Abstract
Sepsis is thought to result from an exaggerated innate immune response to microbial components such as lipopolysaccharide (LPS), but the involvement of a specific mechanism(s) has not been identified. We studied the role of caspase 1 (Cas-1) in the murine innate immune response to infection with gram-negative bacteria and to nonlethal and lethal doses of LPS. cas-1−/− and Cas-1 inhibitor (Ac-YVAD-CHO)-treated cas-1+/+ mice were two- to threefold more susceptible to lethal Escherichia coli infection than cas-1+/+ mice. Administration of Cas-1 products, interleukin-18 (IL-18) or IL-1β, protected three of three and six of seven mice, respectively, from lethal infection with E. coli compared to none of six of untreated mice (P = 0.0082). Therefore, cas-1 is essential for antibacterial host defense. Nonlethal (75 μg) and lethal (500 μg) doses of LPS induce different patterns of gamma interferon, IL-1β, and IL-18 expression. Consequently, the role of Cas-1, which cleaves pro-IL-18 and pro-IL-1β to their active forms, was investigated in these disparate conditions by using enzymatic assay and reverse transcription-PCR. At 75 μg, LPS induced a transient increase in IL-1β and IL-18 levels in serum, whereas at 500 μg it induced a 1.5-fold-higher IL-18 level in serum, which increased till death. At 75 μg of LPS, splenic cas-1 mRNA expression remained unchanged at all time points, but activity increased transiently at 3 h. In lethally treated mice, Cas-1 activity remained elevated until death; however, cas-1 mRNA levels increased at 3 h and decreased to basal levels by 8 h. Treatment with Cas-1 inhibitor protected mice from lethal endotoxemia. Thus, Cas-1 is essential for innate antibacterial host defenses and may represent a mechanism of innate immunity that upon excessive stimulation by microbial components may lead to endotoxic shock.
Sepsis is a leading cause of mortality after microbial infections and is thought to result from a dysregulated innate immune response to microbial products, such as lipopolysaccharide (LPS); however, few specific mechanisms critical to innate immunity have been shown to become altered during the development of sepsis (32). Understanding the regulatory mechanisms that become altered in the development of shock may help in the design of effective therapies for the treatment of sepsis.
Proinflammatory cytokines, such as interleukin-1β (IL-1β) and gamma interferon (IFN-γ), are involved in antimicrobial defenses (15, 16, 20, 40). The recently discovered IFN-γ-inducing factor IL-18 exerts pleotropic activities (7) and plays an important role in defense against a variety of gram-positive and -negative bacterial pathogens (3, 8, 9, 12, 25, 27, 31, 34, 39). Excessive synthesis of IL-1β and IL-18, however, is also associated with septic death (17, 24, 28, 29, 36). This suggests that a controlled expression of IL-18 expression may be critical to the lethal outcome of LPS-induced innate immune response.
Synthesis of IL-1β and IL-18 depends upon the proteolytic cleavage of their precursor polypeptides (pro-IL-1β and pro-IL-18) by the cysteine protease caspase 1 (Cas-1), also called IL-1-converting enzyme (13, 14). Like IL-18−/− mice, cas-1−/− mice do not produce bioactive IL-18 nor do they produce IL-1β and are resistant to LPS toxicity (21, 22). Also, Cas-1 inhibitors suppress IL-1β and IL-18 synthesis and protect mice from endotoxin shock (14, 23). Thus, Cas-1 is considered essential for the toxic effects of LPS. Given its involvement in IL-18 maturation, Cas-1 can also be expected to play an important role in gram-negative antibacterial host defenses (ABHD). However, this is not well characterized.
In the present study, we show that Cas-1 is important in innate host defenses against Escherichia coli infection. However, unlike the case with a nonlethal dose of LPS, high doses of LPS dramatically upregulate cas-1 expression leading to lethality. This association of different levels of expression of one component of the innate immune response to LPS, Cas-1, with both ABHD and lethal inflammation lends support to the hypothesis that sepsis is a dysregulation of innate immune response.
MATERIALS AND METHODS
Materials.
LPS from E. coli O111:B4 (lot no. 60K4078; Sigma Chemical Company, St. Louis, Mo.) was suspended in 0.85% normal saline and sonicated at 30°C for 30 min prior to use. Murine recombinant IL-18 (rmIL-18) and anti-IL-18 monoclonal antibody (clone 93-10C) were purchased from R&D Systems (Minneapolis, Minn.). Cas-1 substrate (Ac-YVAD-pNA) and inhibitor (Ac-YVAD-CHO) were purchased from Alexis Biochemicals, Inc. (San Diego, Calif.).
Mice.
Seven- to twelve-week-old female C3H/HeN (National Cancer Institute, Frederick, Md.) and cas-1−/− mice were housed under a 12-h daylight cycle in controlled humidity and temperature conditions and supplied with food and water ad libitum. One male and three female cas-1+/− mice (with a C3H background [kindly supplied by Tariq Ghayur, Abbott Bioresearch Center, Worcester, Mass., and Paul Jolicoeur, Clinical Research Institute of Montreal, Montreal, Quebec, Canada]) were mixed and allowed to breed naturally and pregnant females were separated from the male immediately. Tail-snip DNA prepared (DNeasy Tissue Kit; Qiagen, Inc., Valencia, Calif.) from 4-week-old pups was genotyped by using cas-1-specific PCR. The primers were (i) forward (5′-GAA GAG ATG TTA CAG AAG CC-3′) and (ii) reverse (for the wild-type gene, 5′-CAT GCC TGA ATA ATG ATC AC-3′, and for the mutated gene, 5′-GCG CCT CCC CTA CCC GG-3′), and the PCR conditions were 94°C for 30 s, 56°C for 30 s, and 72°C for 2 min for 30 cycles.
To determine the cytokine levels in serum, C3H/HeN mice were treated with a predetermined nonlethal (75 μg/mouse) and lethal (500 μg/mouse) doses of LPS in normal saline by the intraperitoneal (i.p.) route, euthanized at different time points, and bled by cardiac puncture. Serum prepared from freshly drawn blood was diluted in phosphate-buffered saline (PBS)-bovine serum albumin and used for cytokine determination. Control mice were treated with normal saline.
Bacteria.
For all live infection experiments, E. coli O18:K1:H7 strain Bort was used as the challenge organism. This clinical isolate, originally isolated from the cerebrospinal fluid of a neonate, has been previously employed in the murine peritonitis model. This isolate is serum resistant and cannot be killed by neutrophils in the absence of type-specific antibody but rather requires activated macrophages (4). A single colony of E. coli, grown overnight on tryptic soy agar, was inoculated into tryptic soy broth and grown to log phase. Bacteria were washed and resuspended in PBS to an approximate concentration of 108 CFU/ml. This cell suspension was diluted further in PBS to the desired dose of bacteria for administration to mice i.p.
