Abstract
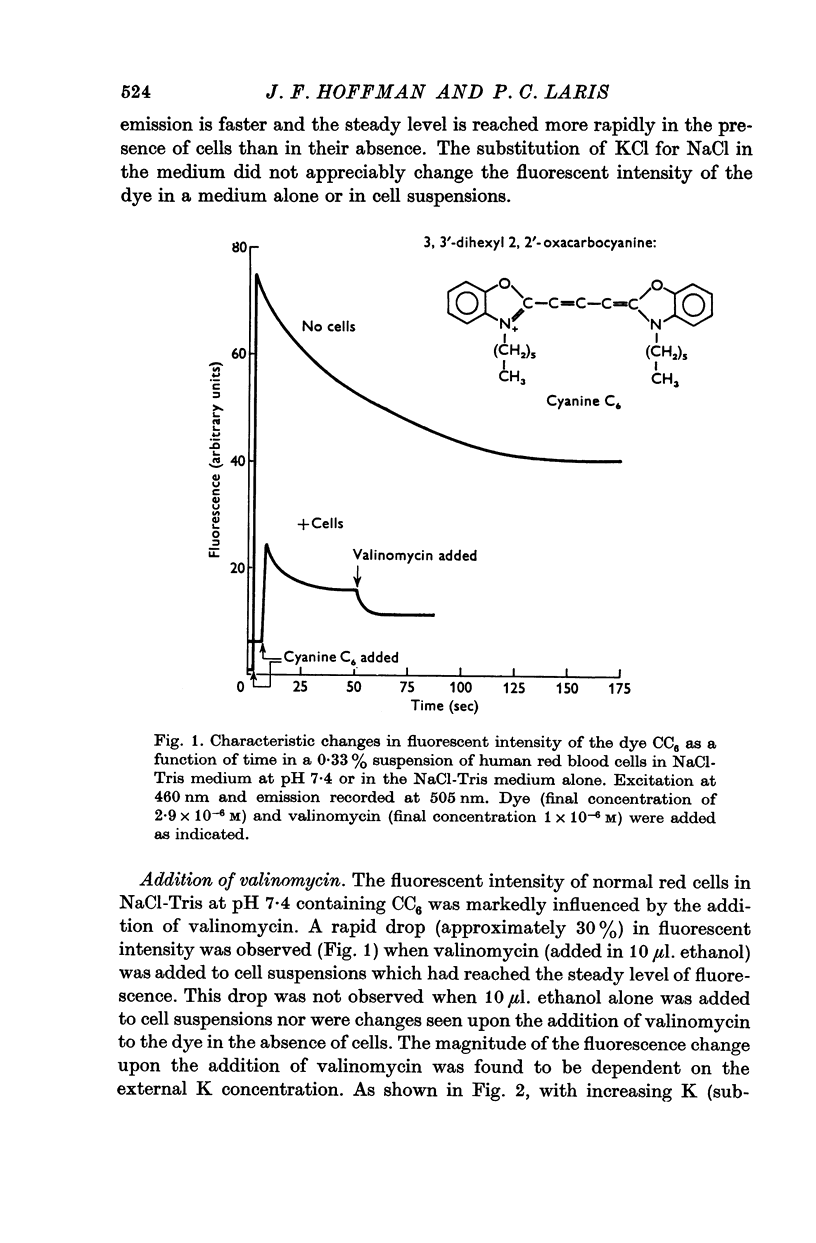
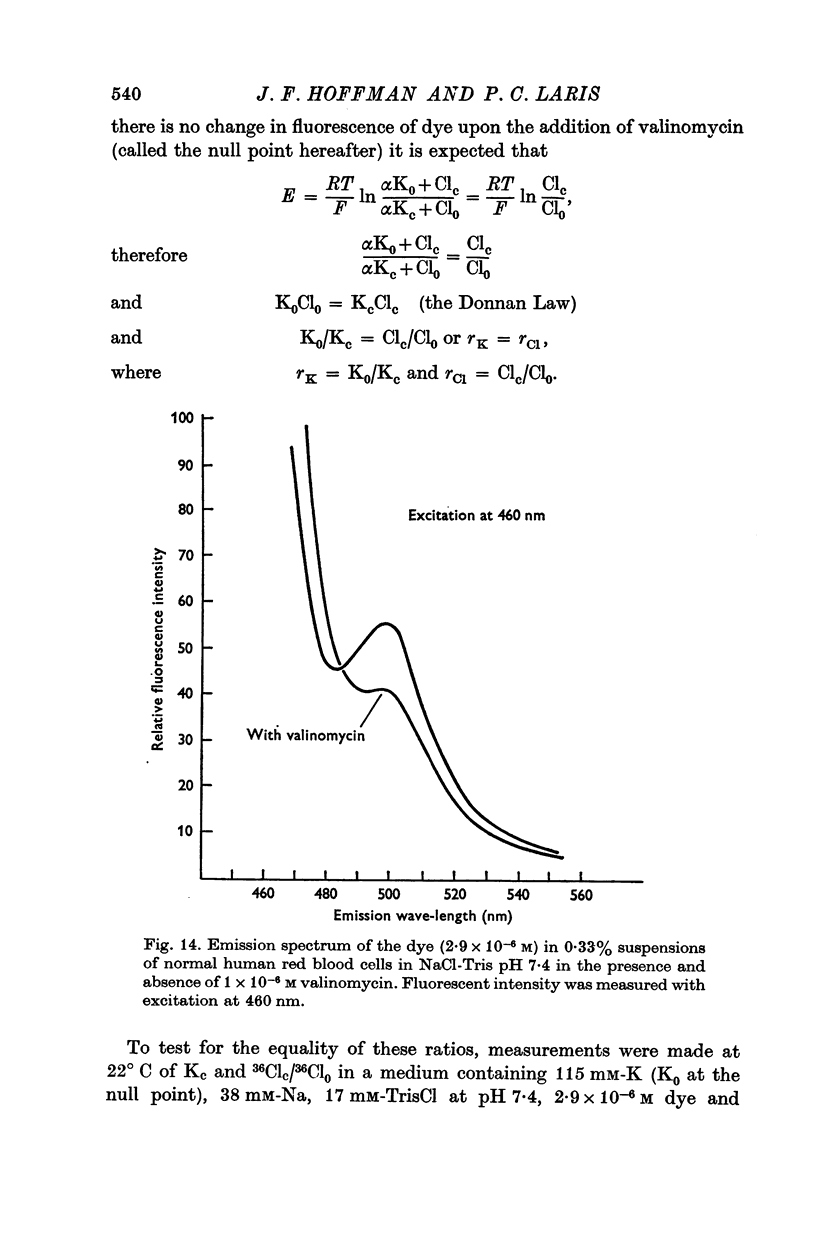
1. Changes in the fluorescent intensity of the dye, 3,3′-dihexyl-2,2′-oxacarbocyanine, added to suspensions of human and Amphiuma red blood cells