18363 | Anaerobe | Adult intestine |
| Enterococcus faecalis | 19916 | Anaerobe | Unknown |
| Lactobacillus plantarum | 67b | Anaerobe | Rectum, healthy |
| Streptococcus mitis | 31611 | Aerobe | Oral cavity |
| Corynebacterium minutissimum | 541 | Aerobe | Erythrasma, trunk |
| Clostridium perfringens | 1795 | Anaerobe | Bovine |
| Gram-negative bacteria | |||
| Bacteroides vulgatus | 4940 | Anaerobe | Unknown |
| Escherichia coli | 24 | Aerobe | Urine, cystitis |
| Pseudomonas aeruginosa | 551 | Aerobe | Unknown |
| Veillonella parvula | 5123 | Anaerobe | Intestinal tract |
| Neisseria sicca | 23929 | Aerobe | Pharynx, healthy |
All bacterial strains used in this study were derived from Culture Collection of the University of Göteborg, except for L. plantarum, which was isolated from human gastrointestinal mucosa.
Culture Collection of the University of Göteborg.
Cell separation.
Umbilical cord blood was obtained from normally delivered full-term babies at Mölndal Hospital (Göteborg, Sweden), and adult blood samples were obtained from nonallergic healthy volunteers without any medication. The study was approved by the Human Research Ethics Committee of the Medical Faculty, Göteborg University. Blood samples were collected in heparinized tubes, and mononuclear cells were isolated by density gradient centrifugation over Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden) (900 g, 20 min, room temperature) within 10 h of collection. The cells were then washed three times in RPMI 1640 (BioWhittaker, Cambrex Company, Verviers, Belgium). CD14+ monocytes were purified from mononuclear cells by magnetic cell sorting using a positive selection technique according to the manufacturer's protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, the cells were incubated with magnetic microbeads conjugated with monoclonal mouse anti-human CD14 antibodies in MACS buffer (PBS with 0.5% human serum albumin [HSA] and 2 mM EDTA) for 15 min. After washing, the cells were run through a MACS column (Miltenyi Biotec) in a magnetic field and rinsed three times with 3 ml of buffer. The column was then removed from the magnet, and the positive cells were flushed out. Analysis with flow cytometry showed that more than 95% of the purified cells expressed CD14.
Cell cultures.
The cells were cultured in serum-free AIM-V medium (Invitrogen, San Diego, Calif.), containing l-glutamine, 50 μg of streptomycin sulfate per ml, 10 μg of gentamicin sulfate per ml, and 20 μM mercaptoethanol, at a concentration of 2 × 106 cells per ml in flat-bottomed 96-well tissue culture plates (Techno Plastic Products, Trasadingen, Switzerland). The mononuclear cells were stimulated with 5 × 107 bacteria per ml for 24 h at 37°C with 5% CO2. One cord and one adult blood sample were prepared and stimulated simultaneously (n = 11 from each group). We also stimulated mononuclear cells from four cord samples and four adult blood samples with combinations of gram-positive (Bifidobacterium adolescentis or L. plantarum) and gram-negative (Escherichia coli) bacteria in equal amounts at a final concentration of 5 × 107 bacteria per ml. Culture supernatants were collected, and triplicates were pooled and kept in −20°C until analyzed by enzyme-linked immunosorbent assay (ELISA). Repeated thawing and freezing were avoided.
Purified monocytes from three cord blood samples and four adult blood samples were diluted to 5 × 105 cells per ml and cultured with L. plantarum, B. adolescentis, E. coli, and Veillonella parvula (5 × 107 bacteria per ml) for 24 h. For blocking experiments, purified monocytes (5 × 105 cells per ml) from three adult samples and three cord blood samples were preincubated with a-CD14 (UCHM-1), a-TLR-2 (TL2.1), a-TLR-4 (HTA125), or isotype control antibody (10 μg per ml) for 1 h in 4°C. The CD14 and TLR-4 antibodies were purchased from Serotec (Oxford, United Kingdom), and the TLR-2 antibody was purchased from Alexis Biochemicals (San Diego, Calif.). The monocytes were then cultured with L. plantarum or E. coli for 5 or 7 h. Initial studies showed that 5 × 106 bacteria per ml was the optimal bacterial concentration for blocking experiments. Culture supernatants were collected as described above.
Cytokine determination.
IL-12, IL-10, TNF-α, and IL-6 in cell culture supernatants were measured by ELISA. IL-12 concentrations were measured by using antibodies specific for the bioactive form p70, which is composed by the two subunits p35 and p40. Plates were coated with IL-12 p70 monoclonal antibody (Ab) (20C2), and biotinylated IL-12 p40/p70 monoclonal Ab (C8.6) was used for detection. IL-10 protein concentrations were determined using anti-human IL-10 monoclonal Ab (JES3-9D7) for capture and biotinylated IL-10 monoclonal Ab (JES3-12G8) for detection. To measure TNF-α concentrations, antihuman monoclonal Ab (MAb1) for capture was paired with biotinylated antihuman monoclonal Ab (MAb11) for detection. IL-6 was measured by using anti-human IL-6 monoclonal Ab (MQ2-13A5) for capure and biotinylated anti-human IL-6 monoclonal Ab (MQ2-39C3) for detection. All antibodies were purchased from Pharmingen (San Diego, Calif.). The standard curves were generated using recombinant human IL-12 p70, IL-10, TNF-α, and IL-6 (Pharmingen).