Susceptibility of mice of to E. coli O18:K1:H7.
C3H/HeN+/+ (wild type), C3H/cas-1+/−, or C3H/cas-1−/− mice were injected with different doses of freshly grown and diluted culture of E. coli O18:K1 i.p. and observed for mortality. The 50% lethal dose (LD50) of E. coli O18:K1 (Bort) was determined by plotting the percent mortality against the dose by using GraphPad Prizm software (GraphPad, San Diego, Calif.). The effect of Cas-1 inhibition on bacterial infection was studied by treating C3H/HeN+/+ mice with 5 mg of Ac-YVAD-CHO/kg i.p. 2 h prior to infecting with different doses of E. coli.
Supplementation of cas-1−/− mice with IL-18 and IL-1β.
cas-1−/− mice were treated i.p. with different doses of rmIL-18 (10 to 200 ng/mouse) or IL-1β (500 U/mouse). These doses of rmIL-18 were previously found to induce circulating IL-18 levels of at least 5 ng/ml (data not shown) at 3 h, which corresponded to the circulating IL-18 concentration induced in mice treated with a nonlethal dose (75 μg) of LPS at that same time. Similarly, 500 IU of IL-1β was shown to be protective against infection with E. coli Bort in C3H/HeJ mice (5). At 3 h after cytokine administration, the mice were infected with 3× LD50 (12,000 CFU of E. coli Bort/mouse) i.p. and then observed for mortality. Untreated cas-1+/− littermate mice were infected with the same dose of E. coli Bort as controls.
Cytokine measurements.
The concentrations of IFN-γ and IL-1β in mouse serum were determined by enzyme-linked immunosorbent assays (ELISAs) with R4-6A2/XMG1.1 (IFN-γ; PharMingen) and PM425B/MM425B (IL-1β; Endogen) antibody pairs as described previously (6). IL-18 was measured by using a mouse IL-18 detection ELISA kit (Medical and Biological Laboratories Co., Ltd., Nagoya, Japan) according to the manufacturer's instructions.
RT-PCR.
The total RNA was prepared from liver and spleen cells of LPS-treated C3H/HeN mice by using the RNAstat-60 reagent (Tel-Test, Inc., Friendswood, Tex.) and was used to set up an reverse transcription-PCR (RT-PCR) by using GeneAMP RNA PCR kit (Perkin-Elmer, Norwalk, Conn.). The primers used were as follows: (i) for IL-18, forward (5′-ACT GTA CAA CCG CAG TAA TAC GG-3′) and reverse (5′-AGT GAA CAT TAC AGA TTT ATC CC-3′), (ii) for cas-1, forward (5′-GAA GAG ATG TTA CAG AAG CC-3′) and reverse (5′-CAT GCC TGA ATA ATG ATC AC-3′), and (iii) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward (5′-TGA AGG TCG GTG TGA ACG GAT TTG GC-3′) and reverse (5′-CAT GTA GGC CAT GAG GTC CAC CAC-3′).
Cas-1 assay.
Cas-1 activity was determined by a colorimetric assay using liver and spleen extracts, prepared according to the method of Thornberry (35), as the enzyme source. Briefly, the liver and spleen from LPS-treated mice were homogenized in a lysis buffer (25 mM HEPES [pH 7.5], 1 mM EDTA, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, 2 mM dithiothreitol) at 5 ml/100 mg of liver tissue and 2 ml/100 mg of spleen tissue. Extracts were centrifuged at 15,000 × g for 30 min at 4°C, and the supernatant was centrifuged again at 200,000 × g for 1 h at 4°C in a Beckman L8M ultracentrifuge. The cytosol was then dialyzed against homogenization buffer containing 10% sucrose (Sigma), 0.1% NP-40 (Sigma), and 2 mM dithiothreitol (Sigma) overnight at 4°C. The dialysate was used for Cas-1 activity measurements. Reactions with enzyme preparation alone, with enzyme mixed with Cas-1 substrate (Ac-YVAD-pNA) or inhibitor (Ac-YVAD-CHO), and with substrate alone were also run as controls. The total increase in the optical density at 405 nm (OD405) versus that of the enzyme-alone wells was calculated.
Western blot analysis of IL-18 and Cas-1 protein.
Equal amounts of tissue protein were separated on a 7.5% sodium dodecyl sulfate (SDS)-polyacrylamide gel. The proteins were transferred to an Immobilon-P membrane by using a semiblot transfer procedure and immunostained with polyclonal antibodies reactive to the mature and precursor forms of murine IL-18 and Cas-1 (Santa Cruz Biologicals, Inc., Santa Cruz, Calif.), according to the manufacturer's protocol. The blots were developed by using TMB membrane substrate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.).
RESULTS
Cas-1-deficient mice are more susceptible to infection.
To assess the importance of Cas-1 activity in ABHD, wild-type C3H/HeN cas-1+/+, heterozygous C3H/HeN cas-1+/−, and C3H/HeN cas-1−/− mice from the same litter were tested for susceptibility to lethal infection with E. coli. The cas-1−/− mice succumbed to bacterial infection with an LD50 of 3,860 CFU/ml. In contrast, the wild-type and cas-1+/− heterozygous mice of the same background were killed by E. coli at an LD50 of 13,700 CFU/mouse, a three- to fourfold difference in susceptibility (Table 1). Thus, Cas-1 activity is required for optimal host defenses against a classically “extracellular” gram-negative bacterium associated with sepsis.
TABLE 1.
Cas-1-deficient mice are more susceptible to infectiona
| Background | Genotype | LD50 (CFU) of E. coli |
|---|---|---|
| C3H/HeN | Wild type | 13,700 |
| C3H/HeN | cas-1+/− | 13,100 |
| C3H/HeN | cas-1−/− | 3,860 |
Mice were infected with different doses of E. coli O18:K1:H7 strain (Bort) i.p. and observed for mortality for 48 h. The percent mortality was plotted against the dose of E. coli (Bort), and the LD50 was calculated from sigmoidal dose-response analysis by using GraphPad Prizm software.
Cas-1 inhibitor renders C3H/HeN mice susceptible to bacterial infection.
