Abstract
In vitro infection of murine peritoneal macrophages with the protozoan Leishmania donovani has been found to alter the signaling parameters of the host. The present study indicates that the enhancement of intracellular ceramide level in macrophages after infection is a major event relating to macrophage dysfunction. We have previously demonstrated that increased ceramide synthesis in host macrophages was involved in the dephosphorylation of extracellular signal-regulated kinase (ERK). In the present study, we further show that downregulation of ERK by ceramide was found to be associated with the inhibition of activated protein 1 (AP-1) and NF-κB transactivation. Pharmacological inhibition of ceramide synthesis by Fumonisin B1 restored the induction of AP-1 and NF-κB DNA-binding activities in infected BALB/c macrophages. On the contrary, in the case of macrophages from the leishmaniasis-resistant C.D2 mice, L. donovani failed to induce sustained ceramide synthesis. Enhanced mitogen-activated protein kinase phosphorylation, AP-1 and NF-κB DNA-binding activity, and the generation of nitric oxide (NO) were observed in L. donovani-infected C.D2 macrophages. ERK activation was necessary for the activation of transcription factors AP-1 and NF-κB, NO generation, and restriction of the parasite burden in the resistant murine host macrophages. Hence, the induction of ceramide synthesis in host macrophages appears to be instrumental and one of the turning points leading to silencing of the macrophage antileishmanial responses.
The success in the intracellular survival of pathogens lies in the effective design of strategies employed by the parasite to evade or impair the elaborate defense mechanism of the host. The protozoan parasite Leishmania donovani, the causative agent of visceral leishmaniasis, exhibits an efficient survival mechanism inside the reticuloendothelial system of the vertebrate host. The intra- and extracellular signal transduction networks provide an effective means of cellular coordination in complex regulatory mechanisms such as immune response. L. donovani has been reported to interfere with the host signal transduction, thereby evading the generation of the reactive oxygen and nitrogen species, impairing antigen presentation by macrophages and T-cell activation (27, 36, 38, 40).
We have previously reported that L. donovani induces elevated levels of ceramide generation in host BALB/c macrophages (16). This ceramide is involved in the downregulation of Ca2+-dependent classical protein kinase C activity and extracellular signal-regulated kinase (ERK) phosphorylation and activity (15). Activation of a tyrosine phosphatase plays a major role in the deactivation of the mitogen-activated protein kinases (MAPKs) in leishmania-infected cells (14, 17).
Most signal transduction pathways in response to extracellular and intracellular cues ultimately impinge on the gene expression via the regulation of the transcription factors. Activated protein-1 (AP-1) and NF-κB are important pleiotropic transcription factors that regulate a variety of gene expressions. It has been reported that influenza A virus infection results in the production of proinflammatory and antiviral cytokines, as well as chemokines, by the recruitment of NF-κB and AP-1 in infected leukocytes (25). Heat-killed serotype III of group B streptococcus, as well as the protozoan Trypanosoma cruzi, induces a host signal transduction pathway that leads to NF-κB and AP-1 activation (22, 48). It is apparent that the shaping of the immune response is highly incumbent on the efficient transactivation of several inducible genes by AP-1 and NF-κB.
AP-1 is a dimer and a member of the Fos and Jun protein family (3, 53), which represents part of the cohort of the “immediate-early” genes (1). The c-fos promoter contains a well-characterized serum responsive element (SRE) whose activity can be stimulated upon mitogenic activation (5, 33). The c-Jun N-terminal kinase (JNK) members of the MAPK superfamily, especially the two splice variants JNK1 and -2, are responsible for the phosphorylation and activation of c-Jun (21, 45). Activation of NF-κB involves prior phosphorylation of the inhibitor protein, IκB (36). Several studies also indicate that activation of NF-κB correlates with the increased activation of the MAPK/JNK pathway (6, 11, 19, 31).
In contrast to many pathogens, which augment the immune activation as in the case of T. cruzi, L. donovani induces an immune “silencing” mechanism for its intracellular survival. Studies on the signal transduction framework leading to the elaborate parasitism have so far been fragmentary. A recent study has reported the ability of L. donovani promastigotes to evade the induction of NF-κB in naive bone marrow macrophages (38). However, the signal transduction pathway leading to the deactivation of the NF-κB remains unclear.
In the present study, we have investigated the effect of enhanced intracellular ceramide due to leishmanial invasion on the host transcription factors such as AP-1 and NF-κB and the intermediate role of MAPK leading to the modulation of these transcription factors in experimental visceral leishmaniasis. In addition, we have related these signaling events to NO generation and the ability of the host cell to kill intracellular amastigotes. Moreover, we thought it would be interesting to find out the effect of L. donovani infection on the intracellular ceramide level and the subsequent downstream activities in a host system that is capable of resisting the infection. The murine strain C.D2 suited our purpose since C.D2 expresses Lshr phenotype, which exhibits innate resistance against Salmonella enterica serovar Typhimurium, Mycobacterium bovis, and L. donovani (32). At the same time, C.D2 is a BALB/c congenic strain and thus shares a similar background with BALB/c (32). C.D2 was shown to exhibit leishmanicidal property (9; D. J. Bradley, Letter, Nature 250:353-354, 1974). Our study implicates that ceramide was majorly responsible for the suppression of AP-1 and NF-κB activation and also the inhibition of NO generation in BALB/c macrophages. Inhibition of phosphorylation of the MAPKs by enhanced ceramide appears to be an important step in evading the activation of transcription factors. Evidently, macrophages from the resistant strain show restricted ceramide synthesis, followed both by the activation of MAPK and transcription factors and by NO generation, with the induction of intracellular parasite clearance. Hence, restriction of ceramide synthesis in the susceptible host can provide a mechanism for immunotherapeutic approach in visceral leishmaniasis.
MATERIALS AND METHODS
Animals and parasites.
BALB/c mice were purchased from National Centre for Laboratory Animal Sciences in India. For each experiment, 8 to 10 mice (4 to 6 weeks old) were used, irrespective of sex. C.D2 mice were a kind gift from Emil Skamene, McGill University, Montreal, Quebec, Canada, and Veneeta Bal, National Institute of Immunology, New Delhi, India.
L. donovani AG-83 (MHOM/IN/1983/AG83) was maintained in vitro in Medium-199 containing 10% fetal calf serum (FCS). Amastigotes were prepared from the spleen of AG-83 infected golden hamster as described by Hart et al. (20). For infection, hamsters were injected with 2 × 107 amastigotes in 0.5 ml of normal saline via the intracardiac route (42). Promastigotes were obtained by suitable transformation. Experiments were performed with stationary-phase promastigotes.
Preparation of peritoneal macrophages and in vitro infection.
The peritoneal macrophages were collected by infusing the peritoneal cavity with ice-cold sterile phosphate-buffered saline (PBS). Cells were cultured as described by Fahey et al. in RPMI supplemented with 10% FCS (13). Infection was administered at a macrophage/parasite ratio of 1:10 for 4 h. After this period, the unbound parasites were washed off and the cells were incubated for variable periods. Infection levels were routinely ca. 2 to 4 parasite per macrophage after 24 h as examined by Giemsa staining, with 86 to 92% of the cells infected. BALB/c and C.D2 cells did not show any remarkable difference in parasite uptake.