Costar plates (Invitrogen) were coated overnight at 4°C with capture antibodies diluted in carbonate buffer (pH 9.6). The plates were washed three times in PBS and blocked for 1 h with 5% bovine serum albumin (Sigma-Aldrich) in PBS. After washing the plates five times in PBS containing 0.01% Tween, 50 μl of samples or standards was added and incubated for 1 h. After washing, biotinylated detector antibodies were added to each well and incubated for 1 h. The plates were washed and incubated with streptavidin-horseradish peroxidase (CLB, Amsterdam, The Netherlands) for 30 min. Thereafter, 3,3′5,5′-tetramethylbenzidine (TMB) liquid substrate (Sigma-Aldrich) was added and incubated for 20 min in the dark, and the color reaction was stopped by adding 1 M H2SO4. The amount of substrate converted to colored product was measured as the optical density at 450 nm in a spectrophotometer (Spectra Max Plus, Molecular Devices). All incubations, except for coating, were carried out on a shaker at room temperature. Samples, standards, biotinylated antibodies and streptavidin-HRP were diluted in high performance ELISA dilution buffer (CLB).
Statistical analysis.
The Wilcoxon signed rank test (GraphPad Prism) was used to compare cytokine production between paired cord cells and adult cells and between cytokine productions in responses to different kinds of bacteria.
RESULTS
IL-12, TNF-α, IL-10, and IL-6 production from cord and adult mononuclear cells after stimulation with different bacterial strains.
In order to study cytokine production after bacterial stimulation, we cultured mononuclear cells from cord and adult blood with six different gram-positive bacterial strains and five different gram-negative strains from the normal flora in the gastrointestinal tract. Protein levels of IL-12, TNF-α, IL-10, and IL-6 were measured in the supernatants.
Gram-positive bacterial strains induced much higher levels of IL-12 and TNF-α than gram-negative bacteria in both cord cells and adult cells (Fig. 1). The highest levels of IL-12 were produced when cells were stimulated by L. plantarum and Streptococcus mitis. All gram-negative bacterium species tested induced levels of IL-12 lower than or just above the detection level of the ELISA (40 pg/ml). Corynebacterium minutissimum and Clostridium perfringens were the gram-positive strains that evoked the lowest levels of IL-12 production. The bacterial strains that were the most potent inducers of IL-12 also evoked the highest levels of TNF-α. All individual species of gram-negative bacteria induced lower production of TNF-α than gram-positive bacteria. Thus, the pattern of TNF-α induced by different bacteria very much resembles the pattern of IL-12.
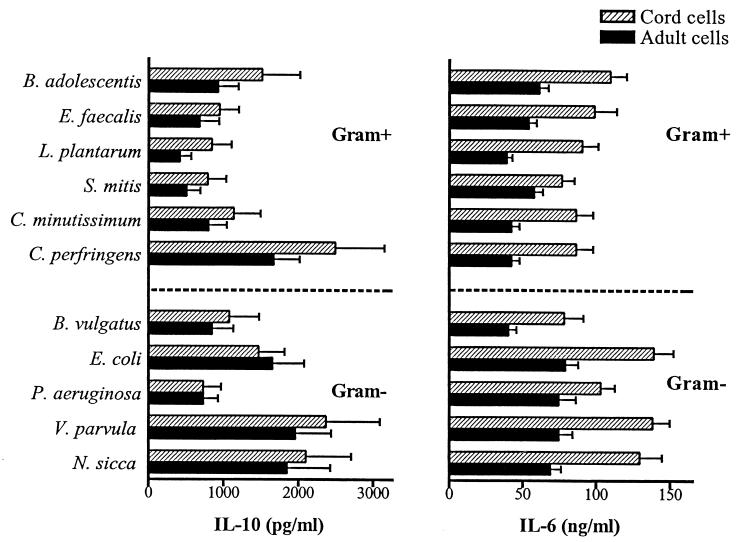
FIG. 1.
IL-12 p70 and TNF-α production from cord cells and adult mononuclear cells (2 × 106 cells/ml) after stimulation for 24 h with different gram-positive (Gram+) or gram-negative (Gram−) bacterial strains (5 × 107 bacteria/ml). Bars represent mean cytokine production from 11 individuals, and the error bars represent standard errors of the means.
In contrast to the pattern of IL-12 and TNF-α production, both gram-positive and gram-negative bacteria induced high levels of IL-10 and IL-6 (Fig. 2). The strains of gram-positive bacteria that evoked low levels of IL-12 and TNF-α generally induced high levels of IL-10 and vice versa. For example, Clostridium perfringens was a poor inducer of IL-12 and TNF-α but evoked the highest levels of IL-10 production among the gram-positive strains. IL-6 seemed to be differentially regulated, and there were no large variations between the different bacterial strains. Cells that were cultured in medium alone did not secrete any cytokines above limits of detection.