were measured in parallel with changes in the membrane potentials of these cells. In these studies the membrane potential was altered in three different ways: by the addition of valinomycin to alter the ratio, PK/PCl, by a change in the pH of the medium to alter the ratio, Clc/Cl0, and by the substitution of impermeant anions for Cl0 again to alter the ratio, Clc/Cl0. In each case hyperpolarization led to a decrease and depolarization to an increase in fluorescent intensity.
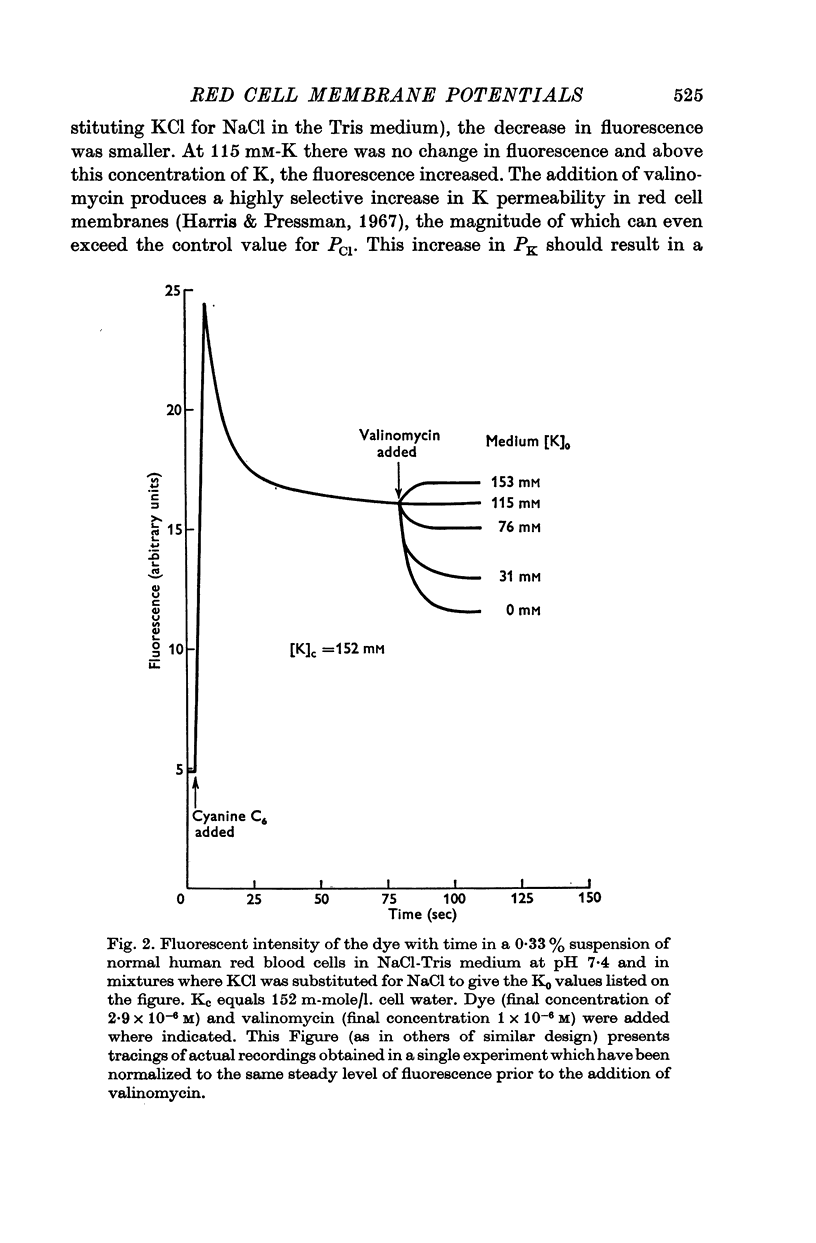
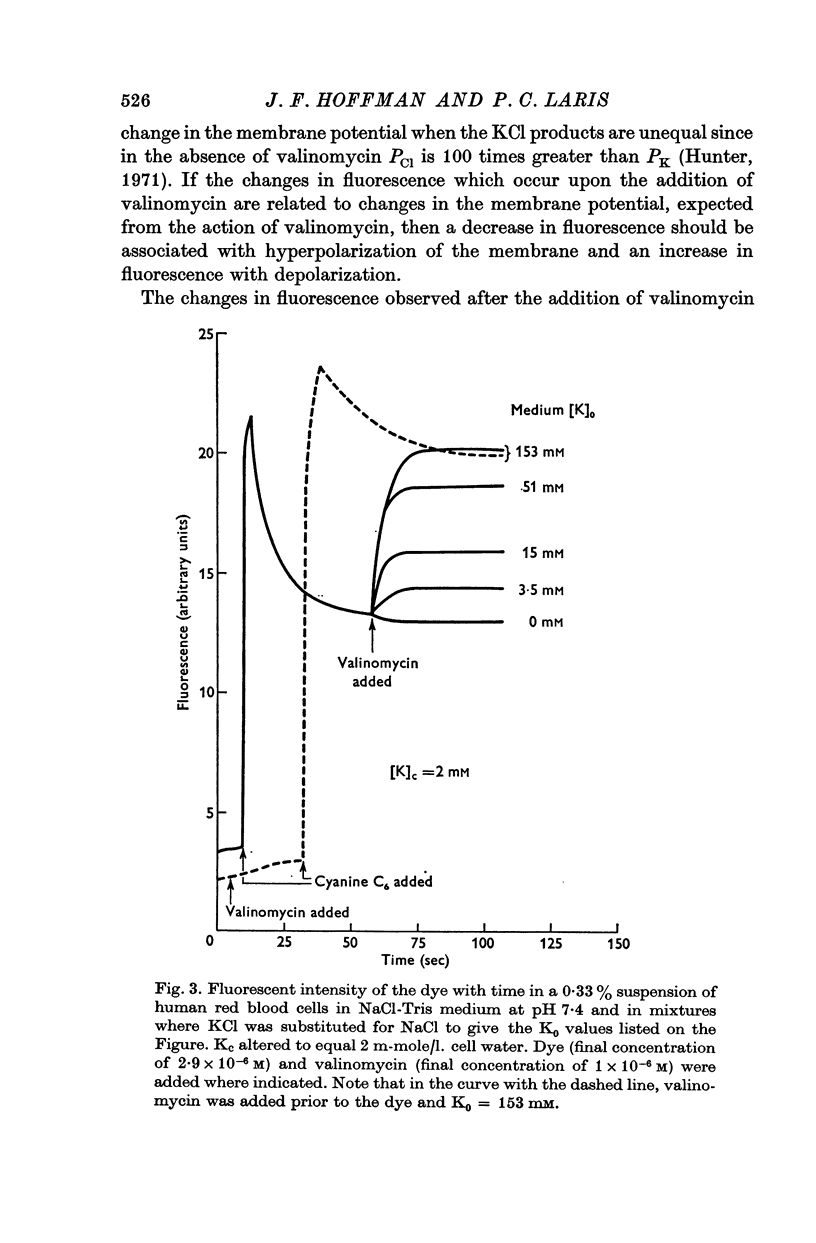
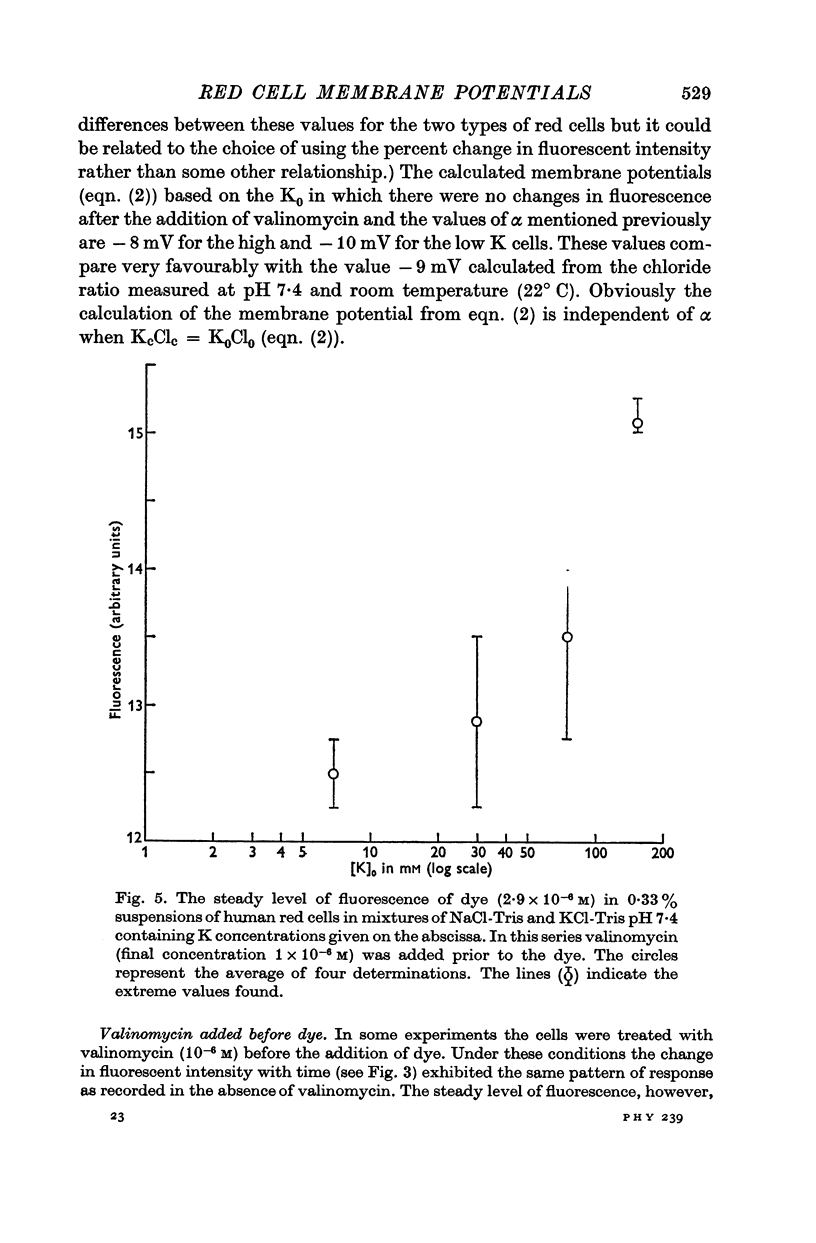
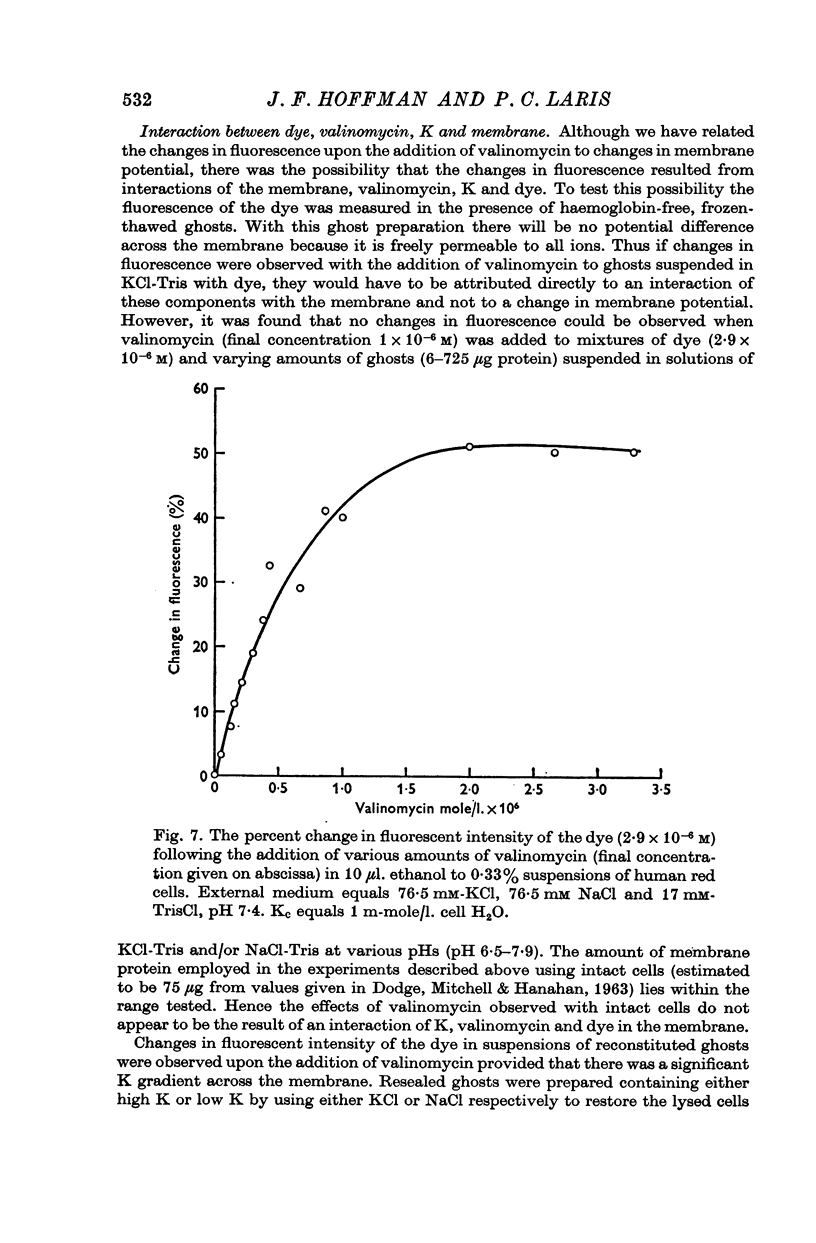
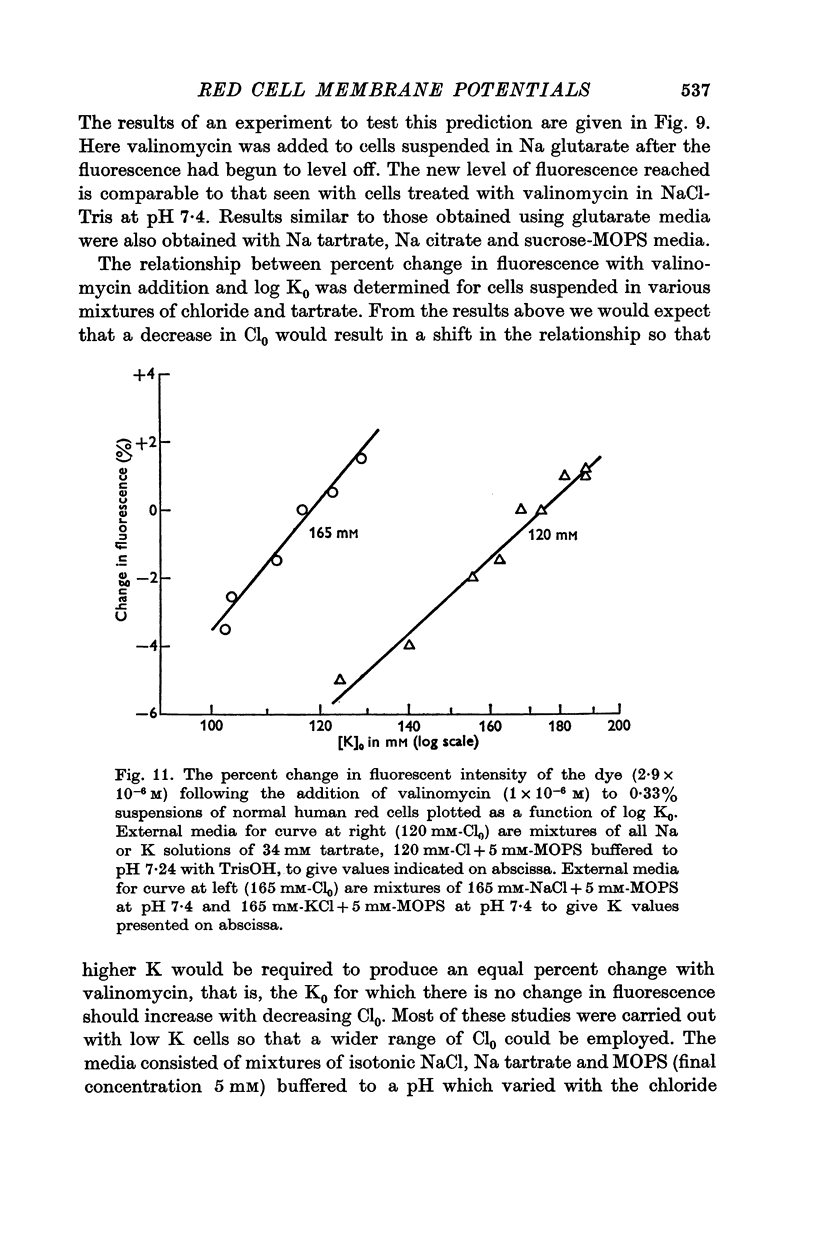
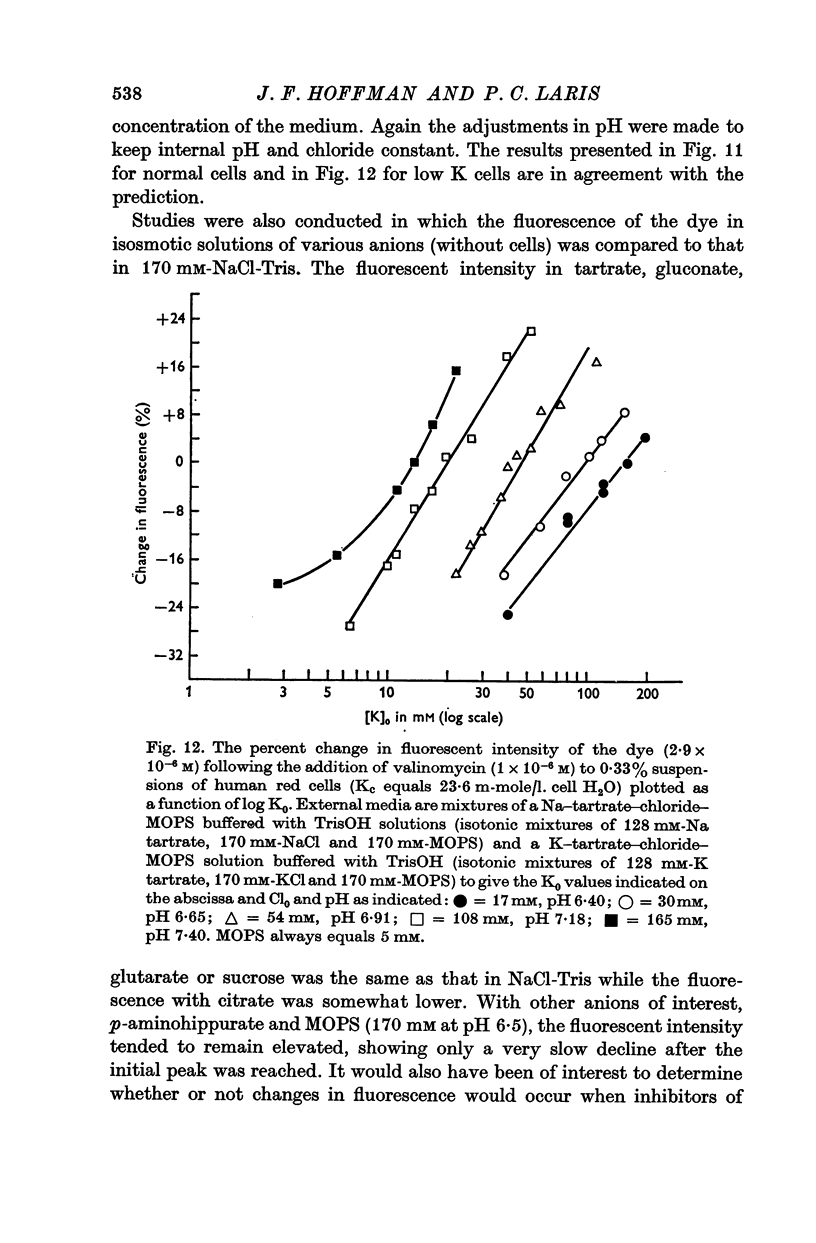