Assay for detection and quantification of ceramide.
Ceramide assay was carried out by using radiolabeled stearic acid as described by Gamen et al. (15). In short, cells (4 × 106) were labeled for 30 h with 5 μCi of [1-14C]stearic acid bound to acid-free serum albumin (a 1:1 molar ratio) in complete medium. Cells were washed with sterile PBS and resuspended in RPMI 1640 with 10% fetal bovine serum and infected with L. donovani promastigotes. Total cellular lipids were extracted at 4°C with chloroform-methanol (2:1 [vol/vol]). The radioactivity content of aliquots from CHCl3 phases were determined by liquid scintillation counting, and equal amounts of radioactivity in each sample were applied to thin-layer chromatography silica gel G plates (Whatman) along with standard C2 and C6 ceramides. Plates were air dried, bands corresponding to the standard were scraped, and radioactive counts were taken in an L.S. Counter with 4 ml of Cocktail-O.
Preparation of cell lysate.
The adherent cell population was scraped and centrifuged at 400 × g for 15 min at 4°C. The cells were then resuspended in ice-cold extraction buffer containing 50 mM Tris-HCl (pH-7.5), 50 mM EGTA, anti-protease mixture, and 50 mM β-mercaptoethanol. Anti-protease mixture consisted of 0.33 mM leupeptin, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), 0.35 mM antipain, 0.24 mg of chymostatin/ml, 0.35 mM pepstatin, and 4.8 trypsin inhibitor units of aprotinin/ml (12, 29). The macrophage-containing suspension was sonicated at 4°C and centrifuged at 4,250 × g for 10 min at 4°C, and then the supernatant was used for experiments.
Electrophoresis and immunoblotting.
Whole-cell sonicate was allowed to centrifuge at 4,250 × g for 10 min at 4°C to remove the nuclear fraction. The supernatant was separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE) and transferred to a nitrocellulose membrane. The membrane was blocked overnight with 3% bovine serum albumin in Tris-saline buffer (pH 7.5), and immunoblotting was done as described by Majumdar and coworkers (12, 30). Immunoreactive bands were visualized by using nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) as a chromogenic substrate for alkaline phosphatase.
Preparation of nuclear extracts.
The cells were collected after incubation for indicated periods by gentle scraping, and nuclear extraction was performed as described previously (4). Briefly, cells were washed in PBS and resuspended in 5× PCV hypotonic lysis buffer containing 10 mM HEPES (pH 7.9) at 4°C, 1.5 mM MgCl2, 10 mM KCl, 0.2 mM PMSF, and 0.5 mM dithiothreitol (DTT). The swollen cells were homogenized, and the nuclei were pelleted by centrifugation. The nuclei were resuspended in 0.5× PNV low-salt buffer containing 20 mM HEPES (pH 7.9), 25% glycerol, 1.5 mM MgCl2, 0.02 M KCl, 0.2 mM EDTA, 0.2 mM PMSF, and 0.5 mM DTT. A high-salt buffer containing a similar composition, except that 0.02 M KCl was substituted by 1.4 M KCl, was added dropwise, and nuclear proteins were extracted in ice for 20 min. The nuclei and debris were removed by centrifugation at 14,000 rpm, and the supernatants containing the nuclear proteins were collected and stored at −80°C.
Electrophoretic mobility shift assay.
NF-κB specific oligonucleotide (5′-TAGTTGAGGGCACTTTCCCAGG-3′) from the NF-κB/RelA DNA-binding domain in murine IκB light-chain gene enhancer and AP-1 specific oligonucleotide (5′-GCTTGATGACTCAGCCCGGAA-3′) probes (synthesized from Gibco-BRL) were labeled with 32P with Klenow by using [α-32P]dATP. Nuclear extracts (15 μg per sample) were incubated with 3 × 105 cpm of 32P-labeled probe (0.2 ng of DNA) in the presence of binding buffer (containing 12.5 mM HEPES [pH 7.9], 10% glycerol, 5 mM MgCl2, 50 mM KCl, 1 mM EDTA, 1 mM DTT, and 300 μg of bovine serum albumin/ml) and 44 μg of salmon sperm DNA for 20 min. For cold competition, 10- and 100-fold excess unlabeled probe was incubated for 15 min at room temperature with the mixture described above before the addition of the labeled probe. Shift complexes were resolved in 6% acrylamide gels at 4°C in 0.5× Tris-borate-EDTA; dried gels were autoradiographed.
Nitrite assay.
The generation of nitrite in the conditioned medium of macrophage culture was assayed by the Griess reaction (18) by using nitric oxide colorimetric assay kit from Boehringer Mannheim. In brief, nitrate present in the sample was reduced to nitrite by NADPH in the presence of the enzyme nitrate reductase. For the assay, macrophages were cultured in a 24-well tissue culture plate (Falcon) at a concentration of 106 cells/ml. Cell-free culture supernatant was collected, and the nitrite level was estimated according to the manufacturer's instruction.
Uptake and intracellular multiplication of L. donovani.
To study the uptake of parasites, after 4 h of promastigote challenge the noningested parasites were removed by extensive washing with complete conditioned RPMI 1640, cultured for the indicated periods, and subjected to cell fixation, followed by Giemsa staining (46).
Densitometric analysis.
Autoradiographs of endogenous protein phosphorylation and immunoblot were analyzed by using a model GS-700 imaging densitometer and Molecular Analyst (version 1.5; Bio-Rad, Hercules, Calif.).
Statistical analysis.
Results were expressed as mean ± the standard deviation for individual sets of experiments. Each experiment was performed three to five times, and the representative data from each set of these experiments was presented in the manuscript. One- or two-tailed Student t test for significance was performed as applicable in each case. A P value of <0.05 was considered significant.
RESULTS
Kinetics of ceramide generation in BALB/c and C.D2 macrophages in response to L. donovani infection.