FIG. 2.
Concentration of IL-10 and IL-6 in supernatants of cord and adult mononuclear cells (2 × 106 cells/ml) stimulated for 24 h with different gram-positive (Gram+) or gram-negative (Gram−) bacterial strains (5 × 107 bacteria/ml). Bars represent mean cytokine production from 11 individuals, and the error bars represent standard errors of the means.
Difference in cytokine production between cord cells and adult mononuclear cells in response to gram-positive and gram-negative bacteria.
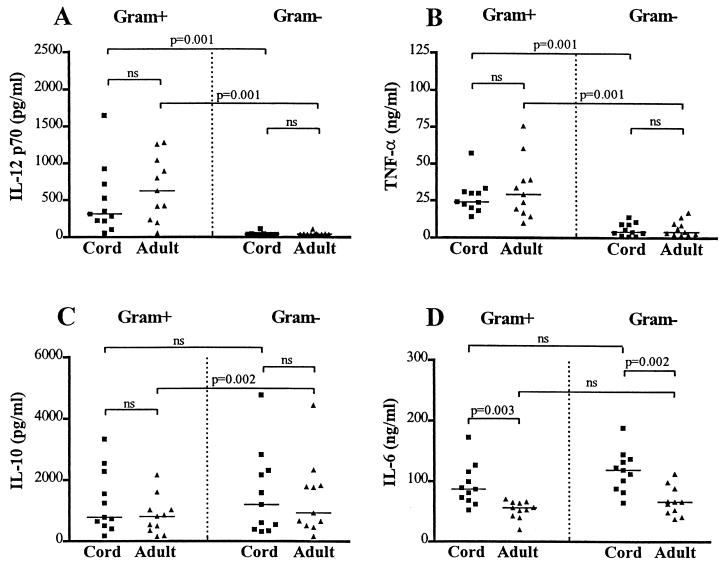
In order to analyze the innate immune responses of newborns to bacteria from the normal flora, we measured the cytokine response of neonatal cells and compared it with that of adult cells. In our experimental setup, one cord and one adult blood sample were stimulated simultaneously, and these were later paired in the statistical analysis. In response to commensal bacteria, cord cells produced similar levels of IL-12, TNF-α, and IL-10, as did adult cells, and produced significantly higher levels of IL-6 (Fig. 3A to D). When the various bacterial strains were analyzed separately for differences between neonates and adults, we found no statistically significant differences in the levels of IL-12, TNF-α, and IL-10 (not shown). However, neonatal cells produced higher levels of IL-6 than adult cells in response to all bacterial strains, except for C. minutissimum and Pseudomonas aeruginosa (data not shown).
FIG. 3.
Production of IL-12 (A), TNF-α (B), IL-10 (C) and IL-6 (D) from cord and adult mononuclear cells after stimulation with gram-positive (Gram+) and gram-negative (Gram−) bacteria. Each point represents mean cytokine production from one individual after 24 h of stimulation with six gram-positive bacterial strains or five gram-negative strains. Cord and adult samples were stimulated simultaneously, and the difference was statistically analyzed as paired observations with the Wilcoxon signed rank test. The difference in the response of each individual to gram-positive and gram-negative bacteria was analyzed as described above. All raw data from each individual are included in the statistical analysis. Horizontal bars represent median cytokine production. A P value of >0.05 was considered not significant (ns).
Since gram-positive and gram-negative bacterial species appeared to induce different patterns of cytokines, we compared the average response of each individual to six gram-positive strains with the average response to five gram-negative strains (Fig. 3A to D). Gram-positive bacteria induced significantly higher levels of IL-12 and TNF-α in both cord cells and adult cells relative to gram-negative strains. In adults, gram-negative bacteria evoked higher levels of IL-10 than did gram-positive strains. In contrast, cord cells responded to gram-negative and gram-positive bacteria with similar amounts of IL-10. There were no significant differences between gram-positive and gram-negative bacterial strains in their capacity to induce IL-6 production. Cells from different individuals produced different amounts of cytokines after bacterial stimulation, and the variation was similar in neonates and adults. Initial kinetic studies showed that differences in IL-12 and TNF-α production between the Gram-positive and Gram-negative strains were significant at 18, 24, 44, and 67 h of stimulation. We used 24 h for our convenience.
Cytokine production after stimulation with combinations of gram-positive and gram-negative bacteria.