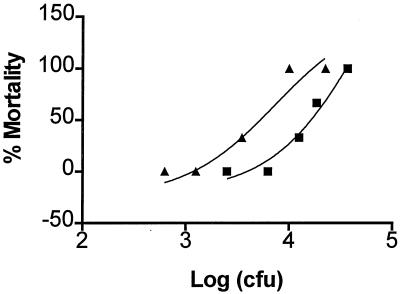
Since knockout mice may have other unidentified defects that may affect the experimental outcome, we also treated wild-type mice with a pharmacological inhibitor of Cas-1. Administration of a single dose (5 mg/kg) of Ac-YVAD-CHO i.p., rendered wild-type C3H/HeN mice >2-fold more sensitive to infection with E. coli compared to untreated mice (Fig. 1). The LD50 of E. coli decreased from 13,700 CFU/mouse in mice not receiving Cas-1 inhibitor to 5,250 CFU/mouse after treatment with Cas-1 inhibitor. Thus, cas-1−/− and wild-type mice treated with Cas-1 inhibitor were more susceptible to infection with E. coli than were untreated wild-type mice.
FIG. 1.
Effect of Cas-1 inhibitor on antibacterial resistance. C3H/HeN mice (five animals per group) were infected with different doses of E. coli 2 h after treatment with Cas-1 inhibitor (Ac-YVAD-CHO) at 5 mg/kg i.p. or saline and then monitored for mortality. The percent mortality was plotted against the E. coli dose, and the LD50 of E. coli was determined. Symbols: ▪, without Cas-1 inhibitor; ▴, with Cas-1 inhibitor.
Recombinant murine IL-18 and IL-1β protect cas-1−/− mice from infection.
If the inability of cas-1−/− mice to produce IL-18 and IL-1β upon exposure to gram-negative bacteria (data not shown) were responsible for their greater susceptibility to bacterial infection, administration of exogenous rmIL-18 and/or rmIL-1β would restore their resistance to infection. In preliminary experiments, cas-1−/− mice were treated with different concentrations of rmIL-18 and 3 h later, the circulating IL-18 levels were determined. Administration of 10 ng of rmIL-18/mouse produced IL-18 concentrations in serum similar to those observed in mice receiving 75 μg of LPS (data not shown). Similarly, our previous studies showed that 500 U of rmIL-1β conferred protection to C3H/HeJ mice against infection (5). Therefore, these doses of rmIL-18 and rmIL1β were used for reconstitution of antibacterial defense in cas-1−/− mice. At 3 h after cytokine administration, mice were challenged with three times the LD50 of E. coli (∼12,000 CFU/mouse) and then observed for mortality. The administration of 500 U of IL-1β completely protected most of the cas-1−/− mice from E. coli infection (one of seven died), whereas 250 U did not (data not shown). Similarly, treatment with 10 ng of IL-18 also protected cas-1−/− mice from E. coli infection (none of three died) (Table 2). Thus, the administration of both products of Cas-1, IL-1β, or IL-18 alone protected mice against lethal bacterial infection.
TABLE 2.
Effects of exogenous IL-1β and IL-18 on antibacterial host defense in cas-1−/− micea
| Treatment | Dose | No. of mice that died/total no. (% mortality) |
|---|---|---|
| Salinec | 6/6 (100) | |
| IL-1βd | 500 IU | 1/7 (14)b |
| IL-18d | 10 ng | 0/3 (0)b |
cas-1−/− mice were treated with recombinant mIL-1β (500 IU/mouse) or mIL-18 (10 ng/ml) 2 h prior to infecting them with 12,000 CFU of E. coli (Bort) per mouse and observed for mortality.
Significantly different from saline-treated cas-1−/− mice: P = 0.0047 for IL-1β treatment and P = 0.0119 for IL-18 treatment by Fisher exact test.
Two of two cas-1−/− mice pretreated with heat-inactivated IL-18 prior to infection with 12,000 CFU of E. coli died by 48 h.
IL-18 and IL-1β contained <1 ng of endotoxin/μg as measured by Limulus amebocyte lysate assay (Associates of Cape Cod, Falmouth, Mass.).
LPS-induced lethality is associated with high levels of IL-18.
The data presented above suggest that Cas-1 activity is essential for ABHD and in its absence, Cas-1 products, IL-18 and IL-1β, complemented the requirement of Cas-1 for ABHD. Earlier, we showed that high IL-18 levels in serum stimulated cas-1 mRNA expression and were associated with lethal endotoxemia (17a). Therefore, we wanted to examine the role of cas-1 expression in the outcome of innate immune responses to gram-negative bacteria and/or LPS. Nonlethal (75 μg) dose of LPS induced the expression of IL-1β, IL-18, and IFN-γ transiently, whereas LPS-induced lethality was associated with a sustained induction of high levels of these cytokines (Fig. 2). The differences between these nonlethal and lethal immune responses may provide insights into the immune dysregulation observed during lethal shock.
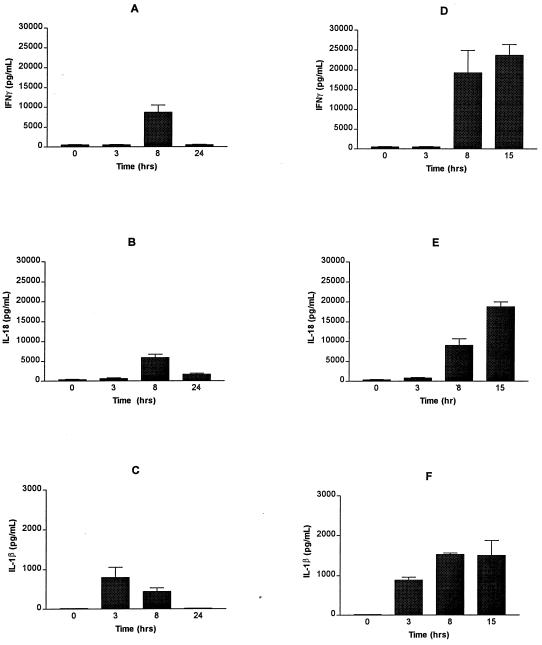
FIG. 2.
Cytokine levels in serum in LPS-treated C3H/HeN mice. C3H/HeN mice were treated with 75 μg (nonlethal dose [A, B, and C]) and 500 μg (lethal dose [D, E, and F]) of LPS i.p., and the IFN-γ (A and D), IL-18 (B and E), and IL-1β (C and F) levels in serum were determined at 3, 8, and 24 h after LPS treatment (75 μg) and 3, 8, and 20 h after LPS treatment (500 μg) from freshly drawn blood (P < 0.0001 by two-way ANOVA analysis). Data represent the means ± the standard errors (SE) of three mice per time point. Mice treated with 500 μg of LPS usually died between 20 and 24 h; therefore, cytokine levels in serum were determined at 20 h and not at 24 h (B, D, and F).
