Abstract
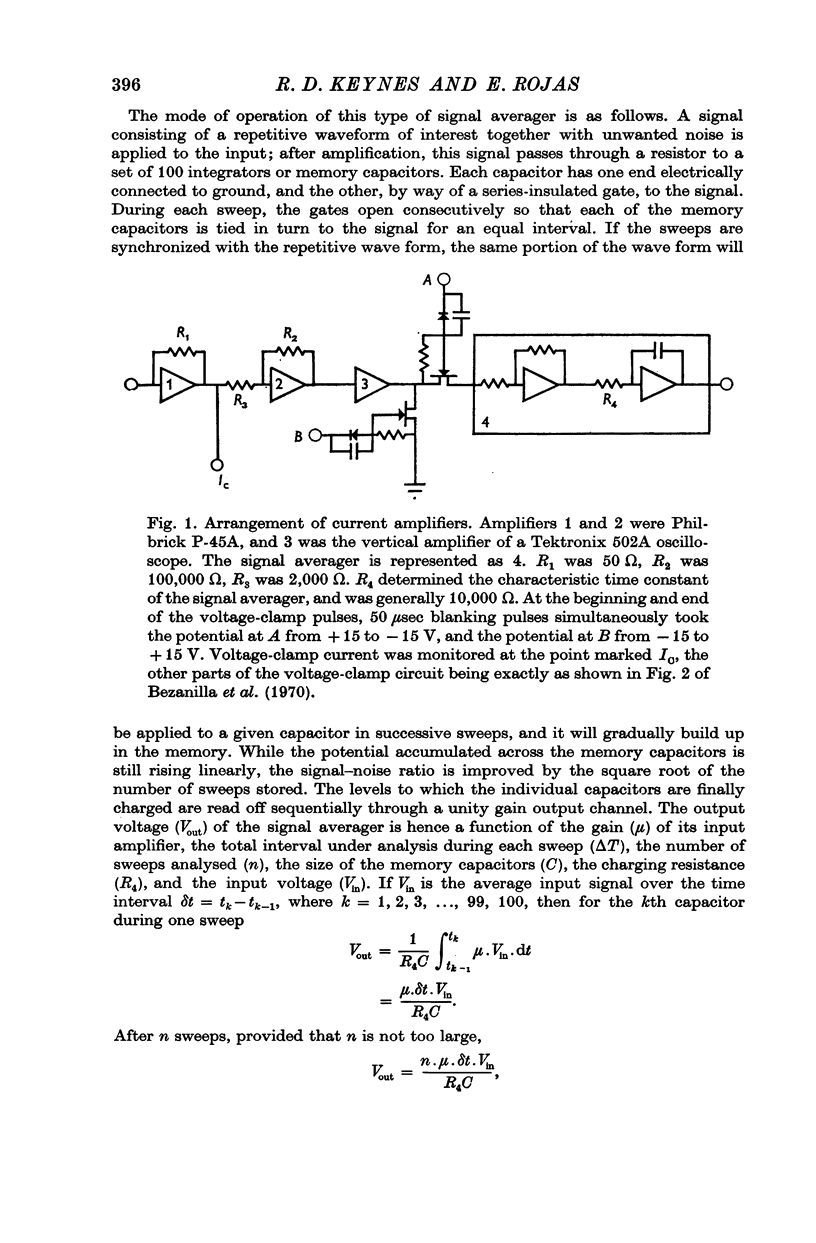
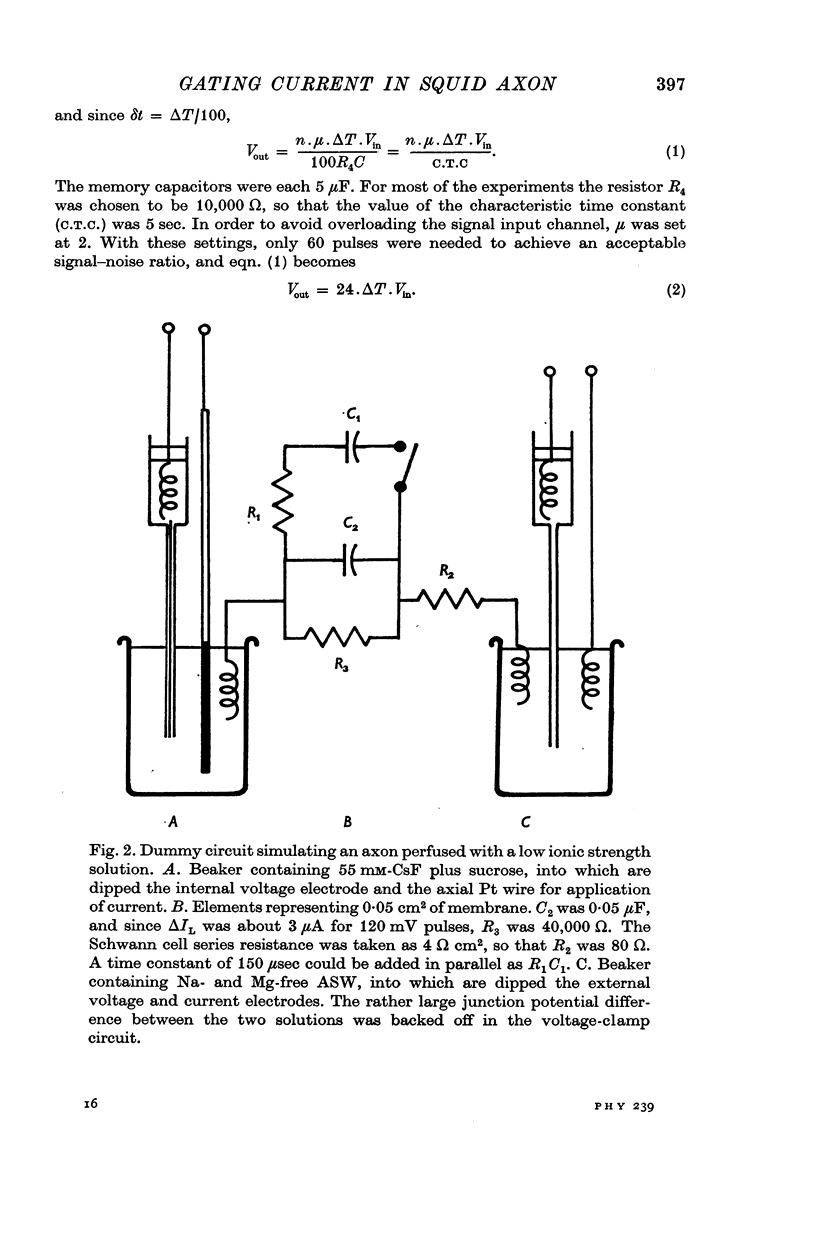
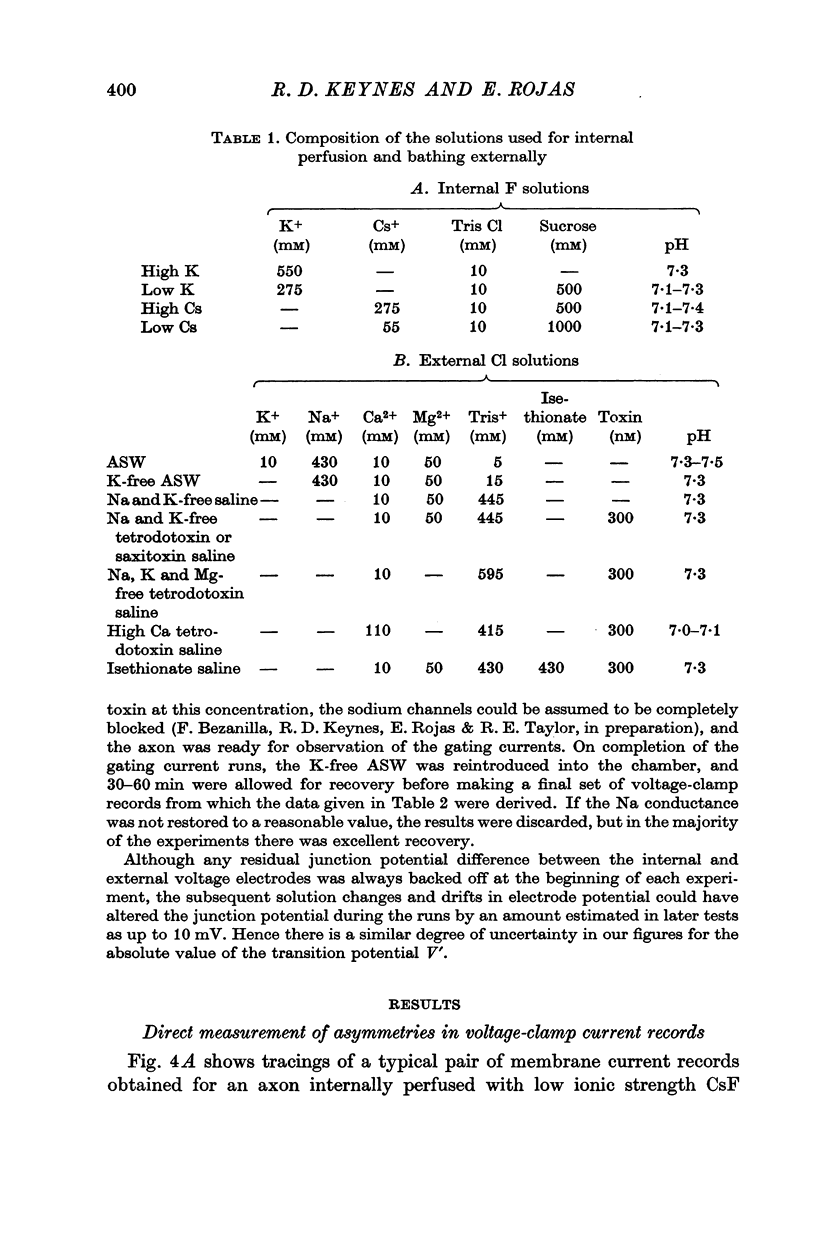
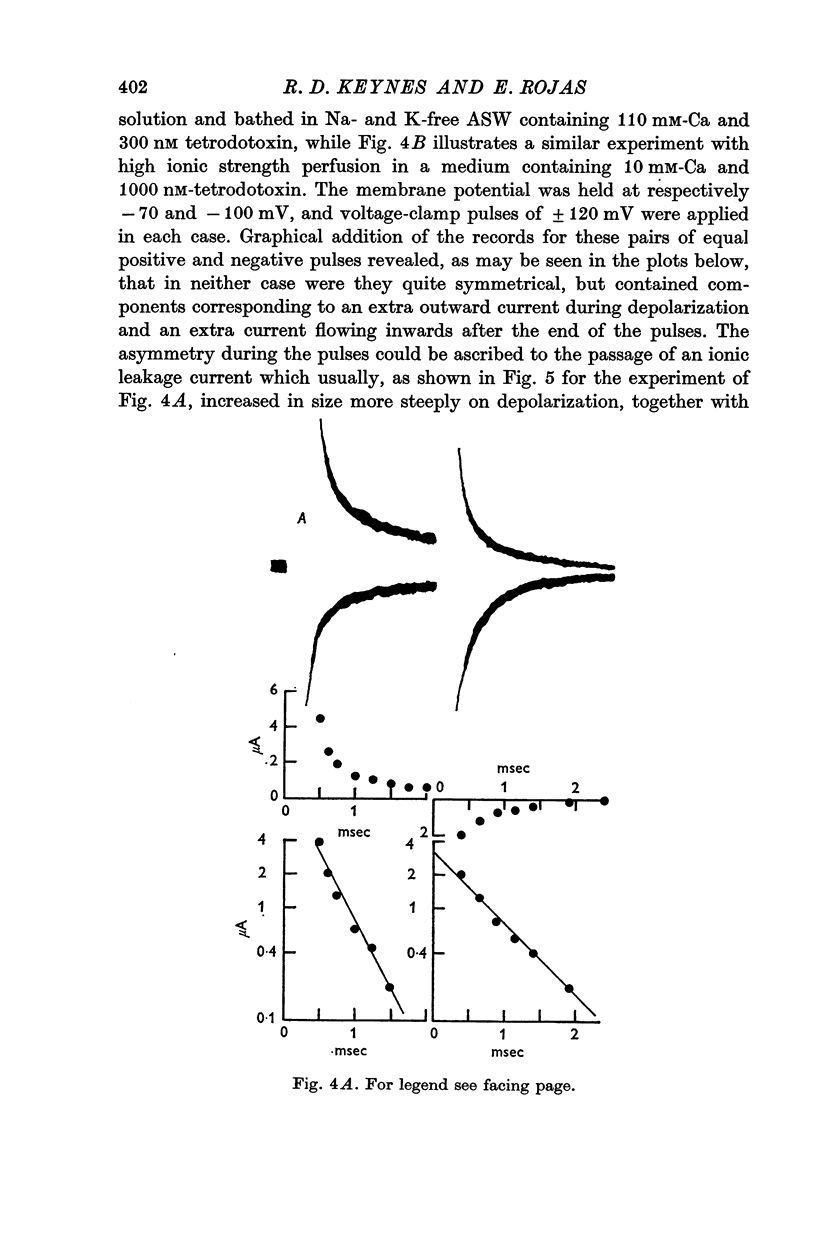
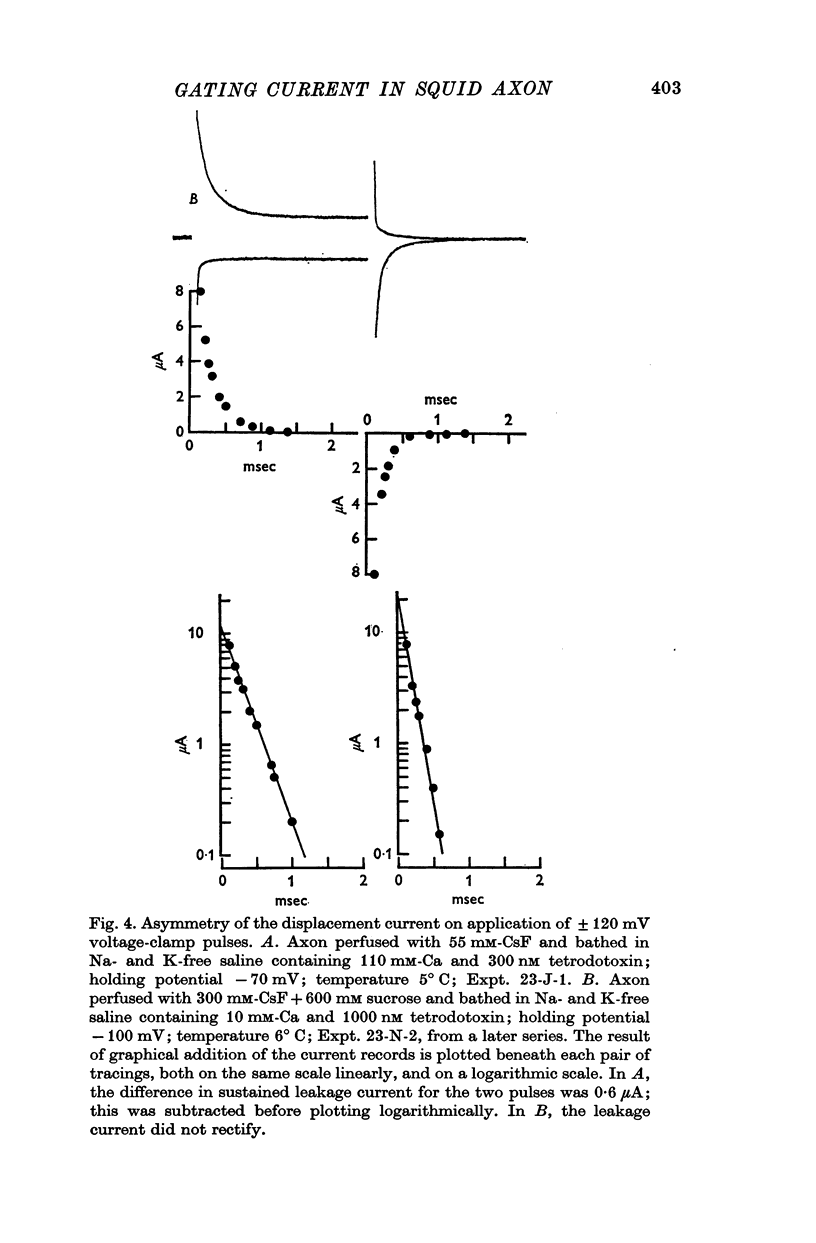
1. Asymmetries in the early time course of the displacement current passing across the membrane after application of equal voltage-clamp pulses in the two directions have been investigated in the squid giant axon. Before making