In previous studies we have demonstrated that intracellular ceramide generation was enhanced in leishmaniasis-susceptible BALB/c macrophages infected with L. donovani in vitro (16). The present study was sought to determine whether there was any alteration in the ceramide level in C.D2 macrophages, which express the Lshr gene responsible for natural resistance to leishmaniasis (9, 32; Bradley, letter). We performed a comparative study in the uninfected and infected macrophages of both BALB/c and C.D2 strains. Figure 1A shows a sustained elevation in the intracellular ceramide in infected BALB/c till 48 h of infection. Similar findings with BALB/c macrophages have been presented as the percent increase over the basal level in observed in an earlier study (16). On the contrary, C.D2 macrophages showed an initial rise by 2.5-fold of the uninfected control by 6 h postinfection, followed by a substantial decrease from 12 h (Fig. 1B). At 24 h, the ceramide level of infected C.D2 was almost comparable to that of the uninfected control (P < 0.02) (Fig. 1), without further increase upon incubation. In both of these cases, the percentage of infected macrophages was routinely examined to be between 86 and 91%, counted 6 h after infection. However, the number of parasites gradually decreased in the C.D2 macrophages with time (at 12 h there were 321 ± 15, at 24 h there were 302 ± 10, and at 48 h there were fewer than 144 ± 21 parasites per 100 macrophages [data not shown]). To further show that the changes in intracellular ceramide levels were independent of the intracellular live parasites, macrophages from BALB/c (Fig. I) and C.D2 (Fig. II) were treated with the whole-cell lysate of L. donovani promastigotes. In keeping with our previous observation in the case of BALB/c macrophages (16), both of these cell types showed a considerable rise in the intracellular ceramide level at 6 h after the treatment (Fig. I and II). This indicated clearly that the changes in ceramide level observed in Fig. 1A and B might be due to some cellular component(s) of the parasites and independent of the presence of live parasites inside the macrophages. The difference in the pattern of ceramide generation due to infection in BALB/c and C.D2, therefore, appeared to be the intrinsic property of the two hosts.
FIG. 1.
Changes in the intracellular ceramide level triggered by L. donovani infection to BALB/c (A) and C.D2 (B) macrophages. The ceramide level was assessed at the indicated periods after infection and is expressed as picomoles of 14C-labeled ceramide per 4 × 106 cells. ✽, P < 0.02; ✽✽, P < 0.05. These data are representative of three independent experiments with identical results. (C) Changes in the ceramide level (percent control) in BALB/c (I) and C.D2 (II) macrophages treated with parasite crude lysate for 6 h (control was taken as 100%).
Phosphorylation of ERK1 and -2 in the BALB/c and C.D2 macrophages in response to L. donovani infection.
Inhibition of MAPK phosphorylation is an important event in leishmanial pathogenesis (35, 38). The observations noted above encouraged us to study the effect of leishmanial invasion on ERK activation since we have formerly reported that enhanced ceramide synthesis was involved in ERK downregulation in infected BALB/c cells (16). Interestingly, a time-dependent comparative study between the two strains revealed enhanced phosphorylation in the infected C.D2 macrophages in contrast to the overall inhibition in the BALB/c (Fig. 2A and B). Lipopolysaccharide (LPS; 1 μg/ml), a well-documented activator of ERKs (19), induced the phosphorylation of ERK1 and -2 in the uninfected BALB/c and C.D2 macrophages (lanes 2 of Fig. 2A and B). This was markedly inhibited in BALB/c macrophages upon infection with or without LPS at all of the time points studied (lanes 3 and 4 of Fig. 2A). In contrast, in the infected C.D2 macrophages, LPS induced enhanced phosphorylation in ERKs at 12, 24, and 36 h, respectively, relative to the LPS control (lanes 4 of Fig. 2B). Moreover, enhanced ERK phosphorylation was evident in infected C.D2 even in the absence of LPS stimulation (lanes 3 of Fig. 2B). At the later periods of incubation the inducibility of p42 phosphorylation was observed to be decreased (Fig. 2B upper panel). In all of the cases, expression of ERK1 and -2 remained unaffected (Fig. 2, lower panel). These results depicted that enhanced ERK phosphorylation was evident in the resistant murine host.
FIG. 2.
Effect of L. donovani infection on ERK activation in BALB/c (A) and C.D2 (B) macrophages. Macrophages with or without L. donovani infection were incubated for 12 h (I), 24 h (II), or 36 h (III) postinfection. LPS (1 μg/ml) was treated where indicated for 30 min prior to the lysis of cells. ERK phosphorylation was studied by Western blot analysis (80 μg of protein sample per lane) with specific antibodies to phospho-ERK (upper panel) and then reprobed with anti-ERK antibody (lower panel). The data shown in this figure are representative of three independent experiments. (C) Effect of PD98059 on ERK phosphorylation in BALB/c and C.D2 cells. BALB/c (I) or C.D2 (II) cells were pretreated with 25 μM PD98059 for 1 h or left untreated (control) and then incubated in fresh RPMI medium supplemented with 10% FCS for 24 h. Before lysis, the cells were stimulated with LPS (1 μg/ml) for 30 min. ERK phosphorylation was observed by Western blotting as described above.
PD98059 is reported to be a pharmacological inhibitor of ERK (14, 50). To record the efficacy of the inhibitor, we pretreated BALB/c macrophages with 25 μM PD98059 for 1 h and then incubated them for 24 h. After 24 h of incubation, the phosphorylation of ERK1 and -2 in the treated cells was shown to be inhibited in presence or absence of LPS (1 μg/ml, treated for 30 min) (Fig. I). Similarly, C.D2 cells also showed inhibition of ERK activity at 24 h after identical treatment with PD98059 (Fig. II). Hence, inhibition of ERK with PD98059 in the next experiments would provide sufficient insight to downstream signaling events in the case of leishmanial infection.
Phosphorylation of the JNKs in BALB/c and C.D2 macrophages.
c-Jun N-terminal kinase (JNK) is another member of Ser/Thr kinase belonging to MAPK superfamily. We have studied the expression and phosphorylation of JNK in the context of experimental visceral leishmaniasis. LPS (1 μg/ml) triggered JNK phosphorylation in uninfected macrophages of both BALB/c and C.D2 (Fig. 3, lanes 2). In infected BALB/c macrophages, there was marked inhibition of LPS-triggered JNK phosphorylation throughout the course of study (Fig. 3A, lanes 4). On the other hand, infection of the resistant C.D2 macrophages induced phosphorylation of JNK in response to LPS (Fig. 3B, lanes 4). Again, L. donovani induced LPS-independent enhanced JNK 2 (p54) phosphorylation at 24 h postinfection (Fig. II, lane 3).
FIG. 3.
(A) Effect of L. donovani infection on JNK activation in macrophages. BALB/c macrophages with or without L. donovani infection were incubated for 12 h (I), 24 h (II), or 36 h (III) postinfection. LPS (1 μg/ml) was treated where indicated for 30 min prior to the lysis of cells. (B) C.D2 macrophages with or without L. donovani infection were incubated for 12 h (I), 24 h (II), or 36 h (III) postinfection. In all of these cases, phosphorylation was studied by Western blot analysis (80 μg of total protein sample per lane) with specific antibodies to phospho-JNK (upper panel) and then reprobed with anti-JNK antibody. The data shown in this figure are representative of three independent experiments.
Effect of L. donovani on the transcription factors AP-1 and NF-κB: role of endogenous ceramide.
