Abstract
1. The spaces occupied by isotopically labelled inulin, polyethylene glycol, mol. wt. 4000 (PEG 4000), polyethylene glycol, mol. wt. 1000 (PEG 1000) and sucrose in metabolizing mammalian kidney and liver slices and in toad bladder epithelial cell preparations incubated in vitro have been examined.
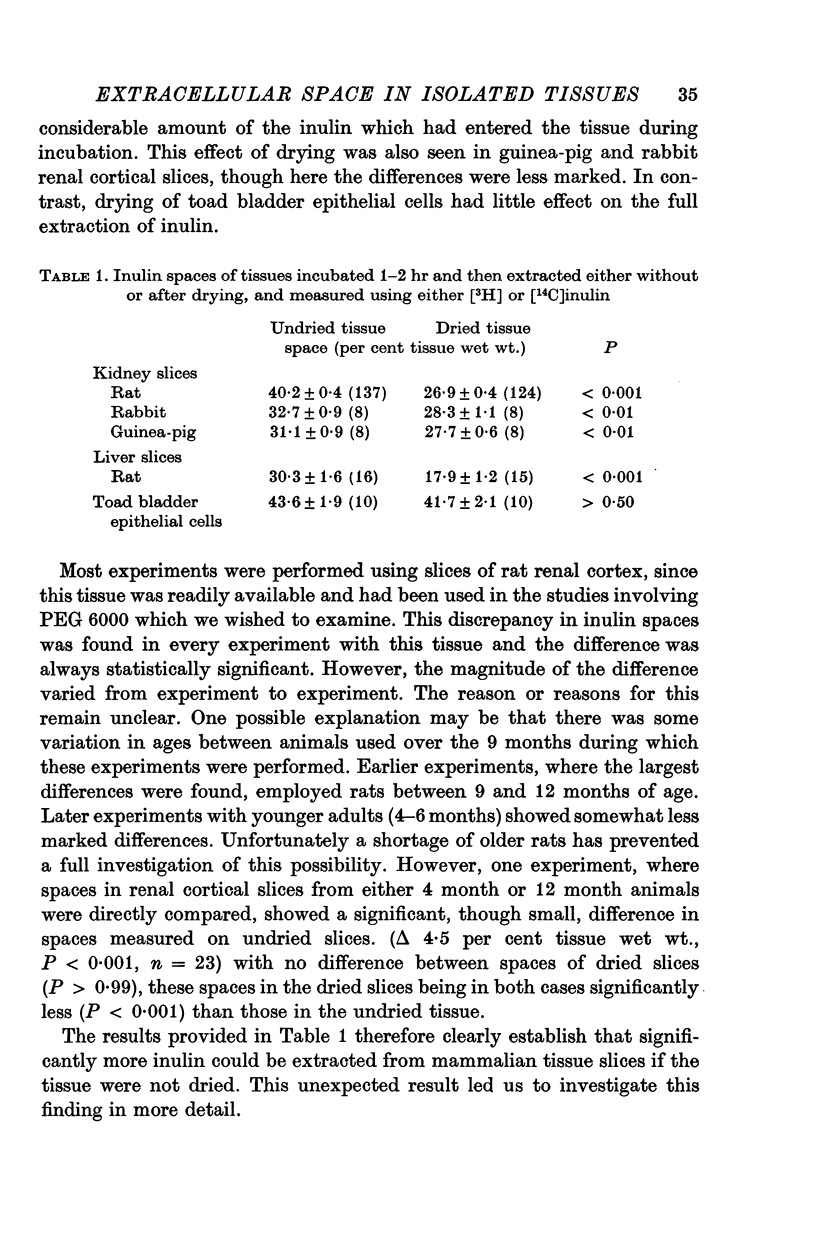