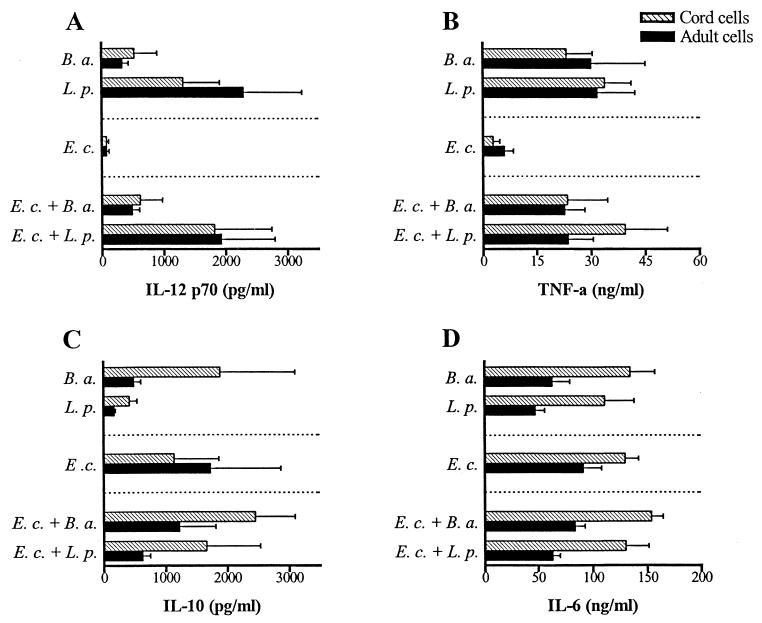
Since gram-positive and gram-negative bacterial strains induce different cytokine patterns, we wanted to examine how combinations of different bacteria affect the cytokine response. As shown in Fig. 4A and B, stimulating mononuclear cells with a mixture of E. coli and B. adolescentis or L. plantarum resulted in similar production of IL-12 and TNF-α relative to stimulation with only gram-positive bacteria. The production of IL-10 increased when the cells were stimulated with a mixture of E. coli and L. plantarum compared with gram-positive bacterium stimulation alone (Fig. 4C), whereas the IL-6 production was not affected by combining different bacterial strains (Fig. 4D).
FIG. 4.
Production of IL-12 (A), TNF-α (B), IL-10 (C), and IL-6 (D) from cord and adult mononuclear cells after stimulation with the gram-positive bacterial strains L. plantarum (L. p.) or B. adolescentis (B. a.) or the gram-negative strain E. coli (E. c.) or with a combination of gram-positive and gram-negative bacteria (5 × 107 bacteria/ml). Bars represent cytokine production from four individuals after 24 h of stimulation, and the error bars represent standard errors of the means.
Cytokine production from purified monocytes after bacterial stimulation.
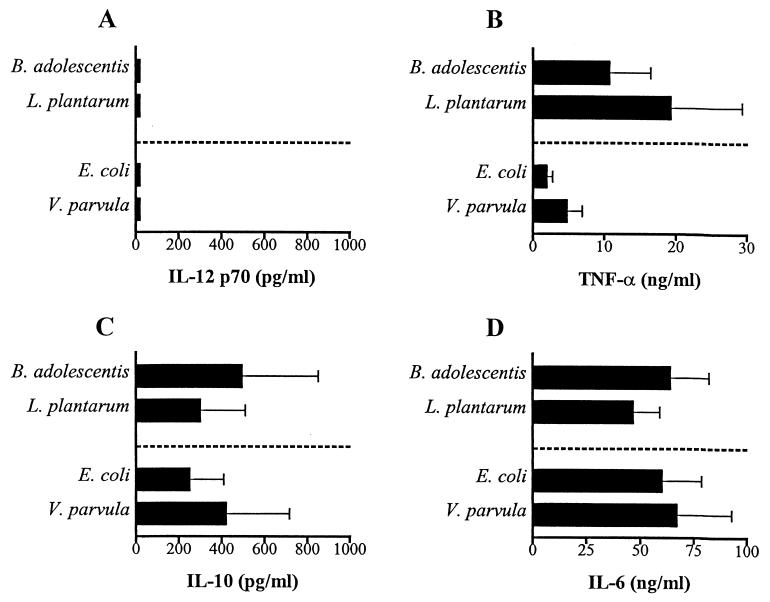
To ensure that the cytokines studied mainly are produced from monocytes, we also used purified monocytes from neonates and adults in cultures with B. adolescentis, L. plantarum, E. coli, and V. parvula. In Fig. 5 we show that the TNF-α, IL-10, and IL-6 pattern produced by neonatal monocytes is similar to that produced by mononuclear cells. In contrast to results with mononuclear cells, IL-12 was not produced by purified CD14+ cells after stimulation with either gram-positive or gram-negative bacteria. This is probably due to the fact that monocytes need help, such as CD40 ligation or soluble factors from T cells or other mononuclear cells, in order to produce IL-12. The same cytokine pattern was observed with purified adult monocytes (not shown).
FIG. 5.
IL-12 (A), TNF-α (B), IL-10 (C), and IL-6 (D) production from purified cord monocytes (5 × 105 cells/ml) after stimulation with B. adolescentis, L. plantarum, E. coli, and V. parvula (5 × 107 bacteria/ml) for 24 h. Bars represent mean cytokine production from three cord blood samples, and error bars represent standard errors of the means.
Inhibition of TNF-α production after blocking CD14, TLR-2, and TLR-4.