The expression of IFN-γ stimulated by 75 and 500 μg of LPS was compared. No IFN-γ was detected for up to 3 h after LPS administration at either dose. At 8 h after LPS treatment, IFN-γ levels increased to 8.7 ± 1.8 ng/ml (75 μg of LPS) and 19.3 ± 5.6 ng/ml (500 μg). However, by 20 h, when lethally endotoxic mice were generally moribund, no IFN-γ was detected in mice treated with 75 μg of LPS, whereas 23.7 ± 2.7 ng of IFN-γ/ml was present in 500 μg of LPS-treated mice (P < 0.0001 by two-way analysis of variance [ANOVA] analysis) (Fig. 2A and D).
Since IFN-γ synthesis is induced by IL-18, we next measured the IL-18 levels in these mice. Similar changes in IL-18 levels were induced by nonlethal and lethal doses of LPS (Fig. 2B and E), as were seen with IFN-γ, suggesting that the IL-18 levels measured with this ELISA detected biologically active IL-18. IL-18 synthesis started soon after LPS treatment, increased up to 5.86 ± 0.91 ng/ml at 8 h and decreased to 1.7 ± 0.2 ng/ml by 24 h in mice treated with 75 μg of LPS (Fig. 2B). In contrast, in mice treated with 500 μg of LPS, IL-18 levels increased to 9.1 ± 1.6 ng/ml at 8 h and continued to increase until death (Fig. 2E). Serum from moribund mice (ca. 20 h post-LPS) contained 18.8 ± 1.2 ng of IL-18/ml (P < 0.0001 by two-way ANOVA analysis of IL-18 levels in mice treated with 75 and 500 μg of LPS for 24 h.).
Expression of bioactive IL-18 requires proteolytic cleavage of its precursor form by Cas-1, which also catalyzes proteolytic maturation of IL-1β (13). Therefore, the IL-1β levels in serum were also measured in these mice. Figure 1C shows that IL-1β levels in serum increased after treatment with 75 μg of LPS to 0.8 ± 0.25 ng/ml at 3 h, decreased to 0.45 ± 0.09 ng/ml at 8 h, and returned to basal levels at 24 h. In contrast, in mice given lethal doses, IL-1β levels in serum increased to 0.88 ± 0.07 ng/ml at 3 h and 1.53 ± 0.04 ng/ml at 8 h and persisted at that level till death (Fig. 2C and F). Thus, unlike the nonlethal dose, lethal doses of LPS induced a sustained increase of Cas-1 products, IL-1β, and IL-18.
Expression of IL-18 protein but not mRNA is affected in LPS-treated mice.
IL-18 is usually expressed as a precursor polypeptide present in the cytoplasmic pool, which, upon cleavage by Cas-1, becomes biologically active IL-18 (13). Increased transcription of the IL-18 gene may not be essential after nonlethal doses of LPS. However, the continuous synthesis of IL-18 triggered by lethal doses of LPS may demand a larger pool of IL-18 message. RT-PCR analysis of the total RNA prepared from liver and spleen tissue of mice treated with nonlethal and lethal doses of LPS revealed no significant differences in the expression of IL-18 mRNA in response to nonlethal or lethal doses of LPS (Fig. 3A and B). This is in agreement with the report that IL-18 mRNA is constitutively expressed and lacks the commonly observed RNA-destabilizing features of other cytokine mRNAs resulting in high constitutive levels of inactive pro-IL-18 precursor protein (7).
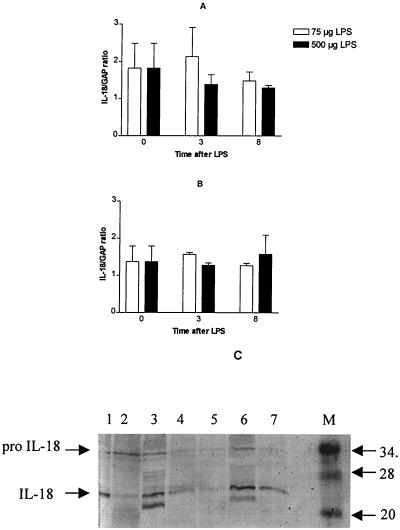
FIG. 3.
Expression of IL-18 mRNA and protein in LPS-treated mice. Total RNA prepared from spleen and liver tissue isolated from C3H/HeN mice before and at 3 and 8 h after administration of 75 or 500 μg of LPS was analyzed by RT-PCR for IL-18 and GAPDH mRNA expression. The levels of IL-18 mRNA in the livers (A) and spleens (B) of mice treated with 75 μg (□) and 500 μg (▪) were plotted as a ratio of IL-18 mRNA to GAPDH mRNA at the time points tested. Each point represents the mean ± SE of results from three mice. (C) Equal amounts of protein from extracts of LPS-treated mouse spleens isolated at different time points were separated on 6 to 14% gradient SDS-polyacrylamide, transferred to Immobilon-P, and immunostained with anti-IL-18 antibody (1:500). Lane 1, zero hours; lanes 2, 3, and 4, at 3, 8, and 15 h after 75 μg of LPS treatment, respectively; and lanes 5, 6, and 7, at 3, 8, and 15 h after 500 μg of LPS treatment, respectively.
Since IL-18 is usually stored in cells in the form of inactive precursor protein (pro-IL-18), we examined whether the differences in LPS-induced IL-18 levels were due to variations in the processing of IL-18 precursor protein (pro-IL-18). Tissue extracts prepared from the livers and spleens of LPS-treated mice were tested for the presence of precursor and mature forms of IL-18 by Western blot analysis. The IL-18 protein contents of spleen extracts of mice treated with 75 and 500 μg of LPS were different (Fig. 3C). In mice treated with 75 μg of LPS, splenic IL-18 levels increased until 8 h but decreased to less than the basal level (lane 1) by 15 h after LPS treatment (Fig. 3C, lanes 2, 3, and 4). In contrast, 500 μg of LPS treatment stimulated greater levels of IL-18 and its precursor at 8 h (Fig. 3C, lane 6), which remained higher than those observed in the spleens of mice treated with 75 μg of LPS at 15 h (Fig. 3, lane 7 versus lane 4). Furthermore, less IL-18 precursor was detected at 3 h in spleen extracts from mice treated with 500 μg of LPS compared to those treated with 75 μg of LPS (Fig. 3, lane 5 versus lane 2). This result suggested that differences in pro-IL-18 processing were responsible for the differences in IL-18 expression in LPS-treated mice. Mice treated with 500 μg of LPS were moribund by 15 h and died between 20 and 24 h. Therefore, no further samples were studied.