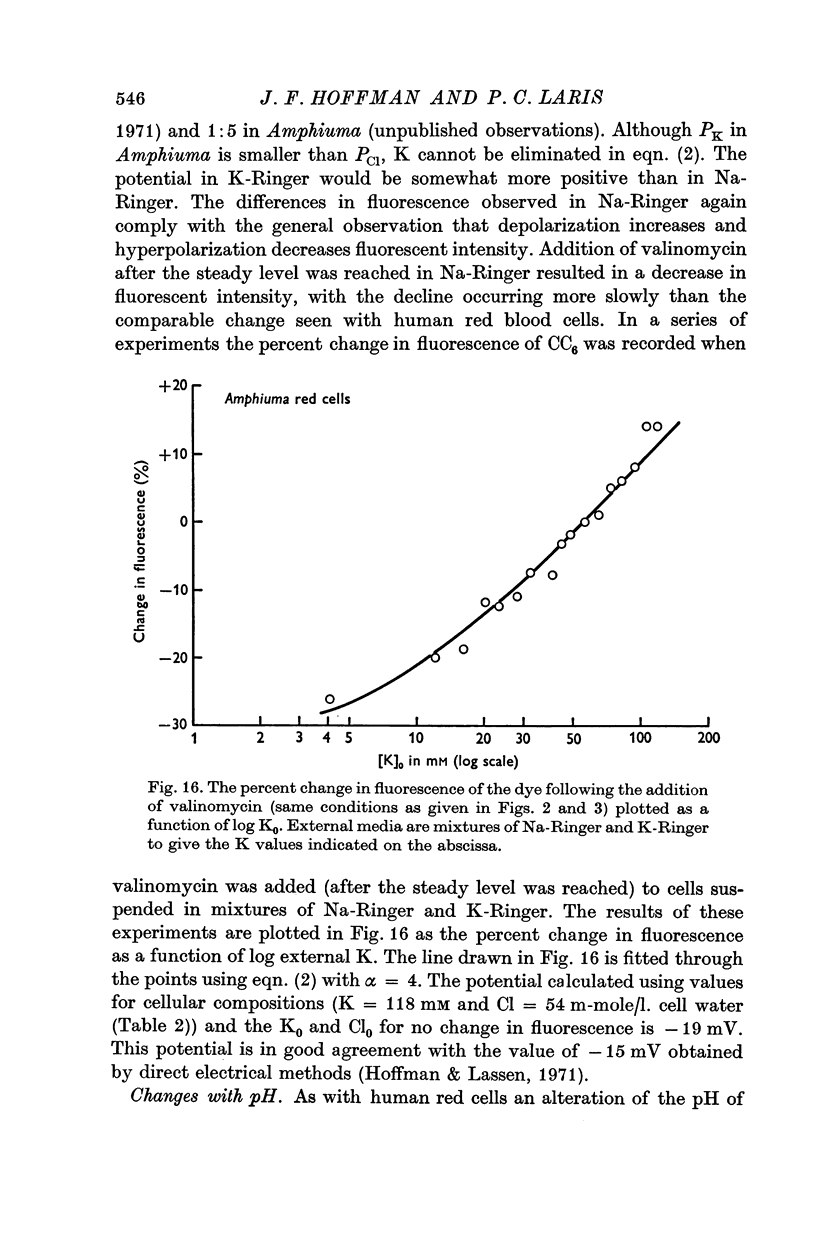
2. The change in fluorescence with the addition of valinomycin was dependent on the concentration of K in both the cells and the medium. Changes in fluorescence were not observed when valinomycin was added to suspensions of frozen-thawed, haemoglobin-free ghosts with dye in KCl or NaCl solutions. Such changes were observed with reconstituted ghosts provided that there was a K concentration gradient across the membrane.