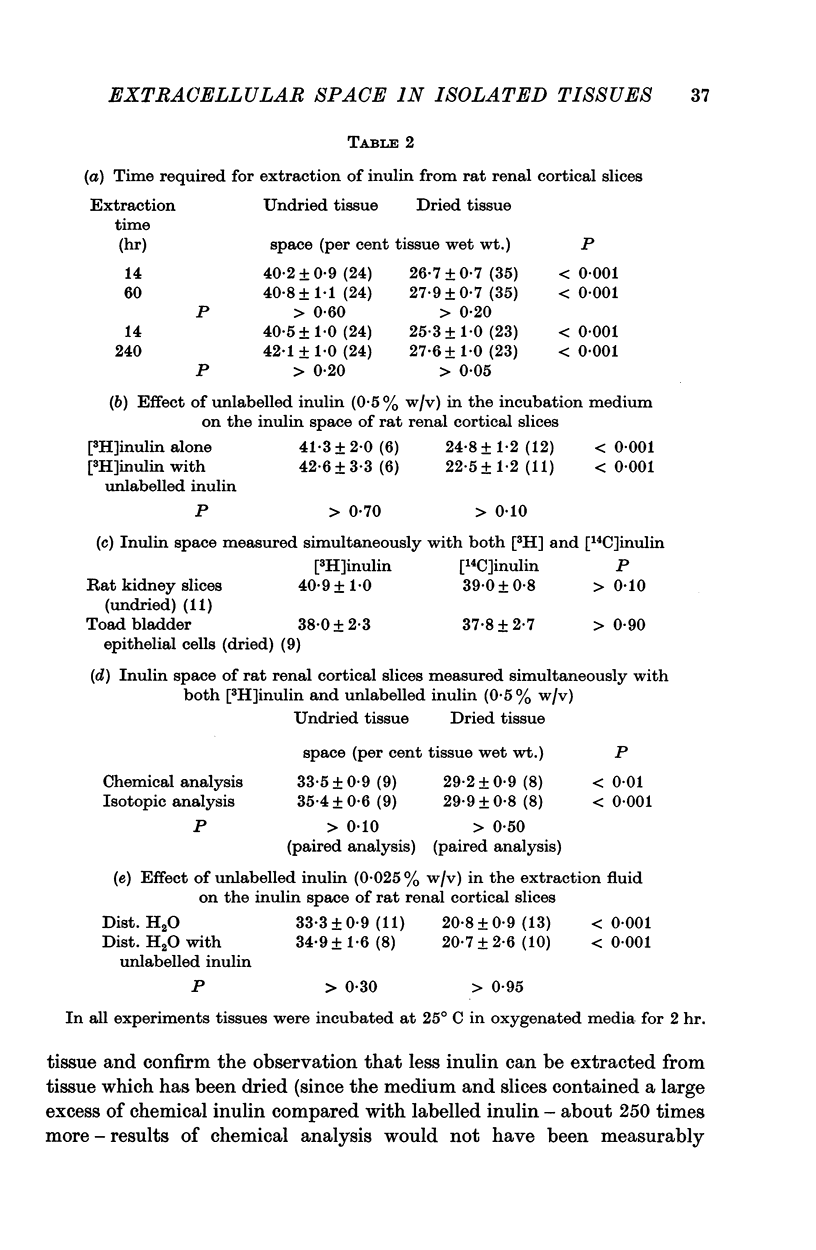
2. In slices of mammalian tissue, and in homogenized liver, it proved impossible to extract inulin completely from tissue which had been dried. However, inulin was recovered as completely from both dried and undried toad bladder epithelial cells scraped from hemibladders incubated in vitro.