TLRs recognize different microbial components and signal through pathways that eventually activate transcription of cytokines. CD14 lacks an intracellular domain but works as a coreceptor with TLRs. In order to study how CD14, TLR-2, and TLR-4 are involved in the recognition of whole bacteria, we blocked these receptors with antibodies prior to stimulation with L. plantarum and E. coli. As shown in Fig. 6 blocking CD14, TLR-2, and TLR-4 inhibited TNF-α production after stimulation with L. plantarum in both cord cells and adult cells. TLR-2 seems to be the most crucial receptor for the recognition of this gram-positive bacterial strain, at least in adults. The corresponding results after stimulating cells with the gram-negative bacterium E. coli showed that antibodies against CD14 and TLR-4 reduced the TNF-α production. In adults, blocking of TLR-2 decreased the production of TNF-α, but this pathway did not operate in neonatal cells. Kinetic studies showed that the optimal time point for the inhibition was 5 to 7 h after bacterial stimulation, while the blocking effect was not seen after 24 h. We reduced the bacterial concentration 10-fold in these experiments, since the blocking effect was optimal using smaller amounts of bacteria.
FIG. 6.
Effect of pretreatment of cord (A) and adult (B) monocytes with antibodies to CD14, TLR-2, or TLR-4 on TNF-α production induced by L. plantarum or E. coli. Monocytes (5 × 105/ml) were incubated with 10 μg of blocking antibody or immunoglobulin G2a control antibody/ml for 1 h prior to bacterial stimulation (5 × 106 bacteria/ml) for 5 to 7 h. The results shown are from one representative experiment out of three.
DISCUSSION
The incidence of atopic allergy is steadily increasing in Western European countries and seems to be due to decreased microbial stimulation of the immune system (43). Studies indicate that exposure to an environment with a high bacterial load is particularly important in the first year of life in order to acquire protection from asthma and other allergic diseases (32). Protection against allergic diseases develops because of induction of immunological tolerance to allergens, which involves differentiation of allergen-specific T cells. In an animal model it has been shown that a normal intestinal flora is necessary for the development of tolerance to dietary antigens (30). Moreover, in humans there is an association between protection against allergy and the presence of antibodies specific for orofecal microbes but not the presence of antibodies against airborne viruses (26). The innate immune response directs T-cell differentiation, thus determining whether tolerance develops. Therefore, it is of interest to study how the immune system of newborns responds to bacteria inhabiting the human gastrointestinal mucosa.
IL-12 is a key cytokine for the differentiation of naïve CD4+ T cells toward a Th1 cytokine pattern, and it has been found to be efficient at inducing IFN-γ production from neonatal CD4+ T cells (44). Although the immune system of newborn children has previously been considered to be Th2 skewed, Marchant et al. have shown that newborn babies vaccinated with bacillus Calmette-Guerin are able to react with an antigen-specific Th1 response and produce similar levels of IFN-γ in response to purified protein derivative (PPD) relative to adults (42). We found that neonatal cells are as potent IL-12 producers as adult cells when stimulated with bacteria from the normal flora. This is in accordance with one study showing comparable levels in cord and adult cells after stimulation with Staphylococcus aureus (33). In contrast, another group has shown that cord cells have a decreased ability to produce IL-12 after stimulation by group B streptococci (19). The latter study included cells from only five blood samples, and in our experience the abilities of different individuals to produce this cytokine vary greatly.
Similar to the IL-12 production, we found that cord cells produce levels of TNF-α as high as those produced by as adults in response to bacteria from commensal flora. The strain Streptococcus agalactiae, which is a major pathogen of neonatal sepsis, has also been shown to induce levels of TNF-α in cord cells that are at least as high as those induced in adult cells (2). Another study showed that cord cells produce levels of TNF-α comparable to those for adult cells when stimulated with LPS (6). We found that IL-6, another proinflammatory cytokine, was produced at high levels from cord cells, in even higher amounts than from adult cells. These findings are supported by others, who observed elevated levels of IL-6 in cord cells after stimulation with S. agalactiae and LPS (2). IL-10 downregulates the inflammatory response, and the suppressive effect is due to the inhibition of proinflammatory cytokines and decreased antigen presentation to T cells (9, 10). Moreover, IL-10 is also able to induce the development of regulatory T cells (15). We found that cells from newborn children produce levels of IL-10 that are as high as those produced by adult cells in response to commensal bacteria. This is in accordance with other studies showing that cord cells produce comparable levels of IL-10 after LPS stimulation (6, 14).
The present study demonstrates that gram-positive bacterial strains induce high levels of IL-12 in both cord cells and adult cells, whereas gram-negative bacterial strains are poor inducers of IL-12. These findings are supported by previous studies on adult cells (16, 18), and several other studies have shown that gram-positive bacterial strains and their cell wall compounds are potent inducers of IL-12 (12, 17, 18, 29). In our experiments, L. plantarum was one of the bacterial strains that induced the highest level of IL-12. Lactobacillus casei has previously been shown to inhibit allergen-induced immunoglobulin E production by murine splenocytes, an effect that may be due to the induction of IL-12 (35). Moreover, we could show that gram-positive bacteria are more potent inducers of TNF-α than gram-negative species, which was also observed by Hessle et al. (personal communication). However, C. perfringens and C. minutissimum evoked lower levels of IL-12 and TNF-α than other gram-positive bacterial strains tested in this study. This could be due to the fact that clostridia often show poor development of their peptidoglycan layer during stationary culture conditions. Peptidoglycan from corynebacteria has previously been shown to be a weaker inducer of IL-12 p40 than peptidoglycan from other bacterial strains (13, 23). Interestingly, clostridia seem to be more prevalent in allergic children than in nonatopic children (4). Our experiments clearly show that gram-negative bacterial strains are poor inducers of IL-12, which was also found by Haller et al. and Hessle et al. (16-18). Monocyte-derived dendritic cells cultured with LPS secrete IL-12 p40, but the bioactive form of IL-12 is produced at very low levels (14). On the other hand, cells that have been primed with IFN-γ prior to LPS stimulation produce IL-12 p70 in both cord cells and adult cells (6).