the measurements, Na current was blocked by removal of external Na and treatment with tetrodotoxin. Potassium current was usually blocked by perfusion with CsF, but some experiments were done with intact axons. A signal averaging technique was used to eliminate the symmetrical components of the membrane current.
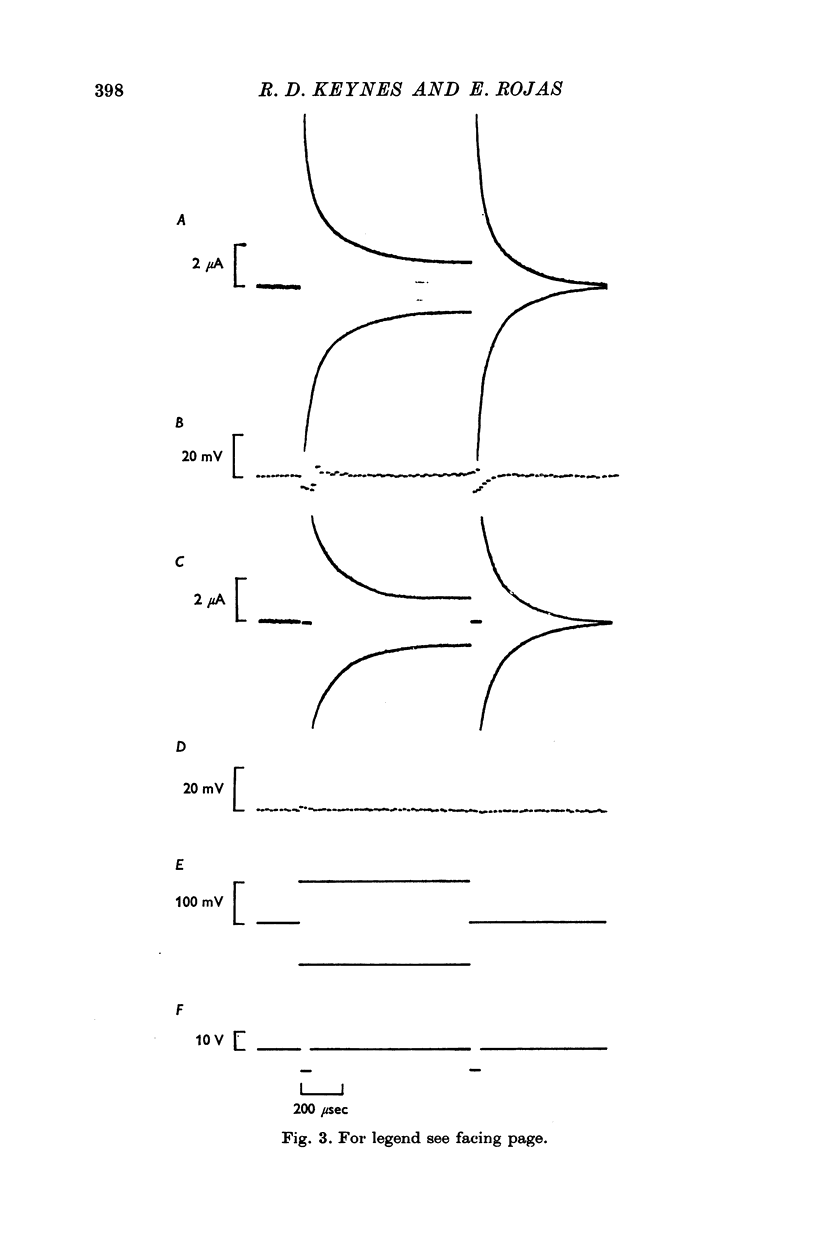
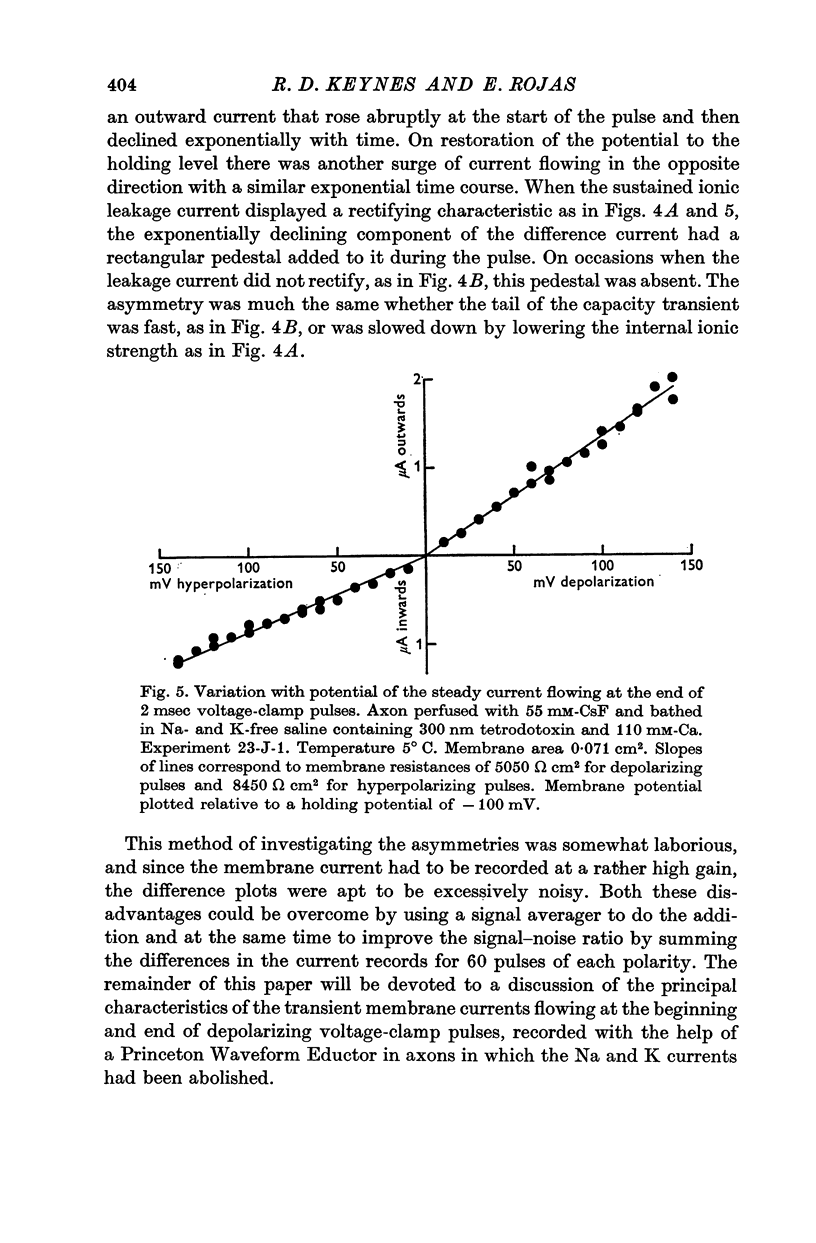
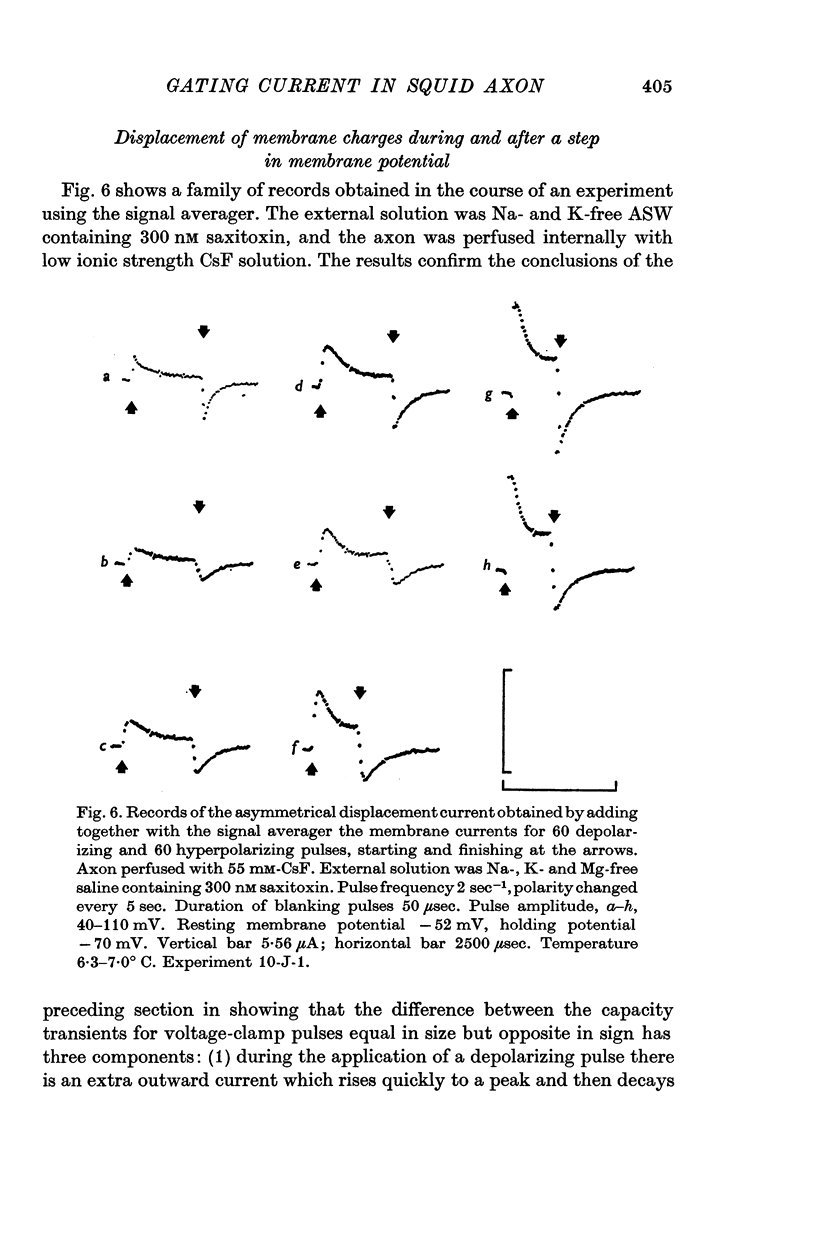