Activation and deactivation signals in the signal transduction cascade ultimately traverse to the nucleus and affect the gene transcription. By regulating the activities of the transcription factors, the MAPK are able to influence the expression of a spectrum of genes (17, 34, 43). From the previous experiments, it was evident that the MAPKs were substantially activated at 24 and 36 h in the C.D2 macrophages upon infection (Fig. 2 and 3). This prompted us to study the effect of the endogenous ceramide on the DNA-binding activity of the two most important transcription factors, AP-1 and NF-κB, at 36 h in case of infection of both BALB/c and C.D2. LPS has been used as a positive control for the tests. LPS-treated uninfected macrophages exhibited remarkable AP-1 and NF-κB activity in both cell types (Fig. 4 and 5, the second lane in each panel). As shown in Fig. 4A and 5A (the sixth lane 6 in each panel), the respective DNA-binding activity of both AP-1 and NF-κB were suppressed in the case of L. donovani-infected BALB/c macrophages. Inhibition of ceramide synthesis by FB1 induced both the DNA-binding activities (Fig. 4A and 5A, seventh lane in each panel). Hence, an important role of ceramide in the regulation of leishmania-induced AP-1 and NF-κB response is evident from the present study.
FIG. 4.
Effect of L. donovani infection on AP-1 transactivation in BALB/c (A) and C.D2 (B) cells. BALB/c and C.D2 macrophages were pretreated with either Fumonisin B1 (50 μM) or PD98059 (25 μM) for 1 h before infection or were left uninfected (controls). At 24 h postinfection, we added RPMI containing FCS, supplemented with 25 μM with and/or 10 μM PD98059 where required to maintain inhibitory effects. LPS (1 μg/ml) was treated to uninfected cells for 24 h. Cells were harvested after 36 h of infection. Nuclear extracts were probed with 32P-labeled AP-1 consensus nucleotide. The specificity of DNA binding was assessed by preincubating extracts with unlabeled probe at a 10-fold molar excess (see panel A, lane 1). The autoradiograms are representative of four independent experiments with identical results.
FIG. 5.
Effect of L. donovani infection on NF-κB transactivation in BALB/c (A) and C.D2 (B) cells. BALB/c and C.D2 macrophages were pretreated with either Fumonisin B1 (50 μM) or PD98059 (25 μM) for 1 h before infection or were left uninfected (controls). At 24 h postinfection, we added RPMI containing FCS, supplemented with 25 or 10 μM PD98059 where required to maintain inhibitory effects. LPS (1 μg/ml) treatment was applied to uninfected cells for 24 h. Cells were harvested after 36 h of infection. Nuclear extracts were probed with 32P-labeled NF-κB consensus nucleotide. The specificity of DNA binding was assessed by preincubating extracts with unlabeled probe at a 10-fold molar excess (Fig. 5A, first lane). The autoradiograms are representative of four independent experiments with identical results.
On the contrary, in the case of the C.D2 macrophages, there was marked increase in both AP-1 and NF-κB DNA-binding activities in the infected cells compared to the uninfected macrophages (Fig. 4B, fourth lane, and 5B, fourth lane, respectively).
These data clearly suggest that the signal transduction pathway involved in disease resistance encompass the activation of AP-1 and NF-κB, which are negatively regulated by the induction of ceramide in the susceptible host cells.
Role of ERKs on AP-1 and NF-κB activation.
We further investigated the effect of ERK on AP-1 and NF-κB activation by using a specific inhibitor of the kinase. When BALB/c cells were pretreated with PD98059, there was no significant changes in the binding activity of either uninfected (Fig. 4A, fifth lane) or infected (data not shown) sets. To understand whether FB1-mediated AP-1 induction (shown in Fig. 4A, seventh lane) occurred via ERK activation, we first blocked the ceramide synthesis in the BALB/c with FB1 and then treated the cells with the ERK inhibitor PD98059 in case of infection. Here we observed significant inhibition of FB1-induced AP-1 activity (Fig. 4A, eighth lane). Therefore, it seems that ERK is essential for the induction of AP-1 activity.
Similarly, the AP-1 DNA-binding activity induced by infection in C.D2 cells was blocked when pretreated with the ERK inhibitor PD98059 prior to infection (Fig. 4B, fifth lane). All of these inhibitor combinations used were tested for their effect on cell cytotoxicity by measuring the LDH release by using cytotoxicity detection assay kit (Boehringer Mannheim). It was observed that the percentage of cell cytotoxicity in infected BALB/c cells with FB1 alone was 2.23 ± 0.68 and that in infected BALB/c cells with FB1 plus PD98059 was 3.05 ± 0.82 at 36 h postinfection (data not shown) and therefore within the permissible range.
As discussed in the case of AP-1, PD98059 in combination with FB1 in BALB/c decreased NF-κB activation in comparison to FB1 treatment alone in the case of infection (Fig. 5A, eighth lane). Similarly, in infected C.D2 macrophages PD 98059 inhibited NF-κB binding (Fig. 5B, fifth lane).
The data presented above clearly indicate that the induction of both AP-1 and NF-κB were dependent on the activation of MAPK pathway and that inhibition of ERK by the enhancement of ceramide in the BALB/c was involved in the suppression of AP-1 and NF-κB DNA-binding activity.
Generation of nitric oxide in infected BALB/c and C.D2 macrophages: role of ERKs and NF-κB.
The generation of nitric oxide (NO) is an important defense mechanism of the host, which largely accounts for the antileishmanial protection mechanisms (8, 39), which is impaired in the susceptible BALB/c during leishmanial infection (39), and the resistant hosts are capable of NO production (7). The changes in the level of nitrite generation by the unstimulated control and infected macrophages were undetectable (data not shown), as found in our previous studies (15). Hence, LPS was used as a positive stimulus for cells to assay the inducible form of the NOS (iNOS) activity in a detectable range. The kinetic study depicted here that L. donovani inhibited LPS induced NO generation in BALB/c macrophages throughout the 48-h postinfection period considered in this study (Fig. 6). In the C.D2 macrophages, LPS-induced NO generation did not show considerable change for up to 24 h of infection. At 36 h postinfection, there was marked rise in LPS-triggered NO, which was enhanced further at 48 h of infection (Fig. 6).
FIG. 6.
Kinetics of NO generation in BALB/c and C.D2 macrophages. Cells (106cells/ml) were cultured in a 24-well plate in presence of LPS (1 μg/ml). The release of NO by LPS was assayed from culture supernatants at indicated intervals. ✽, P < 0.03; ✽✽, P < 0.01. The results presented here are representative of three independent experiments.
When BALB/c macrophages were pretreated with FB1 and then infected with L. donovani, NO generation was triggered in an infected condition (Fig. 7A). However, when both ceramide synthesis and ERK activation were inhibited by the pretreatment with FB1 and PD98059, NO generation was decreased compared to FB1 treatment in infected cells (Fig. 7A). In order to find out the role of ERK in triggering LPS-induced NO generation in infected C.D2 cells, we pretreated macrophages with PD98059, followed by infection. Infected cells failed to generate NO at 48 h postinfection (Fig. 7B). Thus, summarizing the above data, NO generation appeared to be colinearly regulated by the factors that regulate both AP-1 and NF-κB activities. Ceramide played an inhibitory role on NO generation, and ERK was found to be an essential factor for its induction.
FIG. 7.