It has previously been shown that gram-negative bacterial strains are more potent inducers of IL-10 than gram-positive strains in adult cells (17), but our data indicate that the two bacterial groups have the same ability to induce IL-10 production in cord cells. Adult cells secreted higher levels of IL-10 when stimulated by gram-negative bacteria when the data were analyzed as paired observations, but the median level of IL-10 did not differ between gram-positive and gram-negative bacterial stimulation. In general terms, the gram-positive strains that induced the highest levels of IL-10 evoked the smallest amounts of IL-12 and TNF-α. This might be due to the fact that IL-10 inhibits the production of IL-12 and TNF-α (8, 9). However, the explanation for the poor IL-12-stimulating effect of gram-negative bacteria is probably not only the inhibiting capacity of IL-10, since gram-positive bacteria are stronger inducers of IL-12 than gram-negative strains even after the blocking of IL-10 (17).
TLRs recognize bacterial products and signal through pathways that leads to activation of a variety of transcription factors. These factors in turn coordinate the induction of many genes encoding proinflammatory cytokines and other inflammatory mediators. CD14 lacks an intracellular domain but works as a coreceptor with TLRs. We found that TLR-2, TLR-4, and CD14 are important for the inflammatory response to the gram-positive bacterium L. plantarum, in both cord cells and adult cells. TLR-2 seems to be the most crucial receptor for the recognition of this gram-positive bacterial strain, at least in adults. CD14 and TLR-4 are important for the recognition of the gram-negative bacterium E. coli. We observed that E. coli acted through TLR-2 in adults but not in neonatal cells. To our knowledge, this is the first study that shows that blocking of these receptors abolishes the cytokine response to intact bacteria. Our results are supported by those of other groups showing that TLR-2 mainly is involved in responses to gram-positive bacterium components, while TLR-4 is a signaling receptor for gram-negative bacterium cell wall products (11, 36, 38). Furthermore, CD14 has previously been found to be involved in the induction of TNF-α production after stimulation with both gram-positive and gram-negative bacterial compounds (27). TLR-2 can be localized to macrophage phagosomes containing pathogens and trigger an inflammatory response (40). This might explain why the blocking effect was abolished after 24 h, as antibodies block only TLRs on the surface of the cell.
Interestingly, there is a clear individual variation in cytokine production after bacterial stimulation. Some individuals responded with higher levels of IL-12 than of IL-10 when stimulated by gram-positive bacteria, whereas others responded the other way around. Previous studies have demonstrated that mononuclear cells from atopic individuals have a lesser ability to produce IL-12 when stimulated by S. aureus than those from nonatopic individuals (37, 41). Monocyte-derived dendritic cells from atopic patients have also been shown to produce less bioactive IL-12 in response to CD40 ligation (31). In addition, cells from newborns with a high risk of allergy have been reported to produce higher levels of IL-6 than cord cells from low-risk populations (25). In an ongoing prospective study we are examining whether mononuclear cells from newborns that later develop allergies produce higher or lower levels of IL-12, TNF-α, IL-6, and IL-10.
In summary, we found that cord blood mononuclear cells are at least as potent producers of IL-12, TNF-α, IL-10, and IL-6 as adult cells when stimulated by bacteria from the normal flora. Furthermore, the pattern of cytokines produced after stimulation with different strains of bacteria did not differ between neonates and adults. This study shows that antigen-presenting cells in neonates have the ability to potently respond to bacteria from the normal flora in a way that might be important for the maturation of the immune system and that different bacterial strains may have differential effects. It remains to elucidate how commensal bacteria may affect differentiation of T cells and how such modulation might prevent allergy.
Acknowledgments
This work was supported by LUA-SAM, Göteborg University, Harald Jeanssons Stiftelse, Vårdalstiftelsen, Astma och Allergiförbundet, Magnus Bergwalls Stiftelse, and Stiftelsen Wilhelm och Martina Lundgrens Vetenskapsfond.
We thank the staff at Mölndal Hospital Delivery Unit for cord blood as well as the adult volunteers providing blood.