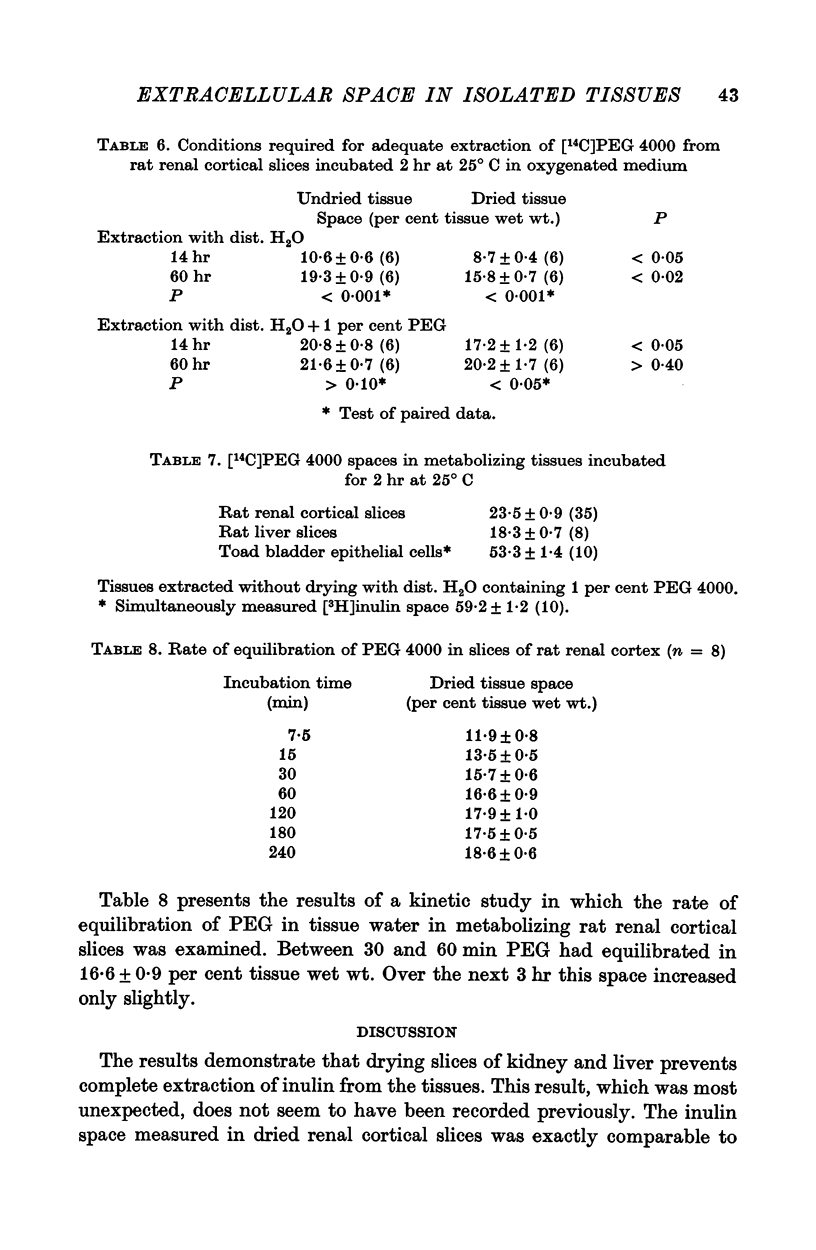
3. PEG 4000 occupied a space in all preparations similar to that from which inulin was extracted in dried tissue.
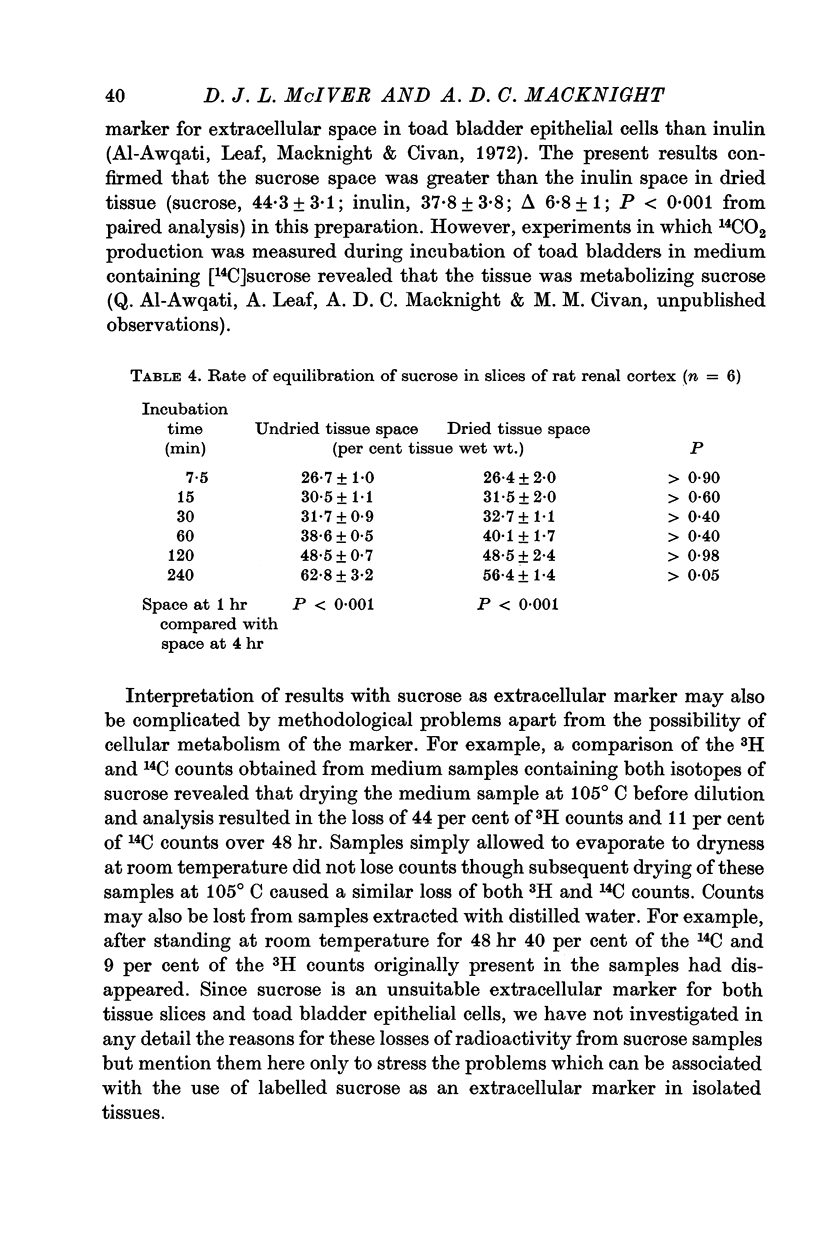
4. PEG 1000 and sucrose entered cellular water in mammalian slices, but PEG 1000 occupied a similar space to inulin in toad bladder epithelial cell preparations.
6. It is concluded that inulin enters cellular water in mammalian slices from which after drying of the slices it cannot be extracted. It thus rather fortuitously provides a measure of extracellular water under these conditions. In preparations of toad bladder epithelial cells inulin seems to be a satisfactory extracellular marker. PEG 4000, which did not appear to enter cellular water also allows a reasonable estimate of extracellular water. PEG 1000 is a suitable extracellular marker for toad bladder epithelial cell preparations but not for mammalian slices. Sucrose entered cellular water in both slices and toad bladder epithelial cells and is not a satisfactory extracellular marker in these tissues.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davey K. J., Skegg D. C. The effects of high concentrations of an electrolyte on the swelling of non-metabolizing tissue slices. J Physiol. 1971 Feb;212(3):641–653. doi: 10.1113/jphysiol.1971.sp009347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX M., THIER S., ROSENBERG L., SEGAL S. IONIC REQUIREMENTS FOR AMINO ACID TRANSPORT IN THE RAT KIDNEY CORTEX SLICE. I. INFLUENCE OF EXTRACELLULAR IONS. Biochim Biophys Acta. 1964 Jan 27;79:167–176. doi: 10.1016/0926-6577(64)90049-x. [DOI] [PubMed] [Google Scholar]