Cas-1 activity differs in mice treated with nonlethal and lethal doses of LPS.
Since LPS treatment did not increase the transcription of IL-18 gene (Fig. 3A and B) but induced different levels of IL-18 and its precursor protein in spleen (Fig. 3C), changes in Cas-1 activity may have been responsible for the differences in LPS-induced IL-18 expression. Cas-1 activity was therefore measured in spleen extracts prepared from LPS-treated mice. As shown in Fig. 4A, Cas-1 activity increased by 3 h after LPS treatment in the spleens of mice receiving both doses of LPS (75 and 500 μg). However, it decreased at 8 and 15 h after LPS treatment in the 75-μg group. In contrast, in mice treated with 500 μg of LPS, the Cas-1 activity increased by 3 h and remained elevated even at 8 and 15 h post-LPS (Fig. 4A). No significant differences in the Cas-1 activity were observed in the livers of mice treated with 75 and 500 μg of LPS (data not shown).
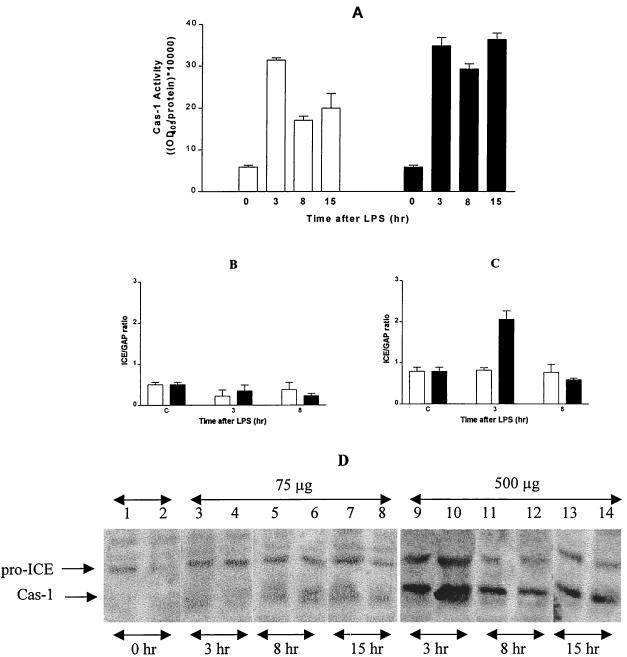
FIG. 4.
Effect of LPS on Cas-1 expression. Spleens and livers of C3H/HeN mice were isolated at 0, 3, and 8 h after treatment with 75 μg (□) and 500 μg (▪) of LPS. Cas-1 activity was determined in spleen extracts prepared by the method of Thornberry (35). Enzyme activity was calculated as follows: (maximum OD405/microgram of protein) × 10,000. Each point represents the mean ± SE of at least two animals. (A) Reactions with enzyme preparation alone and with enzyme plus substrate plus 10 μM Cas-1 inhibitor were used as controls. Total RNA was analyzed by RT-PCR for cas-1 and GAPDH mRNA levels, and the cas-1 mRNA levels in the livers (B) and spleens (C) of mice treated with 75 μg (□) and 500 μg (▪) of LPS were plotted as the ratio of Cas-1 to GAPDH. Spleen extracts containing equal amounts of protein were separated on an SDS-7.5% polyacrylamide gel, transferred to Immobilon-P membrane, and immunostained with polyclonal anti-Cas-1 antibody (1:1,000). Blot represents two separate mice per time point per treatment group. Lanes 1 and 2, zero hour; lanes 3 and 4, 75 μg of LPS at 3 h; lanes 5 and 6, 75 μg of LPS at 8 h; lanes 7 and 8, 75 μg of LPS at 15 h; lanes 9 and 10, 500 μg of LPS at 3 h; lanes 11 and 12, 500 μg of LPS at 8 h; lanes 13 and 14, 500 μg of LPS at 15 h. (D) The figure represents a single representative Western blot containing samples from the spleens of mice treated with 75 and 500 μg of LPS.
Upregulation of cas-1 mRNA and protein in lethally challenged mice.
The levels of cas-1 mRNA were estimated to determine whether transcriptional changes led to the differences in Cas-1 in LPS-treated mice. cas-1 mRNA levels in liver were unaffected by LPS at 3 h and 8 h after treatment with both doses (Fig. 4B). Similarly, cas-1 mRNA levels remained unaltered in the spleens of mice receiving 75 μg of LPS (Fig. 4C). In contrast, cas-1 mRNA expression doubled at 3 h but returned to basal levels by 8 h after treatment with 500 μg of LPS (Fig. 4C). Thus, at the lethal doses of LPS, new Cas-1 precursor expression might explain the sustained processing of pro-IL-18 into mature IL-18.
In agreement with the mRNA levels, moderate levels of mature and precursor forms of Cas-1 protein were detected in spleen extracts from mice treated with 75 μg of LPS (Fig. 4D, lanes 3 to 8, two mice per condition). In contrast, severalfold-higher levels of both these forms of Cas-1 were detected by 3 h in the spleen extracts of mice treated with 500 μg of LPS (Fig. 4D, lanes 9 and 10). Compared to the 3-h levels, these levels decreased at later time points; high levels of Cas-1 and pro-Cas-1 proteins persisted in the spleens of mice treated with 500 μg of LPS for up to 15 h, particularly compared to the nonlethal dose of LPS (Fig. 4D, lanes 11 to 14). These differences in the patterns of cas-1 expression and activity could contribute to the observed differences in IL-18 (and, ultimately, IFN-γ) levels in serum and may be responsible for different outcomes after LPS treatment.
Cas-1 inhibitor protects against LPS lethality.
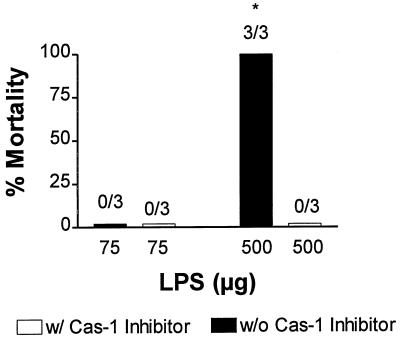
To determine whether the increased level of Cas-1 expression plays a role in lethal endotoxemia, we treated mice with Cas-1 inhibitor 2 h before and after administration of lethal and nonlethal doses of LPS. Figure 5 shows that treatment with 75 μg of LPS alone did not cause death (none of three died) in C3H/HeN mice; however, treatment with 500 μg of LPS caused the death of C3H/HeN mice in less than 24 h (three of three died). However, treatment with 10 mg of Cas-1 inhibitor (Ac-YVAD-CHO)/kg at 2 h before and after LPS treatment, protected all mice from endotoxic death (none of three died; P < 0.0001). This shows that Cas-1, a critical enzyme in ABHD, is also of pivotal importance in the lethal immune responses to LPS.