Effect of ceramide inhibitor and ERK inhibitor on NO release in BALB/c (A) and C.D2 (B) cells. Cells (106 cells/ml) were cultured in a 24-well plate in the presence of LPS (1 μg/ml). Culture supernatants were assayed at 48 h postinfection. Cells were treated with Fumonisin B1 and PD98059 exactly as described in the legend to Fig. 4. Uninfected cells were incubated for 48 h. In the case of infection, cells were incubated with promastigotes for 4 h, washed, and incubated for another 44 h. The results are representative of three experiments. ✽, P < 0.02 versus the infected BALB/c set; ✽✽, P < 0.01 versus the uninfected C.D2 set and P < 0.05 versus the infected C.D2 set.
Differential leishmanicidal activity of BALB/c and C.D2 macrophages.
Control of parasitic multiplication, as well as killing of the existing parasites in the infected macrophages, happens to be the expected outcome in disease resistance. We next sought to study the fate of the intracellular parasites in BALB/c and C.D2 macrophages and the role of the above-mentioned signaling events in promoting disease progression and/or resistance. Figure 8 shows that the parasite uptake by the two strains of host macrophages were comparable. At 36 h, the C.D2 macrophages showed restriction in the number of intracellular parasites, whereas in BALB/c cells the parasite number was increasing. At 48 h, the C.D2 macrophages exhibited effective reduction in the number of amastigotes in infected macrophages (Fig. 8).
FIG. 8.
Comparison of the intracellular parasite burden at different time points in BALB/c and C.D2 macrophages. Cells infected with L. donovani were cultured on glass coverslips. At the indicated periods the cells were fixed and stained with Wright-Giemsa stain, and the number of intracellular parasites counted under a phase-contrast microscope. These results are representative of three experiments. ✽, P < 0.01.
We extended our observations to evaluate the role of ceramide and ERK on the intracellular parasite uptake and survival. When BALB/c cells were treated with FB1, or in combination with PD98059, as in our former experiments, there was no change in the number of promastigotes entering the macrophages (Fig. 9A). C.D2 macrophages also, when treated with PD98059, did not show any change in the uptake of promastigotes in comparison to the untreated macrophages (Fig. 9B). FB1 pretreatment in the case of BALB/c cells leads to restriction of the intracellular parasite number (Fig. 9A). However, PD98059 in combination with FB1 increased the intracellular parasite number (Fig. 9A), indicating that FB1-mediated protection involves the activation of ERK, at least in part. Moreover, the number of amastigotes inside the resistant C.D2 macrophages was enhanced owing to treatment with PD98059 (Fig. 9B). These findings indicate that ERK activation is an important prerequisite for parasite clearance and host resistance. The morphological studies by Giemsa staining of the same experiment discussed above has been represented in Fig. 10.
FIG. 9.
Effect of ERK inhibitor PD98059 (25 μM) on intracellular survival of parasites in BALB/c (A) and C.D2 (B). Cells were pretreated with Fumonisin B1 (50 μM) and PD98059 (25 μM) for 1 h each. Unbound parasites were washed off after 4 h of infection. Cells were further incubated for 18 h, and fresh medium with 25 μM Fumonisin B1 and/or 10 μM PD98059 was added to maintain the inhibitory effect. Macrophages were stained with Giemsa at 6 or 48 h postinfection as mentioned in the text. Parasites were counted under a phase-contrast microscope. The results shown are representative of three experiment. ✽, P < 0.05 versus the infected set; ✽✽, P < 0.04 versus the FB1-treated set and P < 0.05 versus the infected set.
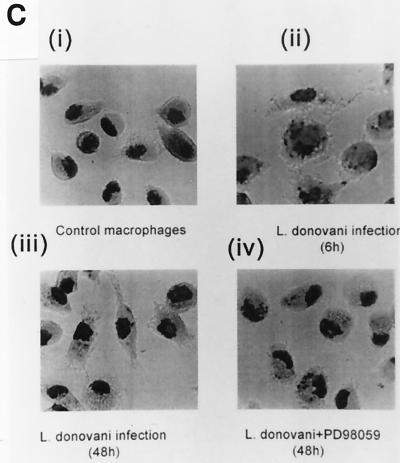
FIG. 10.
Photographs of Giemsa-stained BALB/c macrophages (A; untreated control, infected for 6 h [ii], and infected for 48 h [iii]) and BALB/c macrophages (B) pretreated with Fumonisin B1 (50 μM) (i), followed by infection for 6 h with Fumonisin B1 (50 μM) (ii), followed by infection for 48 h with Fumonisin B1 (50 μM) and PD98059(25 μM) (as described in the legends to Fig. 9), followed by infection for 48 h (iii). (C) C.D2 macrophages: (i) control cells, (ii) cells at 6 h of infection, (iii) cells at 48 h of infection, and (iv) cells that had been pretreated with PD98059 (25 μM) and infected for 48 h. Magnification, ×1,600.
DISCUSSION
The present study clearly underscores the importance of cellular ceramide in host cell deactivation during experimental visceral leishmaniasis. From our previous report (16) it was understood that ceramide deactivates the kinases protein kinase C and ERK and suppresses NO generation in leishmaniasis-susceptible BALB/c macrophages infected with L. donovani. The implications of ERK downregulation are highlighted in the present study since it was found to be instrumental in the induction of transcription factors AP-1 and NF-κB and NO generation. A comparative study in C.D2 has aided our investigations, since C.D2 is a parallel system of murine host conferring resistance phenomena against leishmaniasis (33; Bradley, letter). The natural resistance to leishmanial infection is exhibited by a population of mice, which expresses the resistance allele of Ity/Bcg/Lsh gene (Bradley, letter). C.D2 has been developed as a BALB/c congenic strain (9, 32), expressing the natural resistance gene Ity/Lsh/Bcg (32), and these two strains provide a valuable set for the functional studies of resistant and susceptible host system (7, 40, 47, 54). The candidate gene for the natural resistance Ity/Lsh/Bcg has been cloned and designated Nramp (natural resistance-associated macrophage protein) that has been found to function as a transporter of divalent cations (24, 49, 51).
In our study, Fig. 1 indicates that the Lshr strain exhibited an initial rise in intracellular ceramide level, but it was restricted from a period after 12 h of infection; at 24 h postinfection, ceramide in infected cells returned to the basal level. This showed that elevation of intracellular ceramide level was associated with the successful infection and pathogenesis in case of leishmaniasis. The resistance phenomenon in infected macrophages ensues after NRAMP activation (24). However, the exact molecular mechanism of the downregulation of ceramide synthesis in C.D2 remains to be elucidated.