- Gans J. H., Bailie M. D., Biggs D. L. In vitro metabolism of C-14-labeled amino acids by sheep kidney cortex and medulla. Am J Physiol. 1966 Jul;211(1):249–254. doi: 10.1152/ajplegacy.1966.211.1.249. [DOI] [PubMed] [Google Scholar]
- Glaister D., Kerly M. The oxygen consumption and carbohydrate metabolism of the retractor muscle of the foot of Mytilus edulis. J Physiol. 1936 Jun 10;87(1):56–66. doi: 10.1113/jphysiol.1936.sp003388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KULKA R. G. Colorimetric estimation of ketopentoses and ketohexoses. Biochem J. 1956 Aug;63(4):542–548. doi: 10.1042/bj0630542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLE J. R. DETERMINATION OF WATER AND ELECTROLYTES IN TISSUE SLICES. Anal Biochem. 1964 Jan;7:87–95. doi: 10.1016/0003-2697(64)90122-8. [DOI] [PubMed] [Google Scholar]
- Little J. R., Robinson J. R. Effects of water content upon the respiration and the cations of kidney slices. J Physiol. 1967 Jul;191(1):91–106. doi: 10.1113/jphysiol.1967.sp008238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCILWAIN H., BUDDLE H. L. Techniques in tissue metabolism. I. A mechanical chopper. Biochem J. 1953 Feb;53(3):412–420. doi: 10.1042/bj0530412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight A. D. The extracellular space in rat renal cortical slices incubated at 0.5 degrees and 25 degrees. Biochim Biophys Acta. 1968 Aug;163(1):85–92. doi: 10.1016/0005-2736(68)90035-7. [DOI] [PubMed] [Google Scholar]
- Maude D. L. Effects of K and ouabain on fluid transport and cell Na in proximal tubule in vitro. Am J Physiol. 1969 May;216(5):1199–1206. doi: 10.1152/ajplegacy.1969.216.5.1199. [DOI] [PubMed] [Google Scholar]