2. The asymmetrical current had a contribution of appreciable size attributed to the movement of mobile charges or dipoles in the membrane. This was manifested as an outward current rising rapidly to a peak on depolarization of the membrane and then declining exponentially to zero, followed at the end of the pulse by an inward surge of current with a similar time course. There was also a sustained flow of current outwards during the pulse, arising from ionic leakage with a rectifying characteristic.
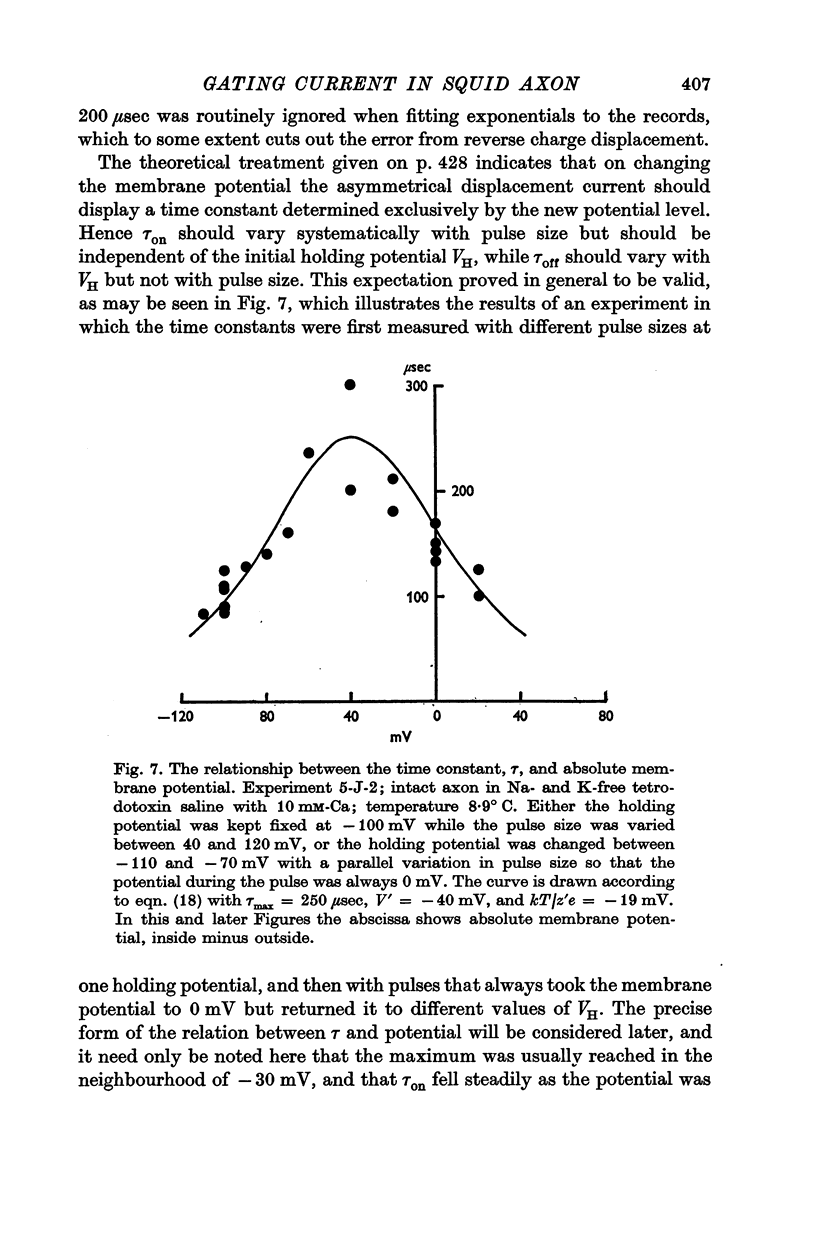
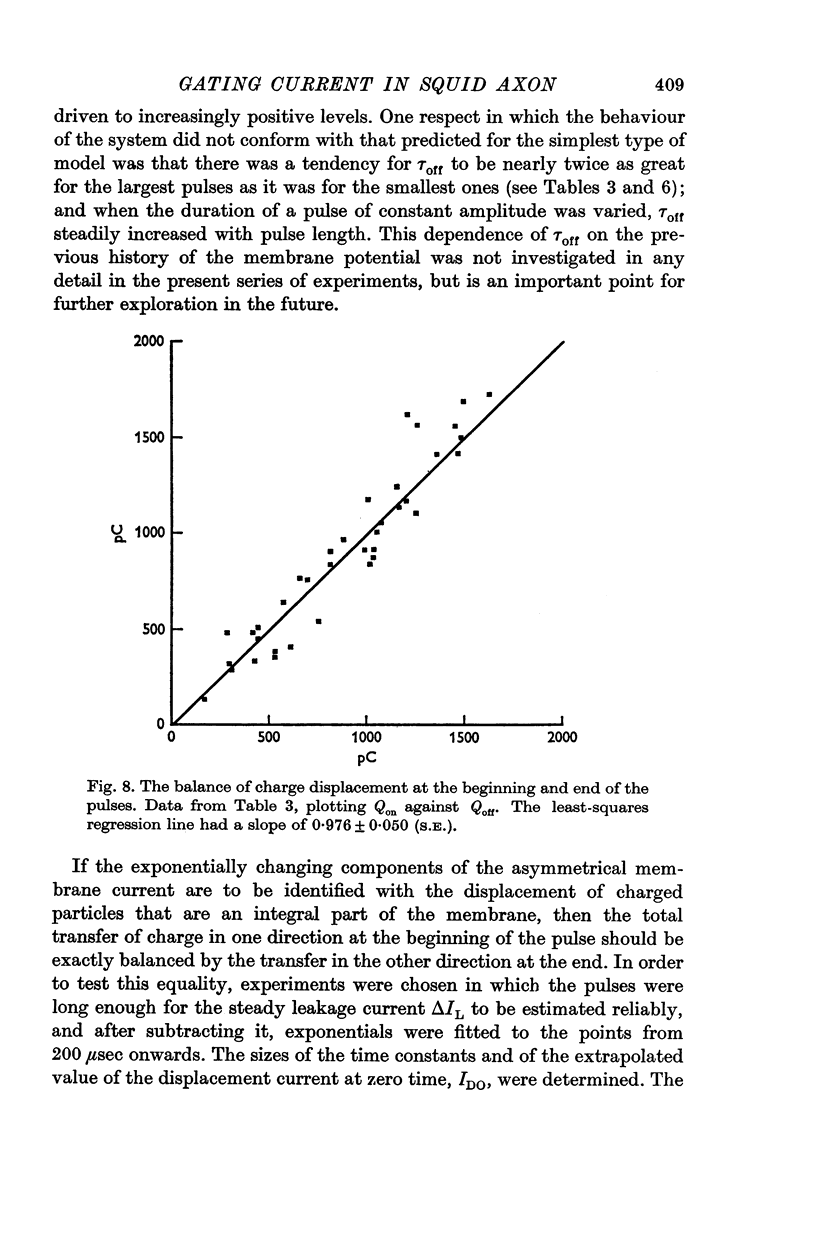
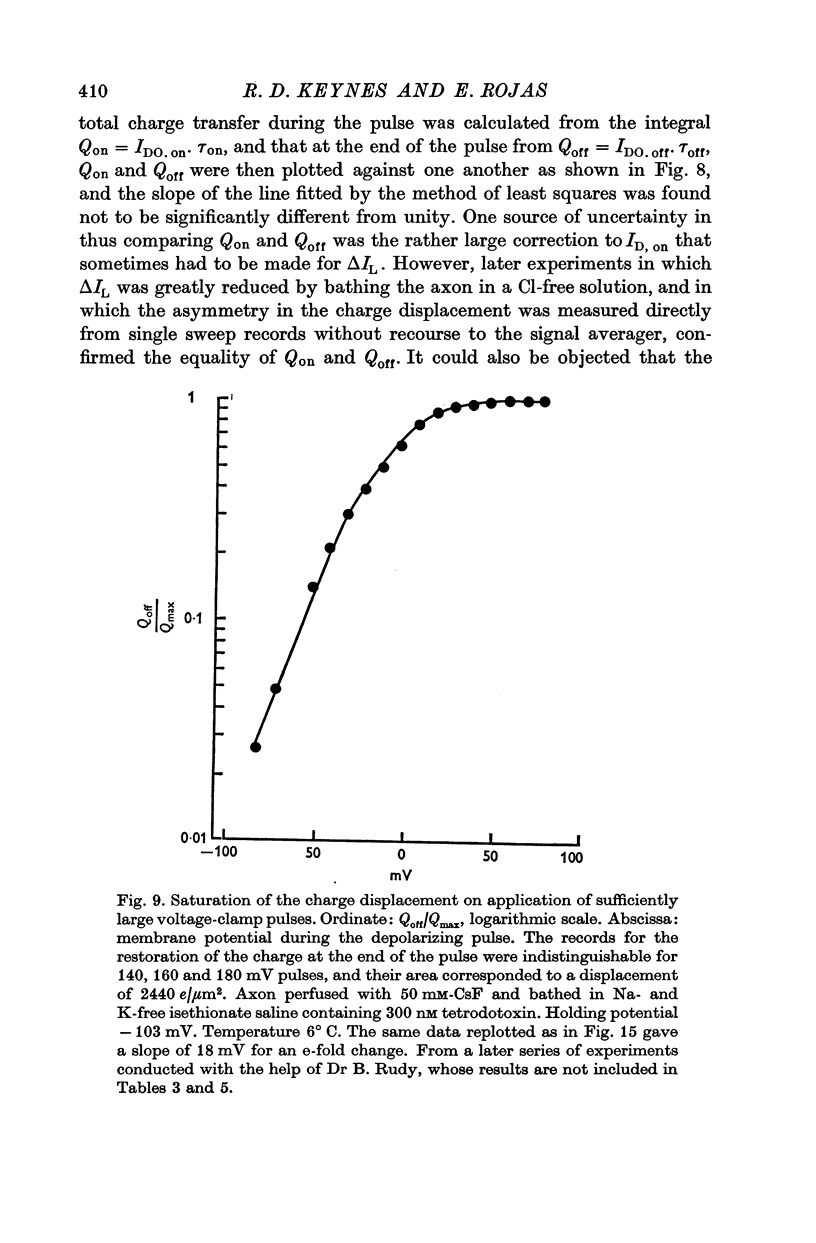
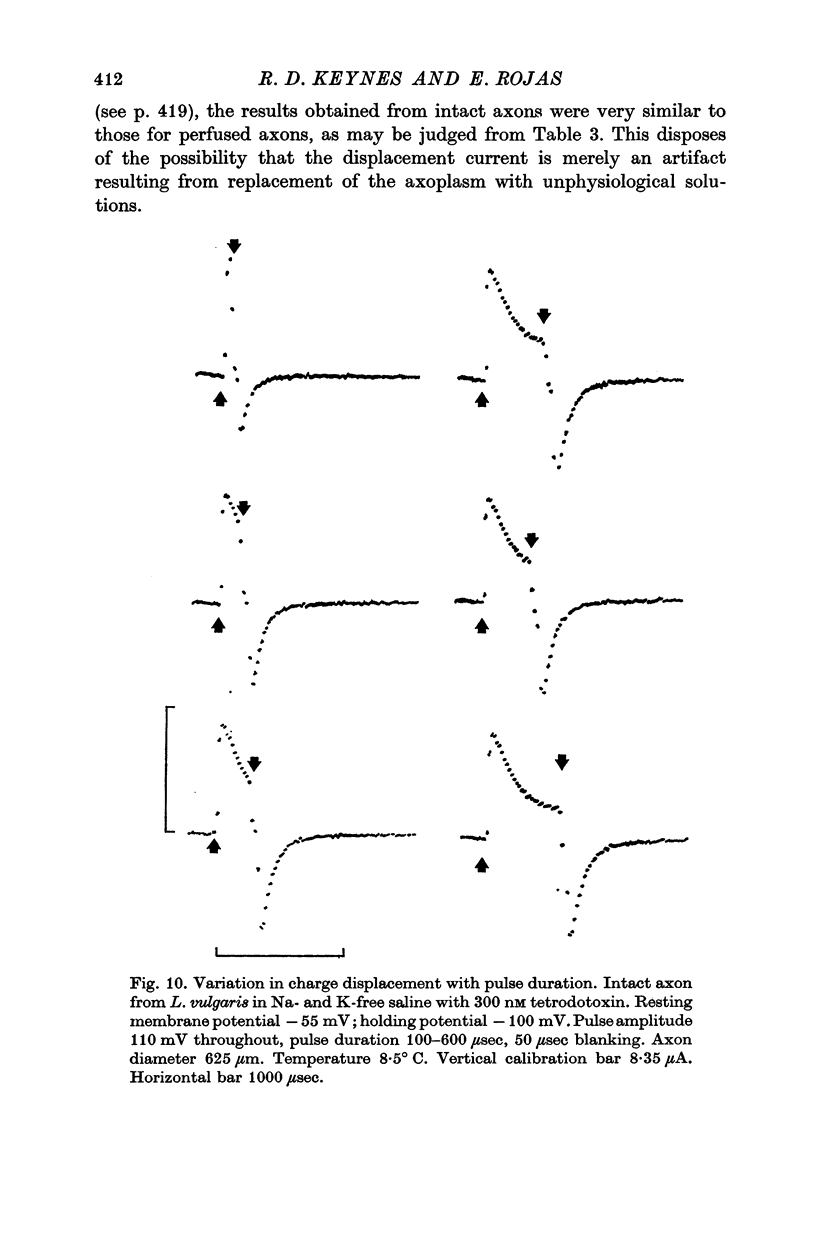
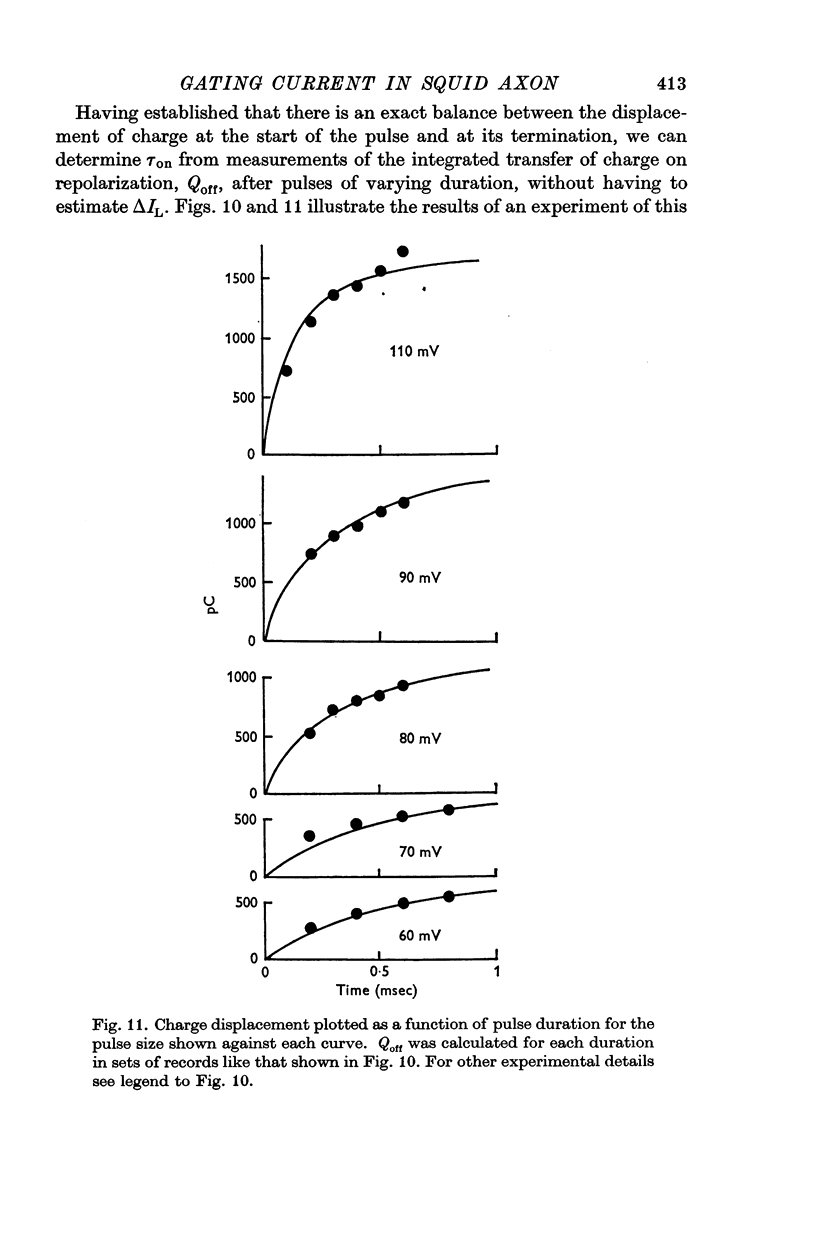
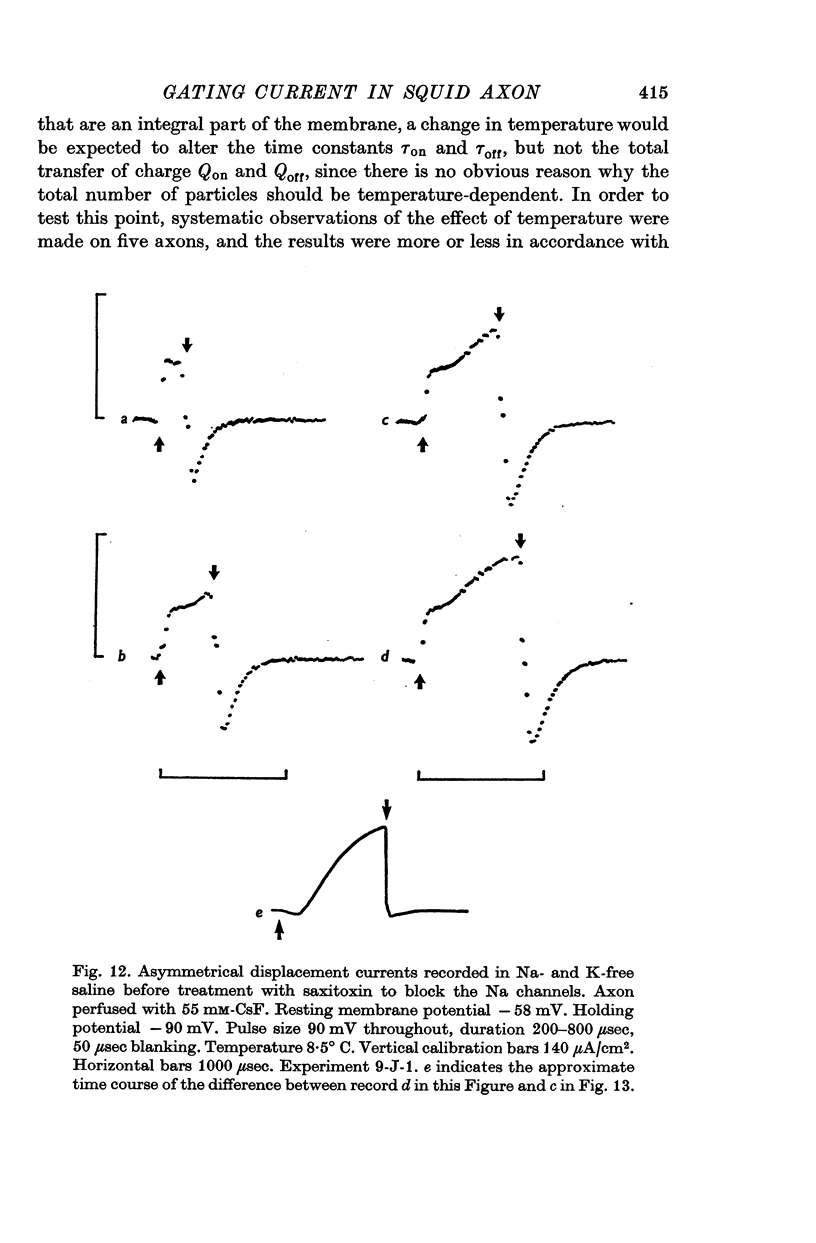
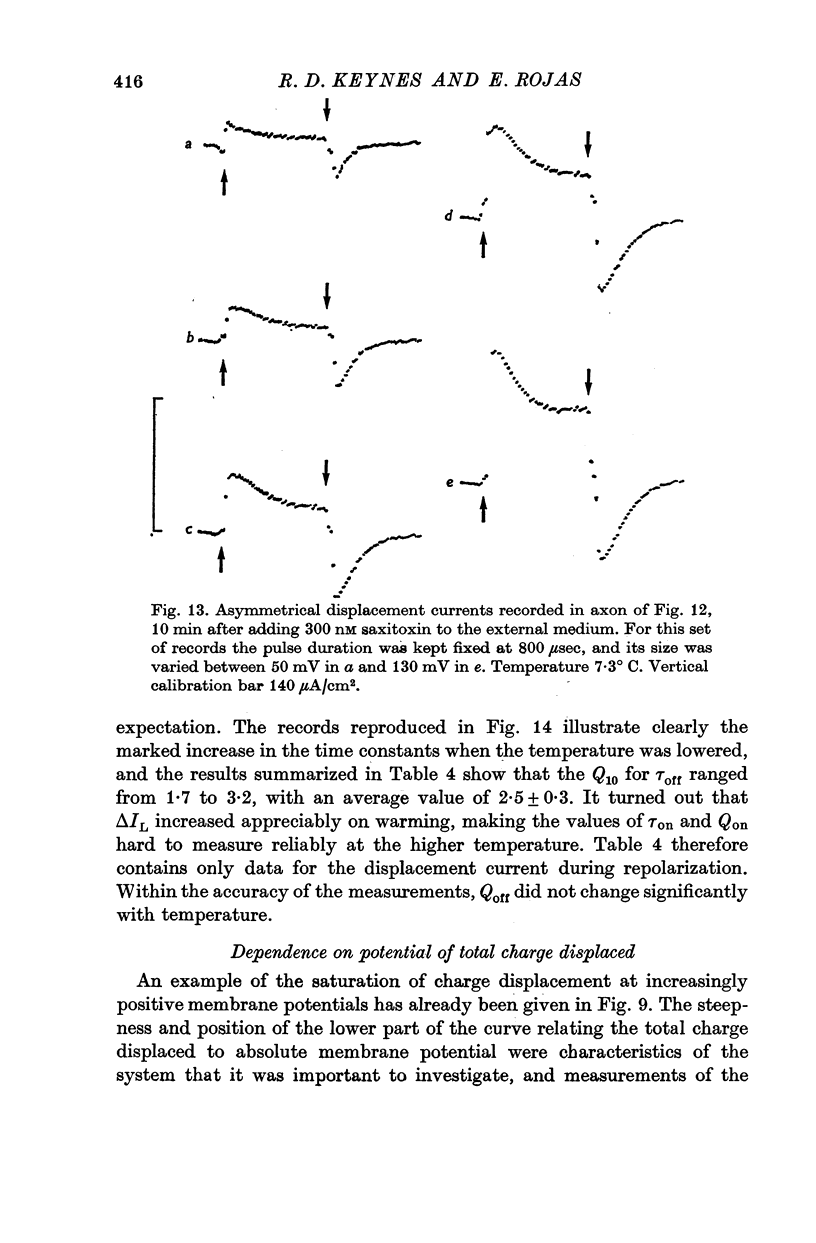
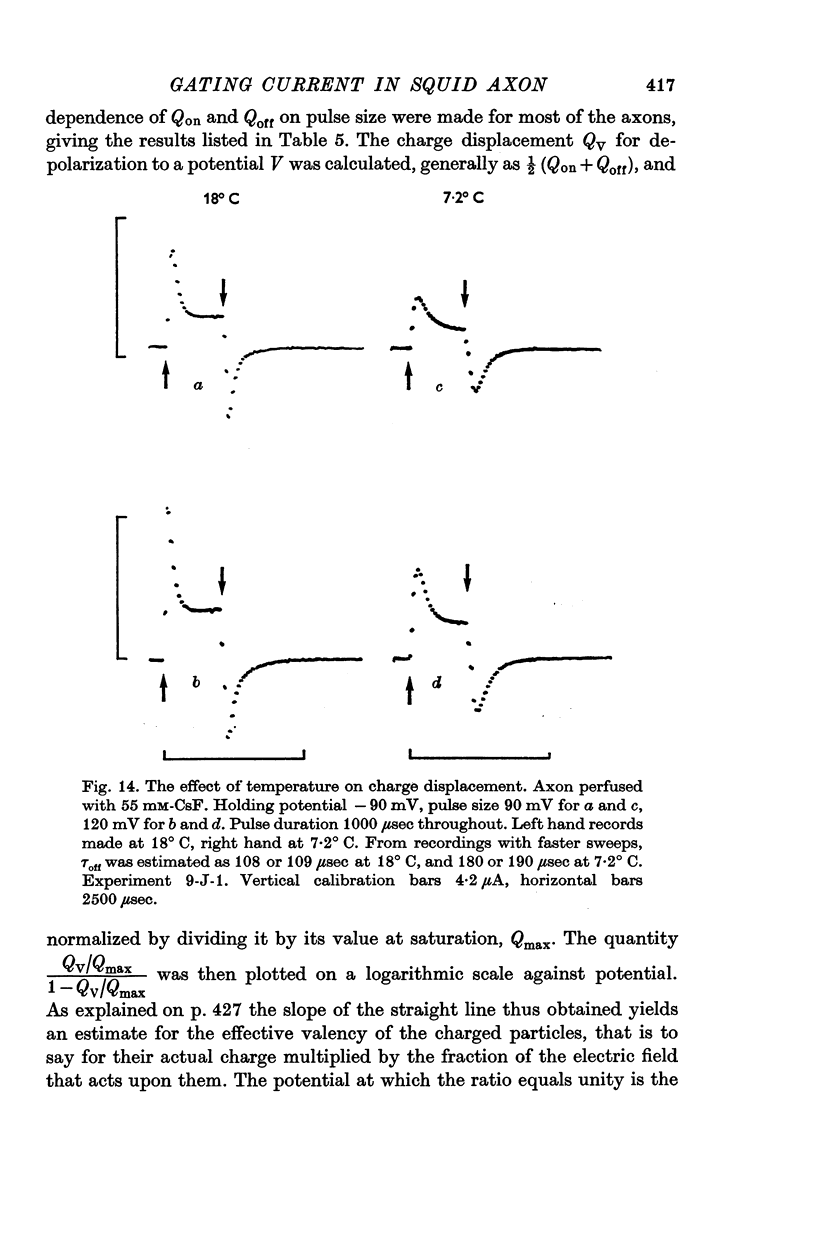
3. The identification of the exponentially changing current component with the displacement of charged particles forming an integral part of the membrane was supported by the demonstration that the total transfer of charge was equal and opposite at the beginning and end of the pulse, that it reached saturation when the internal potential was taken to a sufficient positive value, and that its size was unaffected by temperature, although its time constant had a large temperature coefficient.