Editor: J. D. Clements
REFERENCES
- 1.Ahrne, S., S. Nobaek, B. Jeppsson, I. Adlerberth, A. E. Wold, and G. Molin. 1998. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J. Appl. Microbiol. 85:88-94. [DOI] [PubMed] [Google Scholar]
- 2.Berner, R., P. Welter, and M. Brandis. 2002. Cytokine expression of cord and adult blood mononuclear cells in response to Streptococcus agalactiae. Pediatr. Res. 51:304-309. [DOI] [PubMed] [Google Scholar]
- 3.Björksten, B., P. Naaber, E. Sepp, and M. Mikelsaar. 1999. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin. Exp. Allergy 29:342-346. [DOI] [PubMed] [Google Scholar]
- 4.Björksten, B., E. Sepp, K. Julge, T. Voor, and M. Mikelsaar. 2001. Allergy development and the intestinal microflora during the first year of life. J. Allergy Clin. Immunol. 108:516-520. [DOI] [PubMed] [Google Scholar]
- 5.Böttcher, M. F., E. K. Nordin, A. Sandin, T. Midtvedt, and B. Björksten. 2000. Microflora-associated characteristics in faeces from allergic and nonallergic infants. Clin. Exp. Allergy 30:1590-1596. [DOI] [PubMed] [Google Scholar]
- 6.Chipeta, J., Y. Komada, X. L. Zhang, E. Azuma, H. Yamamoto, and M. Sakurai. 2000. Neonatal (cord blood) T cells can competently raise type 1 and 2 immune responses upon polyclonal activation. Cell Immunol. 205:110-119. [DOI] [PubMed] [Google Scholar]
- 7.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 8.D'Andrea, A., M. Aste-Amezaga, N. M. Valiante, X. Ma, M. Kubin, and G. Trinchieri. 1993. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 178:1041-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Waal Malefyt, R., J. Abrams, B. Bennett, C. G. Figdor, and J. E. de Vries. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Waal Malefyt, R., J. Haanen, H. Spits, M. G. Roncarolo, A. te Velde, C. Figdor, K. Johnson, R. Kastelein, H. Yssel, and J. E. de Vries. 1991. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 174:915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellingsen, E., S. Morath, T. Flo, A. Schromm, T. Hartung, C. Thiemermann, T. Espevik, D. Golenbock, D. Foster, R. Solberg, A. Aasen, and J. Wang. 2002. Induction of cytokine production in human T cells and monocytes by highly purified lipoteichoic acid: involvement of Toll-like receptors and CD14. Med. Sci. Monit. 8:BR149-BR156. [PubMed] [Google Scholar]
- 12.Fujimoto, T., R. B. Duda, A. Szilvasi, X. Chen, M. Mai, and M. A. O'Donnell. 1997. Streptococcal preparation OK-432 is a potent inducer of IL-12 and a T helper cell 1 dominant state. J. Immunol. 158:5619-5626. [PubMed] [Google Scholar]
- 13.Goguel, A. F., G. Lespinats, and C. Nauciel. 1982. Peptidoglycans extracted from gram-positive bacteria: expression of antitumor activity according to peptide structure and route of injection. J. Natl. Cancer Inst. 68:657-663. [PubMed] [Google Scholar]
- 14.Goriely, S., B. Vincart, P. Stordeur, J. Vekemans, F. Willems, M. Goldman, and D. De Wit. 2001. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 166:2141-2146. [DOI] [PubMed] [Google Scholar]
- 15.Groux, H., M. Bigler, J. E. de Vries, and M. G. Roncarolo. 1996. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J. Exp. Med. 184:19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller, D., S. Blum, C. Bode, W. P. Hammes, and E. J. Schiffrin. 2000. Activation of human peripheral blood mononuclear cells by nonpathogenic bacteria in vitro: evidence of NK cells as primary targets. Infect. Immun. 68:752-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hessle, C., B. Andersson, and A. E. Wold. 2000. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infect. Immun. 68:3581-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hessle, C., L. A. Hanson, and A. E. Wold. 1999. Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production. Clin. Exp. Immunol. 116:276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joyner, J. L., N. H. Augustine, K. A. Taylor, T. R. La Pine, and H. R. Hill. 2000. Effects of group B streptococci on cord and adult mononuclear cell interleukin-12 and interferon-gamma mRNA accumulation and protein secretion. J. Infect. Dis. 182:974-977. [DOI] [PubMed] [Google Scholar]
- 20.Kalliomäki, M., P. Kirjavainen, E. Eerola, P. Kero, S. Salminen, and E. Isolauri. 2001. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 107:129-134. [DOI] [PubMed] [Google Scholar]
- 21.Kalliomäki, M., S. Salminen, H. Arvilommi, P. Kero, P. Koskinen, and E. Isolauri. 2001. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357:1076-1079. [DOI] [PubMed] [Google Scholar]