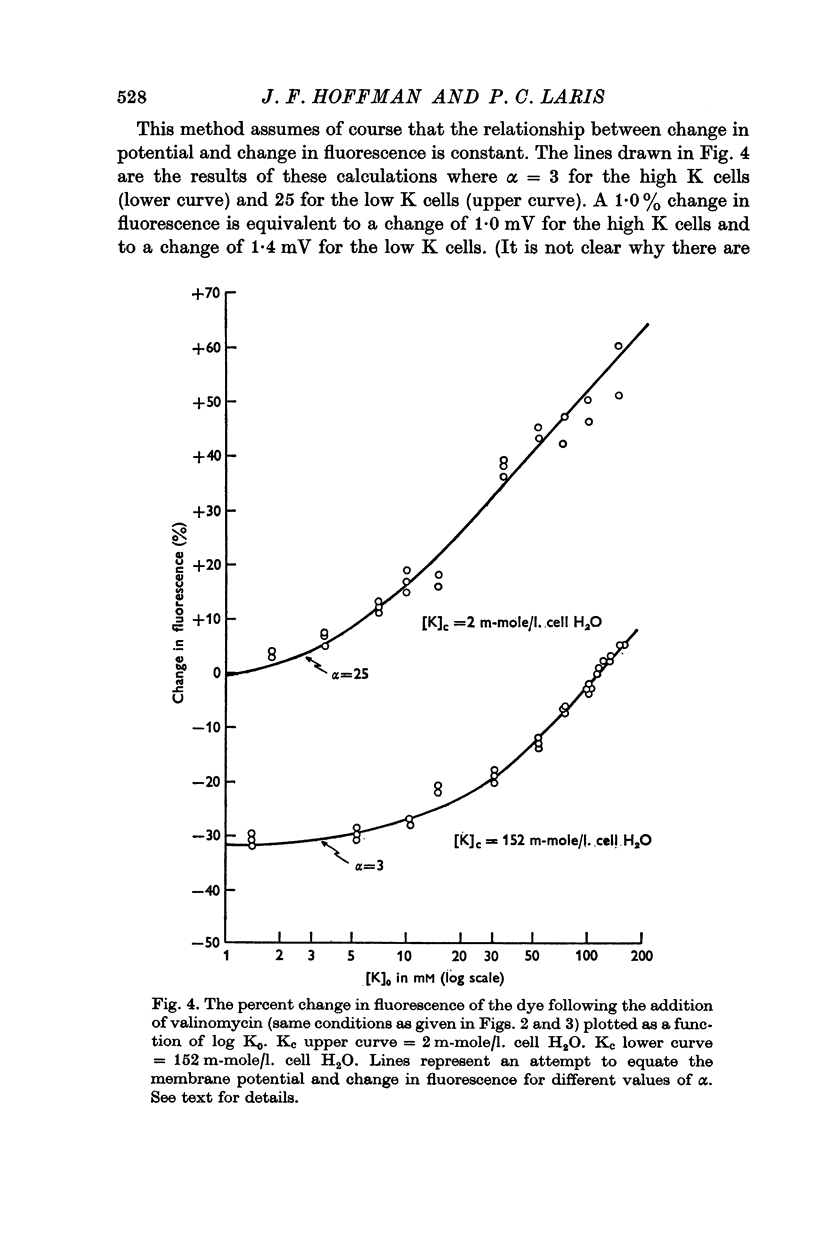
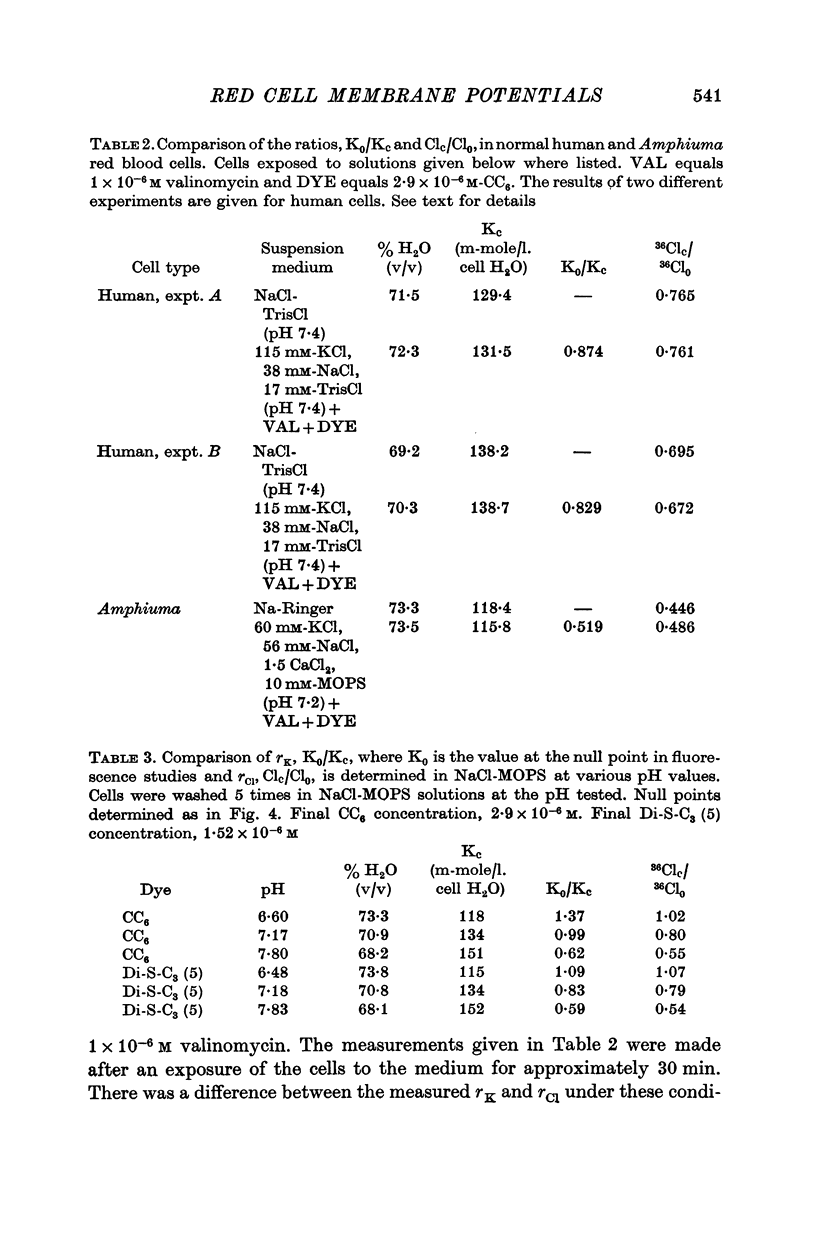