Enhanced ceramide generation had been previously demonstrated by our laboratory to be associated with inhibition of ERK phosphorylation in infected BALB/c cells (16). We were further interested to study the course of ERK activation in the infected BALB/c and C.D2 cells from 12 h postinfection, from which a prominent difference in ceramide levels had been noted in the two systems (Fig. 1). Dual phosphorylation of ERKs in both threonine and tyrosine residues is an essential prerequisite for the enzyme activation (2, 27). By using the antibody which recognizes ERK when phosphorylated at both Thr202 and Tyr204, it was noted that in the resistant C.D2 macrophages, ERK1 (p44) and -2 (p42) were activated due to infection, whereas this event was completely inhibited in the infected BALB/c macrophages (Fig. 2A and B). In the later periods of incubation, the effect of ERK2 was found to be decreased both in the presence of LPS and in infection, a result that might be due to ERK2's sensitivity to the elevation of phosphatases during prolonged culture. Enhancement of ERK phosphorylation, therefore, seemed to be reciprocal to the decrease in ceramide generation in the C.D2 cells upon infection. Previously, we have shown that the activation of a tyrosine phosphatase mediates the inhibition of ERK phosphorylation in infected BALB/c macrophages (16). The important role of SHP-1 in the process has been highlighted in the study by Olivier et al. (14). It can be speculated from these studies that ceramide is involved in the induction of the phosphatase to downregulate the ERK activation in the infected cells.
We also studied the activation status of JNK, which, like ERK is known to influence the nuclear signaling events (21). Phosphorylation of JNK1 (p46) and -2 (p54) at threonine and tyrosine signifies the activation of JNK (44). Phosphorylation of these residues was inhibited in infected BALB/c macrophages as seen in Fig. 3A and B. This observation is supported by the recent study of Prive and Descoteaux, who have reported that the leishmanial parasites failed to induce JNK in naive bone marrow macrophages (38). In contrast, phosphorylation of JNK was evident in the infected C.D2 macrophages, a finding which coincided with the fact that excess ceramide synthesis was inhibited under similar circumstances (Fig. 3C). A previous study clearly indicated the activation of a tyrosine phosphatase in infected BALB/c macrophages, which dephosphorylated ERK1 and -2 (16). Preliminary studies suggest that endogenous ceramide induces phosphatidylinositol 3-kinase, which might selectively activate a tyrosine phosphatase (data not shown), but studies to identify the phosphatase and its characterization are under way. Thus, for the present consideration, selective activation of a tyrosine phosphatase by ceramide could be the putative mediator of ERK and JNK deactivation in L. donovani infection.
L. donovani induced prolonged phosphorylation of ERK and JNK in the C.D2 cells (Fig. 2 and 3). The functional significance of the MAPK pathway encompasses the activation of several transcription factors such as Elk-1/p62TCF, c-Myc, and EBPβ, which can be phosphorylated by MAPK in vitro (17, 35, 43). MAPK enhances the expression of c-fos mRNA in macrophages (51). c-fos happens to be one of the components of the AP-1 transcription factor complex, which may explain, at least in part, our observation that L. donovani infection in BALB/c cells failed to induce AP-1 activity (Fig. 4A). Inhibition of AP-1 activity was influenced by enhanced ceramide in the infected BALB/c cells, since FB1 was shown to induce AP-1 in these cells (Fig. 4A). In contrast, induction of AP-1 was evident in infected C.D2 cells (Fig. 4B). The importance of ERK in AP-1 induction was highlighted by the fact that ectopic inhibition of ERK in the infected C.D2 cells by the specific inhibitor drug PD98059 failed to induce AP-1 DNA-binding activity (Fig. 4). Also, in case of BALB/c cells, when FB1-mediated ERK activation was blocked by PD98059, the AP-1 activity was inhibited, emphasizing the requirement of ERK for AP-1 induction.
BALB/c cells failed to activate NF-κB upon infection with L. donovani whereas, in contrast, C.D2 cells exhibited enhanced NF-κB DNA-binding activity during infection (Fig. 5). Activation of MAPK was also found to be imperative for NF-κB activation (Fig. 5). We show here that inhibition of ERK by PD98059 failed to induce DNA-binding activity of NF-κB in the infected C.D2 cells, indicating ERK as a common regulator of both these two transcription factors in leishmania-infected macrophages.
NO generation in unstimulated control or infected macrophages could be due to expression of the constitutive form of the enzyme nitric oxide synthase, and therefore the minute changes are undetectable with respect to infection or treatments. Hence, LPS is used as positive stimulus for cells to assay iNOS activity in a detectable range. NO generation was inhibited in BALB/c macrophages in response to leishmanial challenge, whereas C.D2 macrophages released elevated level of NO after 36 h of in vitro infection compared to untreated controls (Fig. 6). Inhibition of ceramide by FB1 in infected BALB/c cells induced NO generation (Fig. 7A). FB1-induced NO generation in BALB/c cells was decreased after further inhibition of ERK by PD98059 (Fig. 7A). Inhibition of ERK inhibited NO generation in C.D2 macrophages (Fig. 7B). MAPK- and NF-κB-dependent induction of NO generation has also been reported in the case of tuberculosis (10). It is well established that NF-κB is one of the transcriptional requirement for induction of NO synthesis (23). The promoter of the murine gene encoding iNOS contains NF-κB site beginning 55 bp upstream of the TATA box (54). However, both AP-1 and NF-κB have been implicated in the transactivation of the iNOS promoter in various reports (27, 29).
Comparison of the leishmanicidal activity between BALB/c and C.D2 cells revealed that BALB/c cells harbored intracellular parasites at 48 h of infection, but C.D2 cells induced killing of amastigotes at 48 h (Fig. 8). Figure 9A and B suggest that the induction of parasite killing by macrophage was associated with restricted ceramide generation, followed by the induction of ERK. Thus, collectively, ceramide was found to be involved in the suppression of ERK activation, AP-1 and NF-κB activity, and NO generation, as well as facilitating the intracellular survival of parasites in BALB/c. On the other hand, protection against leishmanial pathogenesis in the leishmaniasis-resistant strain involved suppression of the enhanced ceramide generation after infection. Hence, from our study it becomes apparent that the regulation of the intracellular ceramide generation in macrophage during L. donovani infection might open up a route to evoke antiparasitic cellular responses and thereby restrict the progression of the disease.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Almendral, J. M., D. Sommer, and H. McDonald-Bravo. 1988. Complexity of early genetic response to growth factors in mouse fibroblasts. Mol. Cell. Biol. 8:2140-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, N. G., J. L. Maller, N. K. Tonks, and T. W. Sturgill. 1990. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature 343:651-653. [DOI] [PubMed] [Google Scholar]
- 3.Angel, P., and M. Karin. 1991. Role of Jun Fos and the AP-1 complex in cell proliferation and transformation. Biochim. Biophys. Acta 1072:129-157. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1993. Current protocols in molecular biology, vol 2. John Wiley & Sons, Inc., New York, N.Y.