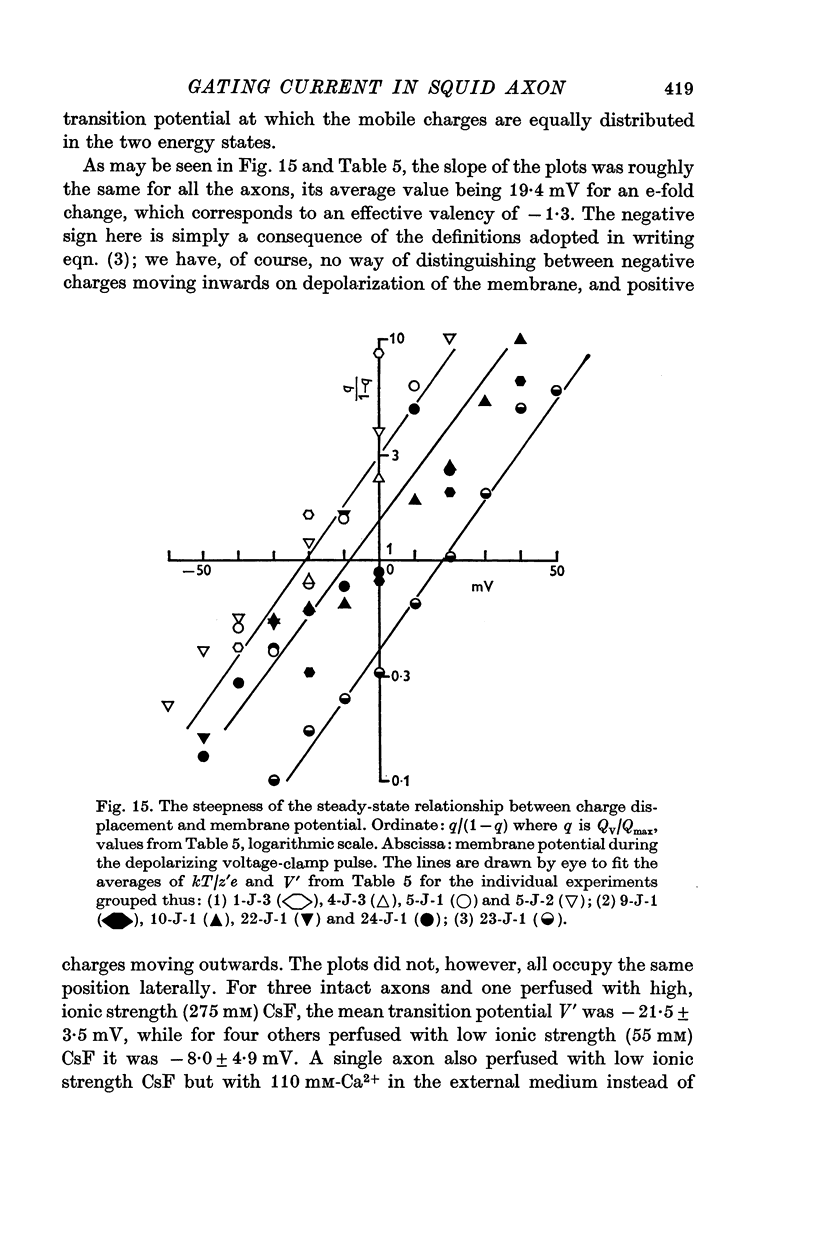
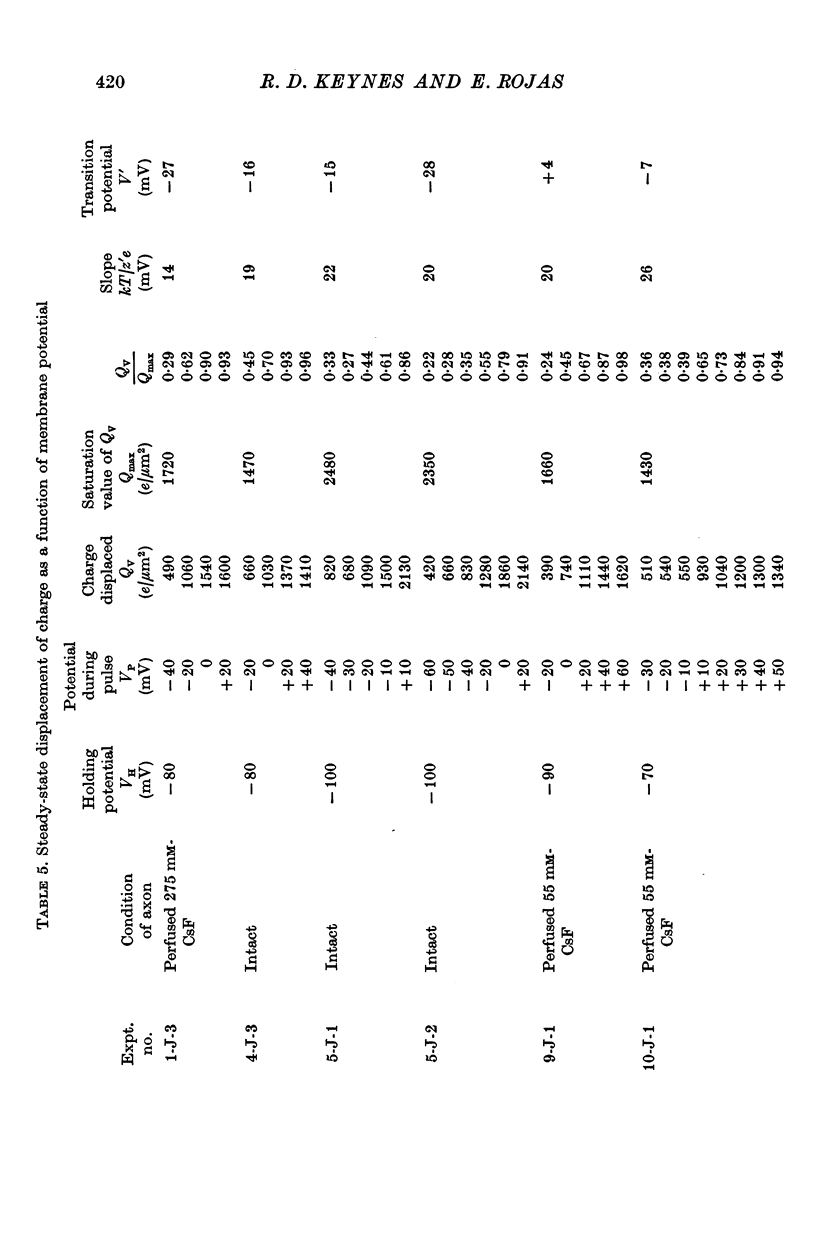
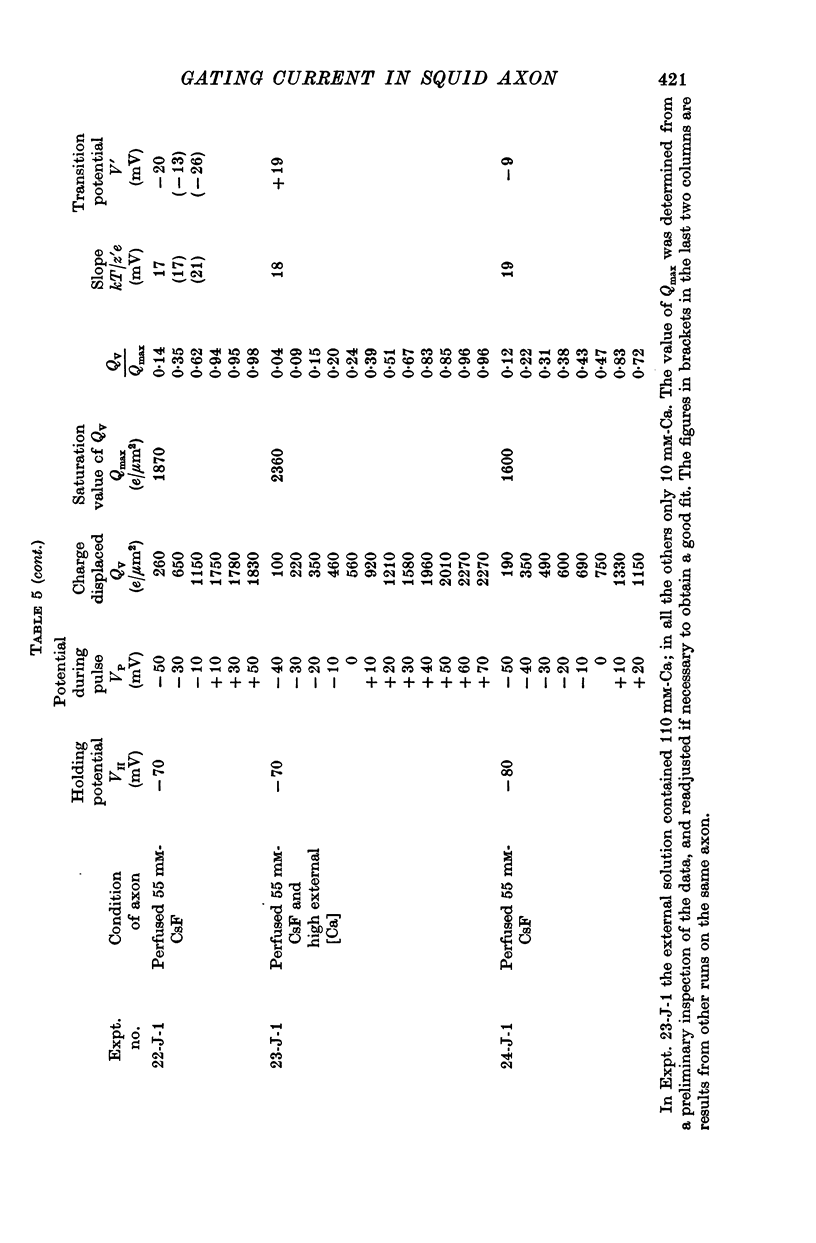
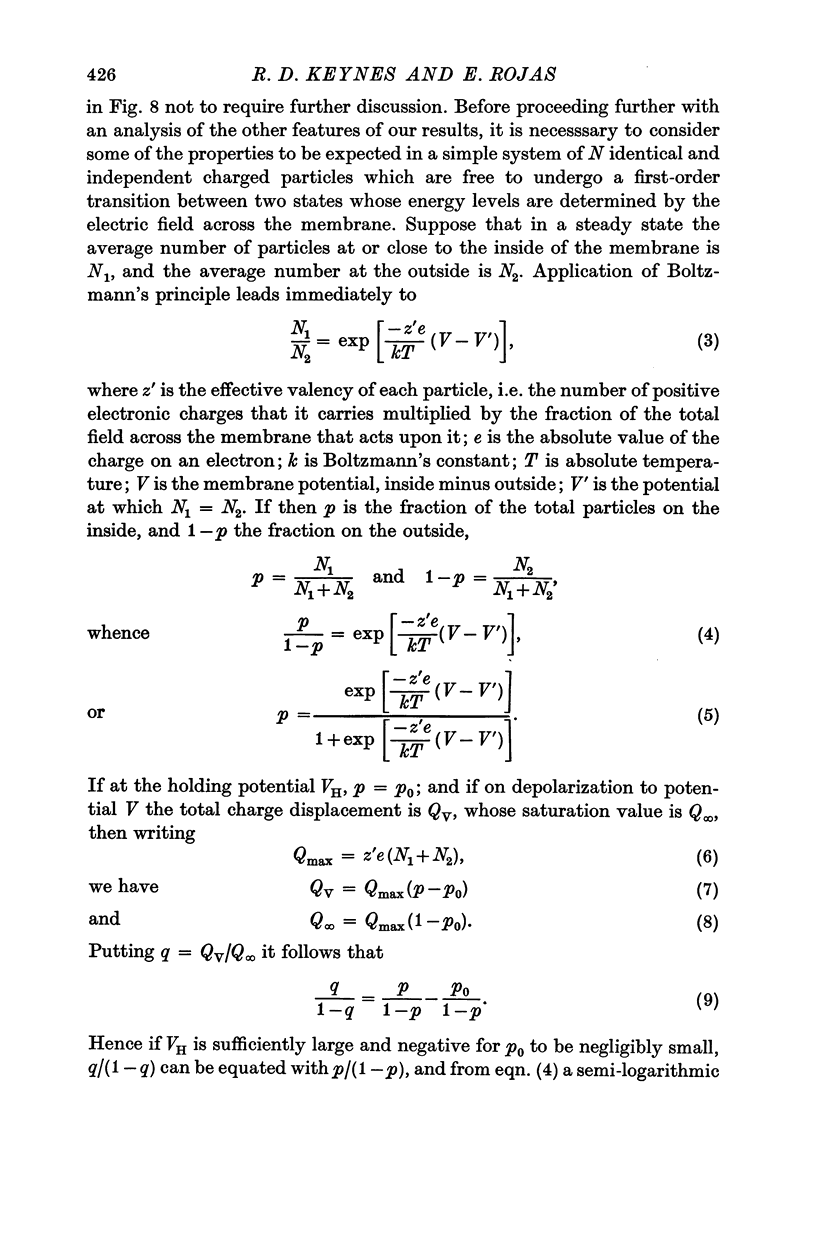
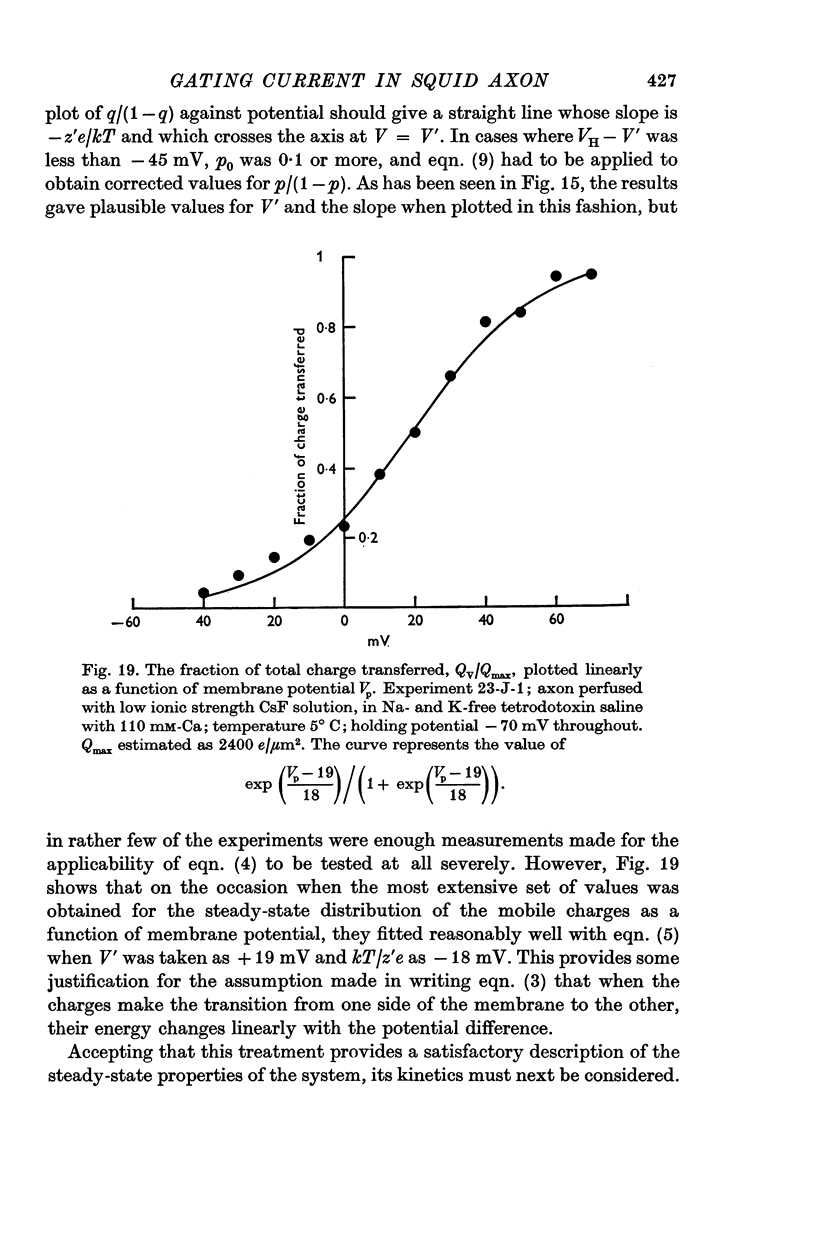
4. The disposition of the mobile charges in the steady state was shown to obey a Boltzmann distribution. At the midpoint of the distribution curve, the proportion of the charge displaced underwent an e-fold change for a 19 mV change in potential. The effective valency of the particles, that is their actual charge multiplied by the fraction of the electric field acting on them, was therefore 1·3.