- 22.Kirschning, C. J., H. Wesche, T. Merrill Ayres, and M. Rothe. 1998. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 188:2091-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrence, C., and C. Nauciel. 1998. Production of interleukin-12 by murine macrophages in response to bacterial peptidoglycan. Infect. Immun. 66:4947-4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, S. M., Y. Suen, L. Chang, V. Bruner, J. Qian, J. Indes, E. Knoppel, C. van de Ven, and M. S. Cairo. 1996. Decreased interleukin-12 (IL-12) from activated cord versus adult peripheral blood mononuclear cells and upregulation of interferon-gamma, natural killer, and lymphokine-activated killer activity by IL-12 in cord blood mononuclear cells. Blood 88:945-954. [PubMed] [Google Scholar]
- 25.Liao, S. Y., T. N. Liao, B. L. Chiang, M. S. Huang, C. C. Chen, C. C. Chou, and K. H. Hsieh. 1996. Decreased production of IFN-γ and increased production of IL-6 by cord blood mononuclear cells of newborns with a high risk of allergy. Clin. Exp. Allergy. 26:397-405. [PubMed] [Google Scholar]
- 26.Matricardi, P. M., F. Rosmini, S. Riondino, M. Fortini, L. Ferrigno, M. Rapicetta, and S. Bonini. 2000. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. BMJ 320:412-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medvedev, A. E., T. Flo, R. R. Ingalls, D. T. Golenbock, G. Teti, S. N. Vogel, and T. Espevik. 1998. Involvement of CD14 and complement receptors CR3 and CR4 in nuclear factor-kappaB activation and TNF production induced by lipopolysaccharide and group B streptococcal cell walls. J. Immunol. 160:4535-4542. [PubMed] [Google Scholar]
- 28.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9:4-9. [DOI] [PubMed] [Google Scholar]
- 29.Miettinen, M., S. Matikainen, J. Vuopio-Varkila, J. Pirhonen, K. Varkila, M. Kurimoto, and I. Julkunen. 1998. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect. Immun. 66:6058-6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreau, M. C., and G. Corthier. 1988. Effect of the gastrointestinal microflora on induction and maintenance of oral tolerance to ovalbumin in C3H/HeJ mice. Infect. Immun. 56:2766-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reider, N., D. Reider, S. Ebner, S. Holzmann, M. Herold, P. Fritsch, and N. Romani. 2002. Dendritic cells contribute to the development of atopy by an insufficiency in IL-12 production. J. Allergy Clin. Immunol. 109:89-95. [DOI] [PubMed] [Google Scholar]
- 32.Riedler, J., C. Braun-Fahrlander, W. Eder, M. Schreuer, M. Waser, S. Maisch, D. Carr, R. Schierl, D. Nowak, and E. von Mutius. 2001. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet 358:1129-1133. [DOI] [PubMed] [Google Scholar]
- 33.Scott, M. E., M. Kubin, and S. Kohl. 1997. High level interleukin-12 production, but diminished interferon-gamma production, by cord blood mononuclear cells. Pediatr. Res. 41:547-553. [DOI] [PubMed] [Google Scholar]
- 34.Sepp, E., K. Julge, M. Vasar, P. Naaber, B. Björksten, and M. Mikelsaar. 1997. Intestinal microflora of Estonian and Swedish infants. Acta Paediatr. 86:956-961. [DOI] [PubMed] [Google Scholar]
- 35.Shida, K., K. Makino, A. Morishita, K. Takamizawa, S. Hachimura, A. Ametani, T. Sato, Y. Kumagai, S. Habu, and S. Kaminogawa. 1998. Lactobacillus casei inhibits antigen-induced IgE secretion through regulation of cytokine production in murine splenocyte cultures. Int. Arch. Allergy Immunol. 115:278-287. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 37.Tang, L., S. Benjaponpitak, R. H. DeKruyff, and D. T. Umetsu. 1998. Reduced prevalence of allergic disease in patients with multiple sclerosis is associated with enhanced IL-12 production. J. Allergy Clin. Immunol. 102:428-435. [DOI] [PubMed] [Google Scholar]
- 38.Tapping, R. I., S. Akashi, K. Miyake, P. J. Godowski, and P. S. Tobias. 2000. Toll-like receptor 4, but not toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J. Immunol. 165:5780-5787. [DOI] [PubMed] [Google Scholar]
- 39.Trinchieri, G. 1993. Interleukin-12 and its role in the generation of TH1 cells. Immunol. Today 14:335-338. [DOI] [PubMed] [Google Scholar]
- 40.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 41.van der Pouw Kraan, T. C., L. C. Boeije, E. R. de Groot, S. O. Stapel, A. Snijders, M. L. Kapsenberg, J. S. van der Zee, and L. A. Aarden. 1997. Reduced production of IL-12 and IL-12-dependent IFN-gamma release in patients with allergic asthma. J. Immunol. 158:5560-5565. [PubMed] [Google Scholar]
- 42.Vekemans, J., A. Amedei, M. O. Ota, M. M. D'Elios, T. Goetghebuer, J. Ismaili, M. J. Newport, G. Del Prete, M. Goldman, K. P. McAdam, and A. Marchant. 2001. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur. J. Immunol. 31:1531-1535. [DOI] [PubMed] [Google Scholar]
- 43.Wold, A. E. 1998. The hygiene hypothesis revised: is the rising frequency of allergy due to changes in the intestinal flora? Allergy 53:20-25. [DOI] [PubMed] [Google Scholar]
- 44.Wu, C. Y., C. Demeure, M. Kiniwa, M. Gately, and G. Delespesse. 1993. IL-12 induces the production of IFN-gamma by neonatal human CD4 T cells. J. Immunol. 151:1938-1949. [PubMed] [Google Scholar]