- 5.Babu, G. J., M. J. Lalli, M. A. Sussman, J. Sadoshima, and M. Periasamy. 2000. Phosphorylation of elk-1 by MEK/ERK pathway is necessary for c-fos gene activation during cardiac myocyte hypertrophy. J. Mol. Cell Cardiol. 32:1447-1457. [DOI] [PubMed] [Google Scholar]
- 6.Baeuerle, P. A., and T. Henkel. 1994. Function and activation of NF-κB in the immune system. Annu. Rev. Immunol. 12:141-179. [DOI] [PubMed] [Google Scholar]
- 7.Barton, C. H., S. H. Whitehead, and J. M. Blackwell. 1995. Nramp transfection transfers Ity/Lsh/Bcg-related pleiotropic effects on macrophage activation: influence on oxidative burst and nitric oxide pathways. Mol. Med. 3:267-279. [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharyya, S., S. Ghosh, P. L. Jhonson, S. K. Bhattacharya, and S. Majumdar. 2001. Immunomodulatory role of interleukin-10 in visceral leishmaniasis: defective activation of protein kinase C-mediated signal transduction events. Infect. Immun. 69:1499-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley, D. J., B. A. Taylor, J. M. Blackwell, E. P. Evans, and J. Freeman. 1979. Regulation of leishmanial populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin. Exp. Immunol. 37:7-14 [PMC free article] [PubMed] [Google Scholar]
- 10.Chan, E. D., K. R. Morris, J. T. Belisle, P. Hill, L. K. Remigio, P. J. Brennan, and D. W. Riches. 2001. Induction of inducible nitric oxide synthase-NO∗ by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-κB signaling pathways. Infect. Immun. 69:2001-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, F., L. M. Demers, V. Vallyathan, M. Ding, Y. Lu, V. Castanova, and X. Shi. 1999. Vanadate induction of NF-κB involves IκB kinase and SAPK/ERK kinase 1 in macrophages. J. Biol. Chem. 274:20307-20312. [DOI] [PubMed] [Google Scholar]
- 12.Das, S., S. Bhattacharyya, S. Ghosh, and S. Majumdar. 2000. Signal transduction mechanism in human neutrophil: comparative study between the ζ- and β-protein kinase isotypes. Mol. Cell. Biochem. 203:143-151. [DOI] [PubMed] [Google Scholar]
- 13.Fahey, T. J., K. J. Tracey, P. T. Olson, L. S. Cousens, W. G. Jones, G. T. Shires, and B. Sherry. 1992. Macrophage inflammatory protein 1 modulates macrophage function. J. Immunol. 148:2764-2769. [PubMed] [Google Scholar]
- 14.Forget, G., K. A. Siminovitch, S. Brochu, S. Rivest, D. Radzioch, and M. Olivier. 2001. Role of host phosphotyrosine phosphatase SHP-1 in the development of murine leishmaniasis. Eur. J. Immunol. 31:3185-3196. [DOI] [PubMed] [Google Scholar]
- 15.Gamen, S., I. Marzo, A. Anel, A. Pineiro, and J. Naval. 1996. CPP32 inhibition prevents Fas-induced ceramide generation and apoptosis in human cells. FEBS Lett. 390:233-237. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh, S., S. Bhattacharyya, S. Das, S. Raha, N. Maulik, D. K. Das, S. Roy, and S. Majumdar. 2001. Generation of ceramide in murine macrophages infected with Leishmania donovani alters macrophage signaling events and aids intracellular parasite survival. Mol. Cell. Biochem. 323:47-60. [DOI] [PubMed] [Google Scholar]
- 17.Gille, H., A. D. Sharrocks, and P. E. Shaw. 1992. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature 358:414-417. [DOI] [PubMed] [Google Scholar]
- 18.Green, S. J., R. M. Crawford, J. T. Hockmeyer, M. S. Meltzer, and C. A. Nacy. 1990. Leishmania major amastigotes initiate the l-arginine-dependent killing mechanism, in IFN-γ-stimulated macrophages by induction of tumor necrosis factor-α. J. Immunol. 145:4290-4297. [PubMed] [Google Scholar]
- 19.Guha, M., and N. Mackman. 2001. LPS induction of gene expression in human monocytes. Cell Signal. 2:85-94. [DOI] [PubMed] [Google Scholar]
- 20.Hart, D. T., K. Vickerman, G. H. Coombs, and A. Qucik. 1981. Simple method for purifying L. mexicana amastigotes in large numbers. Parasitology 82:345-355. [DOI] [PubMed] [Google Scholar]
- 21.Hibi, M., A. Lin, T. Smeal, A. Minden, and M. Karin. 1993. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 11:2135-2148. [DOI] [PubMed] [Google Scholar]
- 22.Huang, H., S. B. Petkova, R. G. Pestell, B. Bouzahzah, J. Chan, H. Magazine, L. M. Weiss, G. J. Christ, M. P. Lisanti, S. A. Douglas, V. Shtutin, S. K. Halonen, M. Wittner, and H. B. Tanowitz. 2000. Trypanosoma cruzi infection (Chagas' disease) of mice causes activation of mitogen-activated protein kinase cascade and expression of endothelin-1 in the myocardium. J. Cardiovasc. Pharmacol. 36:S148-S150. [DOI] [PubMed] [Google Scholar]
- 23.Hur, G. M., Y. S. Ryu, J. H. Hong, S. H. Bae, J. Y. Bae, S. G. Paik, Y. M. Kim, J. H. Seok, and J. H. Lee. 2001. Serum after partial hepatectomy stimulates iNOS gene transcription via downstream NF-κB site. Biochem. Biophys. Res. Commun. 284:607-613. [DOI] [PubMed] [Google Scholar]
- 24.Jabado, N., A. Jankowski, S. Dougaparsad, V. Picard, S. Grinstein, and P. Gros. 2000. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1(Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J. Exp. Med. 192:1237-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julkunen, I., T. Saraneva, J. Pirhonen, T. Ronni, K. Melen, and S. Matikanin. 2001. Molecular pathogenesis of influenza A virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 12:171-180. [DOI] [PubMed] [Google Scholar]
- 26.Kang, S. K., C. J. Tai, K. W. Cheng, and P. C. Leung. 2000. Gonadotropin-releasing hormone activates mitogen-activated protein kinase in human ovarian and placental cells. Mol. Cell Endocrinol. 170:143-151. [DOI] [PubMed] [Google Scholar]
- 27.Kristof, A. S., J. Marks-Konczalik, and J. Moss. 2001. Mitogen-activated protein kinases mediate activator protein-1-dependent human inducible nitric-oxide synthase promoter activation. J. Biol. Chem. 276:8445-8452. [DOI] [PubMed] [Google Scholar]
- 28.Kwan, W. C., W. R. McMaster, N. Wong, and N. E. Reiner. 1992. Inhibition of expression of major histocompatibility complex class II molecules in macrophages infected with Leishmania donovani occurs at the level of gene transcription via a cyclic AMP-independent mechanism. Infect. Immun. 60:2115-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowenstein, C. J., W. A. Evans, P. Raval, A. M. Snowman, S. H. Snyder, S. W. Russell, and W. J. Murphy. 1993. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon and lipopolysaccharide. Proc. Natl. Acad. Sci. USA 90:9730-9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majumdar, S., M. W. Rossi, T. Fujiki, W. A. Phillips, S. Disa, C. F. Queen, R. B. Johnston, O. M. Rosen, B. E. Corkey, and H. M. Korchak. 1991. Protein kinase C isotypes and signaling in neutrophils. J. Biol. Chem. 266:9285-9294. [PubMed] [Google Scholar]