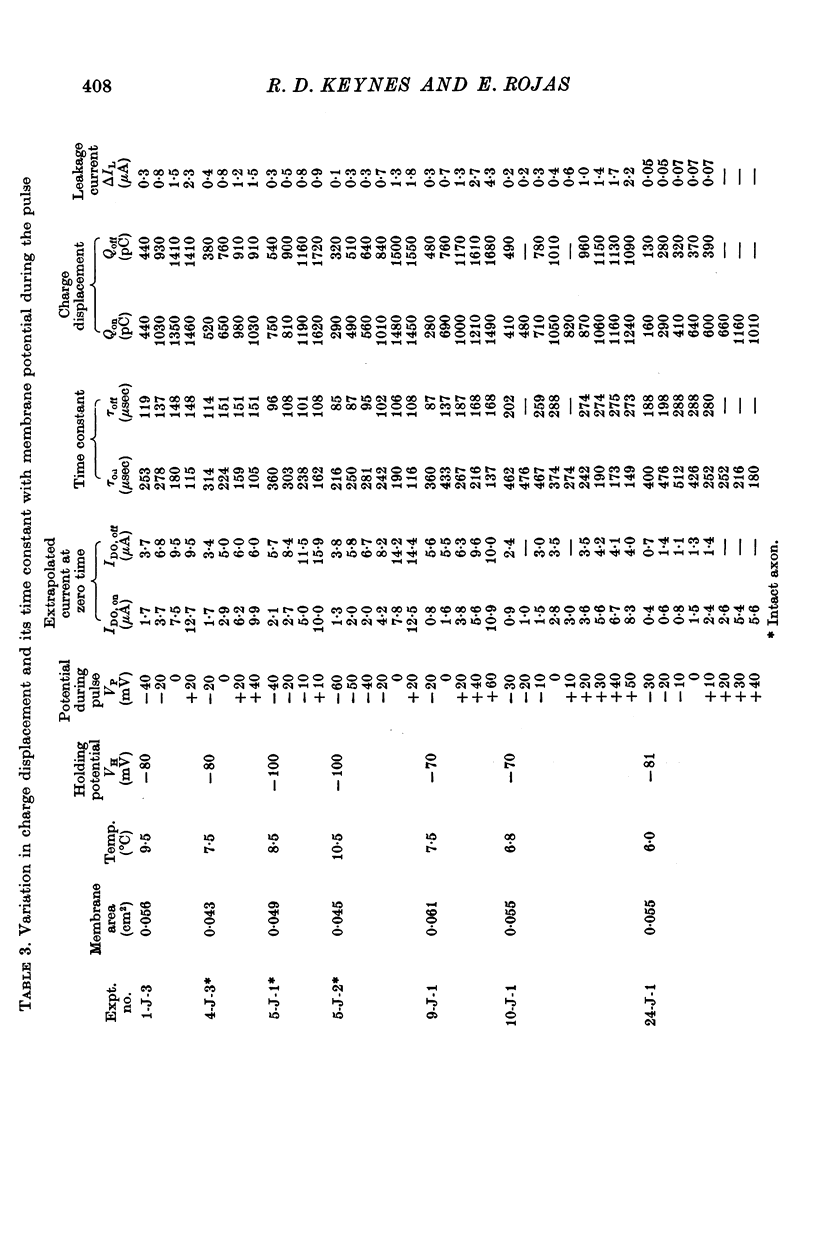
5. The total quantity of mobile charge was estimated as about 1500 × 10-12 C for 0·05 cm2 of membrane, corresponding to some 1900 charges/μm2.
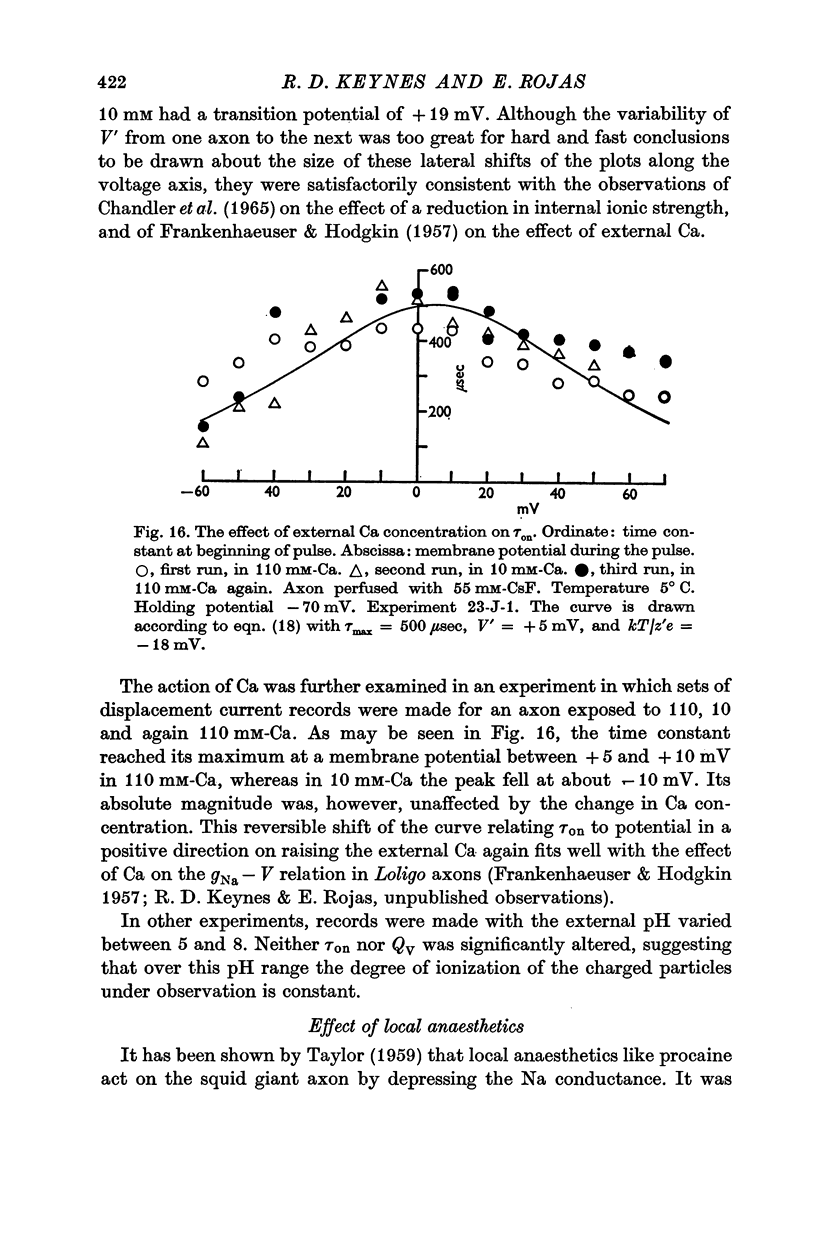
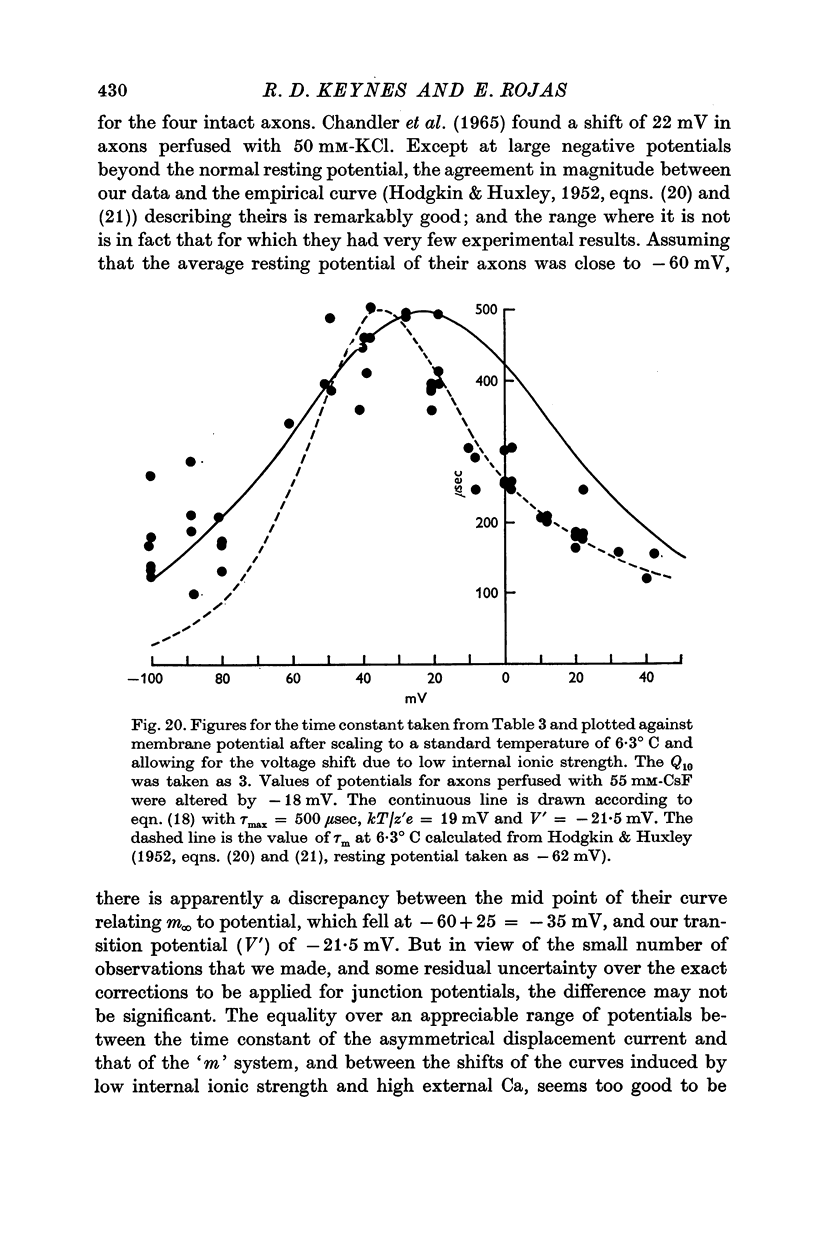
6. The identification of these mobile charges with the gating particles responsible for controlling Na conductance was supported by the findings that (a) their time constants were the same as those of Hodgkin & Huxley's `m' system, both in absolute magnitude and in their dependence on potential and temperature, (b) the transition potential at which the charges were evenly distributed on the two sides of the membrane also agreed with that for the `m' system in intact axons, and its value was similarly shifted in a positive direction by a reduction in internal ionic strength or by raising the external Ca concentration, (c) comparison of the steepness of the curves governing on the one hand the steady-state distribution of the mobile charges and on the other the Na conductance, suggested that an effective cooperation of the charges in groups of three was involved, again in excellent agreement with the `m' system.