- McIver D. J., Raine A. E. The influence of electrolytes on the volume of non-metabolizing renal cortical cells. J Physiol. 1972 Sep;225(3):555–564. doi: 10.1113/jphysiol.1972.sp009955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHELPS C. F. THE PHYSICAL PROPERTIES OF INULIN SOLUTIONS. Biochem J. 1965 Apr;95:41–47. doi: 10.1042/bj0950041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON J. R. Exchanges of water and ions by kidney slices determined by a balance method. J Physiol. 1961 Oct;158:449–460. doi: 10.1113/jphysiol.1961.sp006779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. R. Control of water content of non-metabolizing kidney slices by sodium chloride and polyethylene glycol (PEG 6000). J Physiol. 1971 Feb;213(1):227–234. doi: 10.1113/jphysiol.1971.sp009378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITTAM R. The permeability of kidney cortex to chloride. J Physiol. 1956 Mar 28;131(3):542–554. doi: 10.1113/jphysiol.1956.sp005481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITTEMBURY G. SODIUM EXTRUSION AND POTASSIUM UPTAKE IN GUINEA PIG KIDNEY CORTEX SLICES. J Gen Physiol. 1965 Mar;48:699–717. doi: 10.1085/jgp.48.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIGGINS P. M. SELECTIVE ACCUMULATION OF POTASSIUM ION BY GEL AND KIDNEY SLICES. Biochim Biophys Acta. 1964 Nov 29;88:593–605. doi: 10.1016/0926-6577(64)90102-0. [DOI] [PubMed] [Google Scholar]
- Wiggins P. M. A kinetic study of the state of potassium in kidney tissue. Biochim Biophys Acta. 1965 Nov 29;109(2):454–466. doi: 10.1016/0926-6585(65)90171-8. [DOI] [PubMed] [Google Scholar]
- Wiggins P. M. Efflux of sodium from anaerobic kidney slices. Biochim Biophys Acta. 1967;135(5):894–902. doi: 10.1016/0005-2736(67)90058-2. [DOI] [PubMed] [Google Scholar]