- 31.Manning, A., and A. Rao. 1997. Ikk-1 and Ikk-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278:860-866. [DOI] [PubMed] [Google Scholar]
- 32.Mock, B. A., D. L. Holiday, D. P. Cerretti, S. C. Darnell, A. D. O'Brien, and M. Potter. 1994. Construction of a series of congenic mice with recombinant chromosome 1 regions surrounding the genetic loci for resistance to intracellular parasites (Ity, Lsh, and Bcg), DNA repair responses (Rep-1), and the cytoskeletal protein villin (Vil). Infect. Immun. 62:325-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller, J. M., M. A. Cahill, R. A. Rupec, P. A. Baeuerle, and A. Nordheim. 1997. Antioxidants as well as oxidants activate c-fos via Ras-dependent activation of extracellular-signal-regulated kinase 2 and Elk-1. Eur. J. Biochem. 244:45-52. [DOI] [PubMed] [Google Scholar]
- 34.Nakajima, T., S. Kinoshita, T. Sasagawa, K. Sasaki, M. Naruto, T. Kishimoto, and S. Akira. 1993. Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL6. Proc. Natl. Acad. Sci. USA 90:2207-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nandan, D., R. Lo, and N. E. Reiner. 1999. Activation of phosphotyrosine phosphatase attenuates mitogen-activated protein kinase signaling and inhibits c-Fos and nitric oxide synthase expression in macrophages infected with Leishmania donovani. Infect. Immun. 67:4055-4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newton, R., D. A. Stevens, L. A. Hart, M. Lindsay, I. M. Adcock, and P. J. Barnes. 1997. Superinduction of COX-2 mRNA by cycloheximide and interleukin-1β involves increased transcription and correlates with increased NF-κB and JNK activation. FEBS Lett. 418:135-138. [DOI] [PubMed] [Google Scholar]
- 37.Olivier, M., R. W. Brownsey, and N. E. Reiner. 1992. Defective stimulus-response coupling in human monocytes infected with Leishmania donovani is associated with altered activation and translocation of protein kinase C. Proc. Natl. Acad. Sci. USA 89:7481-7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prive, C., and A. Descoteaux. 2000. Leishmania donovani promastigotes evade the activation of mitogen-activated protein kinases p38, c-Jun N-terminal kinase, and extracellular signal-regulated kinase-1/2 during infection of naive macrophages. Eur. J. Immunol. 8:2235-2244. [DOI] [PubMed] [Google Scholar]
- 39.Proudfoot, L., A. Nikolaev, G. J. Feng, X. Q. Wei, M. A. Ferguson, J. S. Brimacombe, and F. Y. Liew. 1996. Regulation of the expression of nitric oxide synthase and leishmanicidal activity by glycoconjugates of Leishmania lipophosphoglycan in murine macrophages. Proc. Natl. Acad. Sci. USA 93:10984-10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramarathinam, L., D. W. Niesel, and G. R. Klimpel. 1993. Ity influences the production of IFN-γ by murine splenocytes stimulated in vitro with Salmonella typhimurium. J. Immunol. 150:3965-3972. [PubMed] [Google Scholar]
- 41.Reiner, N. E., W. Ng, and W. R. McMaster. 1987. Parasite-accessory cell interactions in murine leishmaniasis. II. Leishmania donovani suppresses macrophage expression of class I and class II major histocompatibility complex gene products. J. Immunol. 138:1926-1932. [PubMed] [Google Scholar]
- 42.Saha, B., H. Roy, A. Pakrahsi, R. N. Chakraborti, and S. Roy. 1991. Immunological studies on experimental visceral leishmaniasis. I. Changes in lymphoid organs and their possible role in pathogenesis. Eur. J. Immunol. 21:577-581. [DOI] [PubMed] [Google Scholar]
- 43.Seth, A., F. A. Gonzalez, and S. Gupta. 1992. Signal transduction within nucleus by mitogen-activated protein kinase. J. Biol. Chem. 267:24796-24804. [PubMed] [Google Scholar]
- 44.Sluss, H. K., T. Barrett, B. Derijard, and R. J. Davis. 1994. Signal transduction by tumor necrosis factor mediated by JNK protein kinases. Mol. Cell. Biol. 14:8376-8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smeal, T., B. Binetruy, D. A. Mercola, M. Birrer, and M. Karin. 1991. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature 354:494-496. [DOI] [PubMed] [Google Scholar]
- 46.Stauber, L. A. 1958. Host resistance to the Khartoum strain of Leishmania donovani. Rice Institute Pamphlet 45:80. [Google Scholar]
- 47.Swanson, R. N., and A. D. O'Brien. 1983. Genetic control of the innate resistance of mice to Salmonella typhimurium: Ity gene is expressed in vivo by 24 hours after infection. J. Immunol. 131:3014-3020. [PubMed] [Google Scholar]
- 48.Vallejo, J. G., P. Knuefermann, D. L. Mann, and N. Sivasubramanian. 2000. Group B streptococcus induces TNF-α gene expression and activation of transcription factors NF-κB and activator protein-1 in human cord blood myocytes. J. Immunol. 165:419-425. [DOI] [PubMed] [Google Scholar]
- 49.Vidal, S., M. L. Tremblay, G. Govoni, S. Gauthier, G. Sebastiani, D. Malo, E. Skamene, M. Olivier, S. Jothy, and P. Gros. 1995. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J. Exp. Med. 182:655-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, X., and G. P. Studzinski. 2001. Activation of extracellular signal-regulated kinases (ERKs) defines the first phase of 1,2,5-dihydroxyvitamin D3-induced differentiation of HL-60 cells. J. Cell. Biochem. 80:471-482. [DOI] [PubMed] [Google Scholar]
- 51.Wardrop, S. L., and D. R. Richardson. 2000. Interferon-gamma and lipopolysaccharide regulate the expression of NRAMP2 and increase the uptake of iron from low relative molecular mass complexes by macrophages. Eur. J. Biochem. 267:6586-6593. [DOI] [PubMed] [Google Scholar]
- 52.Wilson, N. J., A. Jaworowski, A. C. Ward, and J. A. Hamilton. 1998. cAMP enhances CSF-1-induced ERK activity and c-fos mRNA expression via a MEK-dependent and Ras-independent mechanism in macrophages. Biochem. Biophys. Res. Commun. 244:475-480. [DOI] [PubMed] [Google Scholar]
- 53.Woodgett, J. R. 1990. fos and jun: two into one will go. Semin. Cancer Biol. 4:389-397. [PubMed] [Google Scholar]
- 54.Xie, Q. W., Y Kashiwabara, and C. Nathan. 1994. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J. Biol. Chem. 269:4705-4708. [PubMed] [Google Scholar]
- 55.Zwilling, B. S., M. Massie, L. Vespa, M. Kwasniewski, J. Nath, and W. LaFuse. 1988. Continuous expression of I-A by murine peritoneal macrophages is linked to the Bcg gene. Curr. Top. Microbiol. Immunol. 137:316-324. [DOI] [PubMed] [Google Scholar]