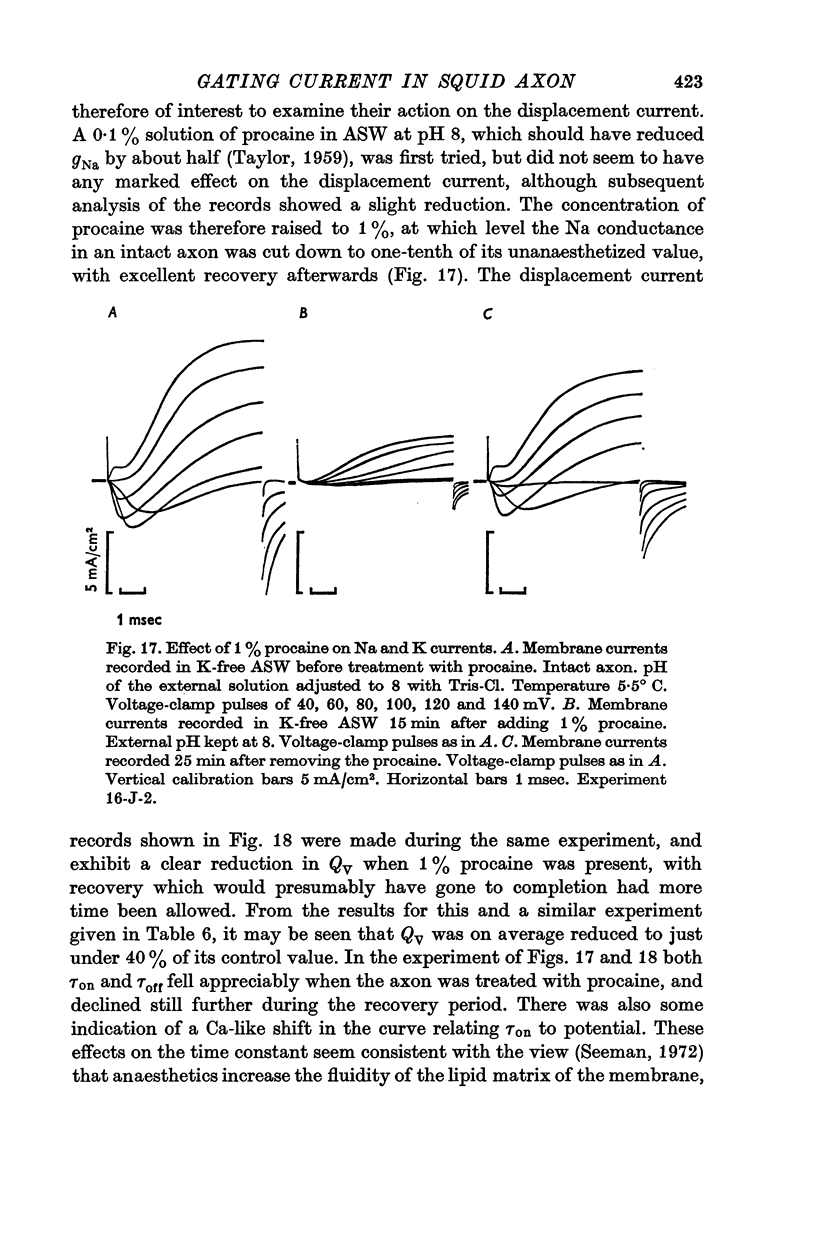
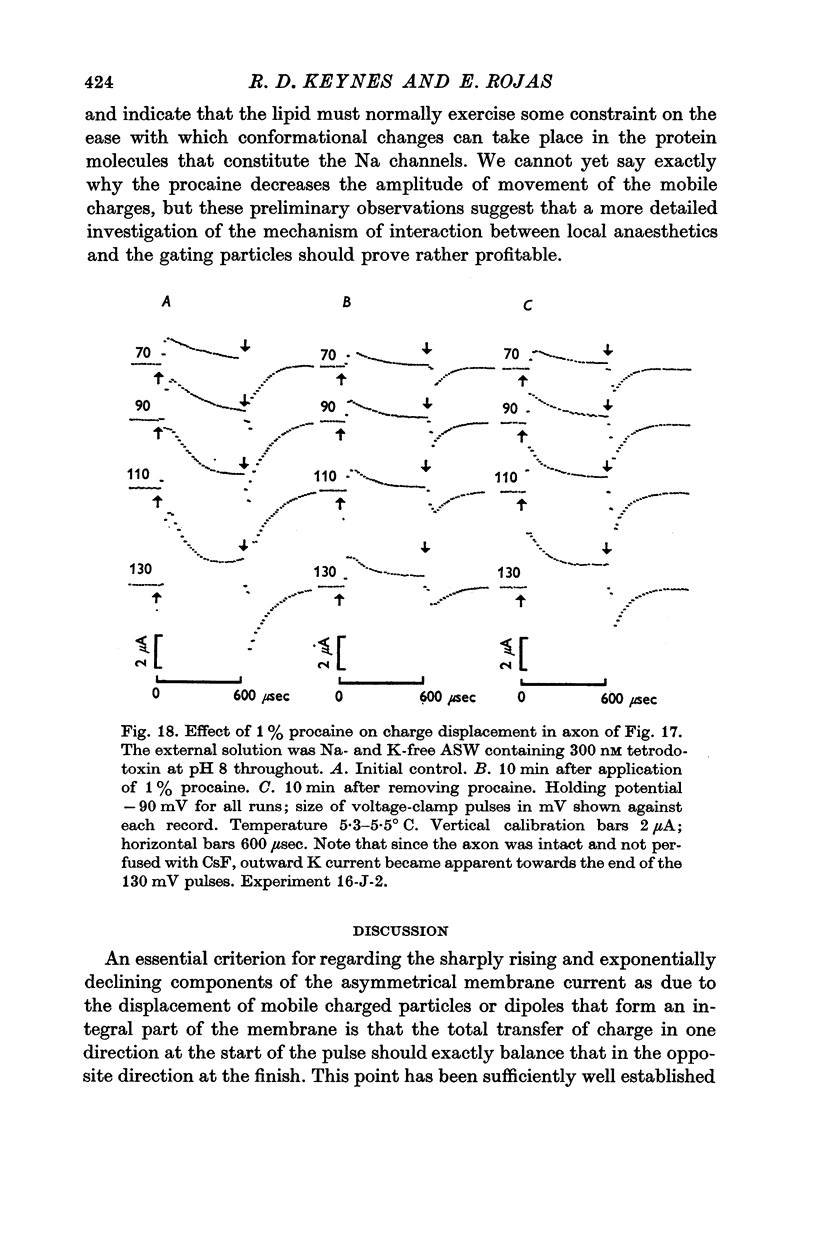
7. Displacement of the mobile charges was unaffected by external pH over the range 5-8, but preliminary observations showed that 1% procaine reduced the total charge transfer to somewhat less than 40% of the initial value, and roughly halved the time constant.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973 Apr 13;242(5398):459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Rojas E., Taylor R. E. Sodium and potassium conductance changes during a membrane action potential. J Physiol. 1970 Dec;211(3):729–751. doi: 10.1113/jphysiol.1970.sp009301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys J. 1960 Sep;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Evidence for two types of sodium conductance in axons perfused with sodium fluoride solution. J Physiol. 1970 Dec;211(3):653–678. doi: 10.1113/jphysiol.1970.sp009298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Sodium and potassium currents in squid axons perfused with fluoride solutions. J Physiol. 1970 Dec;211(3):623–652. doi: 10.1113/jphysiol.1970.sp009297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Hille B., Keynes R. D., Landowne D., Rojas E. Analysis of the potential-dependent changes in optical retardation in the squid giant axon. J Physiol. 1971 Oct;218(1):205–237. doi: 10.1113/jphysiol.1971.sp009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Henderson R., Ritchie J. M. The binding of labelled tetrodotoxin to non-myelinated nerve fibres. J Physiol. 1972 Dec;227(1):95–126. doi: 10.1113/jphysiol.1972.sp010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. Characteristics of the sodium gating current in the squid giant axon. J Physiol. 1973 Aug;233(1):28P–30P. [PubMed] [Google Scholar]
- Moore J. W., Narahashi T., Shaw T. I. An upper limit to the number of sodium channels in nerve membrane? J Physiol. 1967 Jan;188(1):99–105. doi: 10.1113/jphysiol.1967.sp008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Taylor R. E., Atwater I., Bezanilla F. Analysis of the effects of calcium or magnesium on voltage-clamp currents in perfused squid axons bathed in solutions of high potassium. J Gen Physiol. 1969 Oct;54(4):532–552. doi: 10.1085/jgp.54.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- TAYLOR R. E. Effect of procaine on electrical properties of squid axon membrane. Am J Physiol. 1959 May;196(5):1071–1078. doi: 10.1152/ajplegacy.1959.196.5.1071. [DOI] [PubMed] [Google Scholar]