FIG. 5.
Cas-1 inhibition protects from lethal endotoxemia. C3H/HeN mice were challenged with nonlethal (75 μg) or lethal (500 μg) doses of LPS in the presence or absence of Cas-1 inhibitor (Ac-YVAD-CHO given at 10 mg/kg i.p., either 2 h before or after LPS treatment) and observed (for mortality) for the next 24 h. ✽, P < 0.0001 (Fisher exact test).
DISCUSSION
In the present study, we show that Cas-1 is critical to innate host defenses against E. coli and in its absence, Cas-1 products, IL-18 or IL-1β, restore ABHD. We further demonstrate that sustained activation of Cas-1, perhaps through mRNA upregulation, after exposure to a lethal dose but not a nonlethal dose of a microbial product, LPS, was associated with lethality. Thus, Cas-1 may represent one component of the innate immune response, whose level of expression is associated with the outcome after exposure to gram-negative bacteria or its LPS. The altered regulation of Cas-1 expression at lethal doses of LPS may contribute importantly to the development of sepsis, and the inhibition of Cas-1 activity may offer a strategy for the treatment of sepsis.
IL-18 was originally discovered as an IFN-γ-stimulating factor and has been shown to play an important role in antibacterial defense (3, 39). Another cytokine, IL-1β, also participates in murine antibacterial defenses (5) (20, 40). However, these cytokines have also been associated with endotoxin-induced liver injury and death (17, 28, 30, 33). Since their expression requires Cas-1-mediated maturation of their inactive precursor proteins, we hypothesized that understanding the expression of Cas-1 may help to explain the dual involvement of IL-18 and IL-1β in host defense and lethal endotoxemia.
We observed that cas-1−/− mice were three- to fourfold more sensitive to bacterial infection than wild-type controls and treatment with Cas-1 inhibitor, Ac-YVAD-CHO rendered wild-type mice susceptible to infection. These observations differ from those of Sansonetti et al., who showed that Shigella spp. utilize host Cas-1 for invasion (34). Here we show that Cas-1 is essential for defense against infections with E. coli. Our findings are in contrast to the report that Cas-1 deficiency does not affect host defense against primary infections with Candida albicans (26). The differences in these observations may represent basic differences between immune mechanisms involved in host defense against infections with prokaryotic and eukaryotic microbes.
Although more susceptible to bacterial infection, cas-1−/− mice did not appear to be as susceptible to infection with E. coli as LPS-hyporesponsive mice such as C3H/HeJ, which can die after infection with as few as 10 bacteria (4). This may be due to the role of other LPS-induced, Cas-1-independent cytokines in antibacterial defense. For example, IL-12 confers protection against yersiniosis in BALB/c mice (2, 26). Alternatively, other caspases and proteases may mediate the synthesis of IL-1β (1, 18) and IL-18 (1, 10, 14) in cas-1−/− mice and maintain basal antibacterial resistance (11, 26). In agreement with the previously reported involvement of IL-18 or IL-1β in ABHD (3, 39), exogenous IL-18 and IL-1β rendered cas-1−/− mice resistant to bacterial infection (Table 2).
Administration of excessive IL-18 to cas-1−/− mice, however, led to mortality after bacterial infection (data not shown). This was consistent with our earlier observation that the presence of insufficient or an excess amount (i.e., greater than an “optimal” level) of circulating IL-18 caused mortality after bacterial infection. Supra-optimal IL-18 levels induced cas-1 mRNA expression, thus initiating a positive regulatory loop, which led to the synthesis synthesis of even higher IL-18 levels (and mortality) after treatment with a high dose of LPS (17a). Although LPS presumably induced these levels by its effect on Cas-1 activity, we wanted to study the effect of Cas-1 upregulation on immune responses more directly.
We observed that the patterns of Cas-1 activity after the different doses of LPS paralleled that of IL-18 expression. Both lethal and nonlethal doses of LPS induced Cas-1 enzymatic activity at 3 h. However, Cas-1 activity decreased by 8 h after treatment with 75 μg of LPS, whereas it remained elevated for 15 h in animals given lethal doses. Thus, the ability to downregulate Cas-1 activity appears critical for a well-regulated response to LPS.
Cas-1 is usually expressed constitutively in an inactive (pro-Cas-1) form that requires proteolytic activation through Cas-11 (37). This posttranslational processing alone appeared responsible for increased Cas-1 activity in animals treated with 75 μg of LPS. In contrast, increased transcription, translation, and proteolytic activation of Cas-1 were all found to contribute to the sustained enhancement of Cas-1 expression and activity in mice treated with 500 μg of LPS.
The mechanism(s) responsible for this reduction in Cas-1 activity and consequently the levels of IL-1β, IL-18, and IFN-γ in serum after treatment with nonlethal doses of LPS may have been overwhelmed by the lethal dose of LPS. The increased cas-1 mRNA and protein expression observed in the spleens of mice given lethal doses of LPS at 3 h (Fig. 3C and D) was not reflected in a proportional increase in splenic Cas-1 enzyme activity (Fig. 3A). This low enzyme activity despite high level of protein expression may be due to the presence of endogenous inhibitors of Cas-1 activity such as nitric oxide (19) or the serpin proteinase inhibitor 9 (42). If true, this may suggest that in mice challenged with a lethal dose of LPS, the endogenous Cas-1 inhibitor(s) is exhausted, resulting in a late, unopposed Cas-1 activity. We are investigating this possibility further.
Sustained activity and protein levels of Cas-1 may be critical for the development of LPS-induced lethality. We showed that high levels of IL-18 in serum obtained after a lethal dose of LPS induced Cas-1 expression, thus initiating a novel positive regulatory feedback loop and resulting in synthesis of even greater levels of circulating IL-18 (17a). Cas-1 activation can inhibit apoptosis of neutrophils (38), thereby promoting neutrophil mediated-tissue injury, a process that may be important in lethal endotoxemia (28). Excess Cas-1 protein may also cause inflammatory injury through the deleterious accumulation of proinflammatory cytokines (IL-18, IL-1β, and consequently IFN-γ) (41). Indeed, Cas-1-deficient mice are resistant to LPS-induced shock (14, 21, 22)
In conclusion, we have shown that Cas-1 is a component of innate ABHD; however, excessive Cas-1 expression is accompanied by LPS lethality in the absence of infection. A better understanding of the mechanisms that regulate Cas-1 expression may provide early clues to the development of sepsis and suggest additional targets for therapeutic intervention.
Acknowledgments
This work was supported by National Institutes of Health grants AI40568 (to A.S.C.), CA71401, and CA78282 (to D.V.K.).
Editor: J. D. Clements
REFERENCES
- 1.Akita, K., T. Ohtsuki, Y. Nukada, T. Tanimoto, M. Namba, T. Okura, R. Takakura-Yamamoto, K. Torigoe, Y. Gu, M. S. Su, M. Fujii, M. Satoh-Itoh, K. Yamamoto, K. Kohno, M. Ikeda, and M. Kurimoto. 1997. Involvement of caspase-1 and caspase-3 in the production and processing of mature human interleukin 18 in monocytic THP.1 cells. J. Biol. Chem. 272:26595-26603. [DOI] [PubMed] [Google Scholar]
- 2.Bohn, E., and I. B. Autenrieth. 1996. IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-γ production in NK cells and CD4+ T cells. J. Immunol. 156:1458-1468. [PubMed] [Google Scholar]
- 3.Bohn, E., A. Sing, R. Zumbihl, C. Bielfeldt, H. Okamura, M. Kurimoto, J. Heesemann, and I. B. Autenrieth. 1998. IL-18 (IFN-γ-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J. Immunol. 160:299-307. [PubMed] [Google Scholar]
- 4.Cross, A., L. Asher, M. Seguin, L. Yuan, N. Kelly, C. Hammack, J. Sadoff, and P. Gemski, Jr. 1995. The importance of a lipopolysaccharide-initiated, cytokine-mediated host defense mechanism in mice against extraintestinally invasive Escherichia coli. J. Clin. Investig. 96:676-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross, A. S., J. C. Sadoff, N. Kelly, E. Bernton, and P. Gemski. 1989. Pretreatment with recombinant murine tumor necrosis factor alpha/cachectin and murine interleukin 1α protects mice from lethal bacterial infection. J. Exp. Med. 169:2021-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Boer, M. L., J. Hu, D. V. Kalvakolanu, J. D. Hasday, and A. S. Cross. 2001. IFN-γ inhibits lipopolysaccharide-induced interleukin-1β in primary murine macrophages via a Stat1-dependent pathway. J. Interferon Cytokine Res. 21:485-494. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello, C. A. 1999. Interleukin-18. Methods 19:121-132. [DOI] [PubMed] [Google Scholar]
- 8.Dybing, J. K., N. Walters, and D. W. Pascual. 1999. Role of endogenous interleukin-18 in resolving wild-type and attenuated Salmonella typhimurium infections. Infect. Immun. 67:6242-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckmann, L., and M. F. Kagnoff. 2001. Cytokines in host defense against Salmonella. Microbes Infect. 3:1191-1200. [DOI] [PubMed] [Google Scholar]
- 10.Fantuzzi, G., and C. A. Dinarello. 1999. Interleukin-18 and interleukin-1β: two cytokine substrates for ICE (caspase-1). J. Clin. Immunol. 19:1-11. [DOI] [PubMed] [Google Scholar]
- 11.Fantuzzi, G., A. J. Puren, M. W. Harding, D. J. Livingston, and C. A. Dinarello. 1998. Interleukin-18 regulation of interferon gamma production and cell proliferation as shown in interleukin-1β-converting enzyme (caspase-1)-deficient mice. Blood 91:2118-2125. [PubMed] [Google Scholar]
- 12.Foss, D. L., M. J. Zilliox, and M. P. Murtaugh. 2001. Bacterially induced activation of interleukin-18 in porcine intestinal mucosa. Vet. Immunol. Immunopathol. 78:263-277. [DOI] [PubMed] [Google Scholar]
- 13.Ghayur, T., S. Banerjee, M. Hugunin, D. Butler, L. Herzog, A. Carter, L. Quintal, L. Sekut, R. Talanian, M. Paskind, W. Wong, R. Kamen, D. Tracey, and H. Allen. 1997. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature 386:619-623. [DOI] [PubMed] [Google Scholar]
- 14.Gu, Y., K. Kuida, H. Tsutsui, G. Ku, K. Hsiao, M. A. Fleming, N. Hayashi, K. Higashino, H. Okamura, K. Nakanishi, M. Kurimoto, T. Tanimoto, R. A. Flavell, V. Sato, M. W. Harding, D. J. Livingston, and M. S. Su. 1997. Activation of interferon-gamma inducing factor mediated by interleukin-1β converting enzyme. Science 275:206-209. [DOI] [PubMed] [Google Scholar]
- 15.Heinzel, F. P., M. D. Sadick, B. J. Holaday, R. L. Coffman, and R. M. Locksley. 1989. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis: evidence for expansion of distinct helper T-cell subsets. J. Exp. Med. 169:59-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinzel, F. P., M. D. Sadick, S. S. Mutha, and R. M. Locksley. 1991. Production of interferon gamma, interleukin-2, interleukin-4, and interleukin-10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc. Natl. Acad. Sci. USA 88:7011-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochholzer, P., G. B. Lipford, H. Wagner, K. Pfeffer, and K. Heeg. 2000. Role of interleukin-18 (IL-18) during lethal shock: decreased lipopolysaccharide sensitivity but normal superantigen reaction in IL-18-deficient mice. Infect. Immun. 68:3502-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Joshi, V. D., J. D. Hasday, R. J. Hebel, and D. V. Kalvakolanu. 2002. Serum IL-18 levels determine outcome of innate immune response to LPS through an positive regulatory feedback loop with caspase-1. J. Immunol. 169:2536-2544. [DOI] [PubMed] [Google Scholar]
- 18.Kamens, J., M. Paskind, M. Hugunin, R. V. Talanian, H. Allen, D. Banach, N. Bump, M. Hackett, C. G. Johnston, P. Li, et al. 1995. Identification and characterization of ICH-2, a novel member of the interleukin-1β-converting enzyme family of cysteine proteases. J. Biol. Chem. 270:15250-15256. [DOI] [PubMed] [Google Scholar]
- 19.Kim, Y. M., R. V. Talanian, J. Li, and T. R. Billiar. 1998. Nitric oxide prevents IL-1β and IFN-γ-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1β-converting enzyme). J. Immunol. 161:4122-4128. [PubMed] [Google Scholar]
- 20.Labow, M., D. Shuster, M. Zetterstrom, P. Nunes, R. Terry, E. B. Cullinan, T. Bartfai, C. Solorzano, L. L. Moldawer, R. Chizzonite, and K. W. McIntyre. 1997. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J. Immunol. 159:2452-2461. [PubMed] [Google Scholar]
- 21.Li, P., H. Allen, S. Banerjee, S. Franklin, L. Herzog, C. Johnston, J. McDowell, M. Paskind, L. Rodman, J. Salfeld, et al. 1995. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80:401-411. [DOI] [PubMed] [Google Scholar]
- 22.Li, P., H. Allen, S. Banerjee, and T. Seshadri. 1997. Characterization of mice deficient in interleukin-1β converting enzyme. J. Cell Biochem. 64:27-32. [DOI] [PubMed] [Google Scholar]
- 23.Livingston, D. J. 1997. In vitro and in vivo studies of ICE inhibitors. J. Cell. Biochem. 64:19-26. [PubMed] [Google Scholar]
- 24.Mancilla, J., P. Garcia, and C. A. Dinarello. 1993. The interleukin-1 receptor antagonist can either reduce or enhance the lethality of Klebsiella pneumoniae sepsis in newborn rats. Infect. Immun. 61:926-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mastroeni, P., S. Clare, S. Khan, J. A. Harrison, C. E. Hormaeche, H. Okamura, M. Kurimoto, and G. Dougan. 1999. Interleukin-18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect. Immun. 67:478-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mencacci, A., A. Bacci, E. Cenci, C. Montagnoli, S. Fiorucci, A. Casagrande, R. A. Flavell, F. Bistoni, and L. Romani. 2000. Interleukin 18 restores defective Th1 immunity to Candida albicans in caspase 1-deficient mice. Infect. Immun. 68:5126-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neighbors, M., X. Xu, F. J. Barrat, S. R. Ruuls, T. Churakova, R. Debets, J. F. Bazan, R. A. Kastelein, J. S. Abrams, and A. O'Garra. 2001. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on interferon gamma production. J. Exp. Med. 194:343-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Netea, M. G., G. Fantuzzi, B. J. Kullberg, R. J. Stuyt, E. J. Pulido, R. C. McIntyre, Jr., L. A. Joosten, J. W. van der Meer, and C. A. Dinarello. 2000. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J. Immunol. 164:2644-2649. [DOI] [PubMed] [Google Scholar]
- 29.Ohlsson, K., P. Bjork, M. Bergenfeldt, R. Hageman, and R. C. Thompson. 1990. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature 348:550-552. [DOI] [PubMed] [Google Scholar]
- 30.Okamura, H., H. Tsutsi, T. Komatsu, M. Yutsudo, A. Hakura, T. Tanimoto, K. Torigoe, T. Okura, Y. Nukada, K. Hattori, et al. 1995. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 378:88-91. [DOI] [PubMed] [Google Scholar]
- 31.Pasquali, P., R. Adone, L. C. Gasbarre, C. Pistoia, and F. Ciuchini. 2002. Effect of exogenous interleukin-18 (IL-18) and IL-12 in the course of Brucella abortus 2308 infection in mice. Clin. Diagn. Lab. Immunol. 9:491-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rangel-Frausto, M. S. 1999. The epidemiology of bacterial sepsis. Infect. Dis. Clin. N. Am. 13:299-312. [DOI] [PubMed] [Google Scholar]
- 33.Sakao, Y., K. Takeda, H. Tsutsui, T. Kaisho, F. Nomura, H. Okamura, K. Nakanishi, and S. Akira. 1999. IL-18-deficient mice are resistant to endotoxin-induced liver injury but highly susceptible to endotoxin shock. Int. Immunol. 11:471-480. [DOI] [PubMed] [Google Scholar]
- 34.Sansonetti, P. J., A. Phalipon, J. Arondel, K. Thirumalai, S. Banerjee, S. Akira, K. Takeda, and A. Zychlinsky. 2000. Caspase-1 activation of IL-1β and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12:581-590. [DOI] [PubMed] [Google Scholar]
- 35.Thornberry, N. A. 1994. Interleukin-1β converting enzyme. Methods Enzymol. 244:615-631. [DOI] [PubMed] [Google Scholar]
- 36.Wakabayashi, G., J. A. Gelfand, J. F. Burke, R. C. Thompson, and C. A. Dinarello. 1991. A specific receptor antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits. FASEB J. 5:338-343. [DOI] [PubMed] [Google Scholar]
- 37.Wang, S., M. Miura, Y. K. Jung, H. Zhu, E. Li, and J. Yuan. 1998. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92:501-509. [DOI] [PubMed] [Google Scholar]
- 38.Watson, R. W., O. D. Rotstein, J. Parodo, R. Bitar, J. C. Marshall, R. William, and G. Watson. 1998. The IL-1β-converting enzyme (caspase-1) inhibits apoptosis of inflammatory neutrophils through activation of IL-1β. J. Immunol. 161:957-962. [PubMed] [Google Scholar]
- 39.Wei, X. Q., B. P. Leung, W. Niedbala, D. Piedrafita, G. J. Feng, M. Sweet, L. Dobbie, A. J. Smith, and F. Y. Liew. 1999. Altered immune responses and susceptibility to Leishmania major and Staphylococcus aureus infection in IL-18-deficient mice. J. Immunol. 163:2821-2828. [PubMed] [Google Scholar]
- 40.Yamada, H., S. Mizumo, R. Horai, Y. Iwakura, and I. Sugawara. 2000. Protective role of interleukin-1 in mycobacterial infection in IL-1 alpha/beta double-knockout mice. Lab. Investig. 80:759-767. [DOI] [PubMed] [Google Scholar]
- 41.Yamanaka, K., M. Tanaka, H. Tsutsui, T. S. Kupper, K. Asahi, H. Okamura, K. Nakanishi, M. Suzuki, N. Kayagaki, R. A. Black, D. K. Miller, K. Nakashima, M. Shimizu, and H. Mizutani. 2000. Skin-specific caspase-1-transgenic mice show cutaneous apoptosis and pre-endotoxin shock condition with a high serum level of IL-18. J. Immunol. 165:997-1003. [DOI] [PubMed] [Google Scholar]
- 42.Young, J. L., G. K. Sukhova, D. Foster, W. Kisiel, P. Libby, and U. Schonbeck. 2000. The serpin proteinase inhibitor 9 is an endogenous inhibitor of interleukin 1β-converting enzyme (caspase-1) activity in human vascular smooth muscle cells. J. Exp. Med. 191:1535-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]