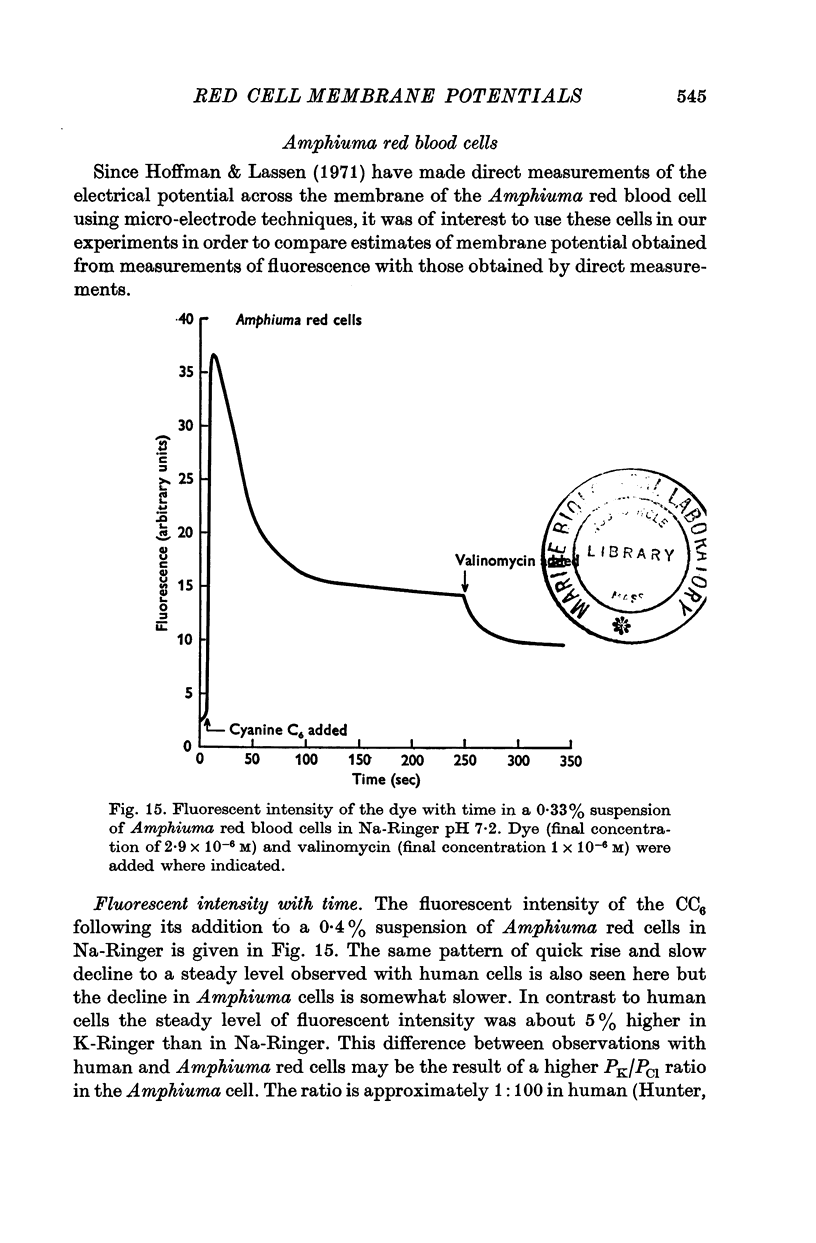
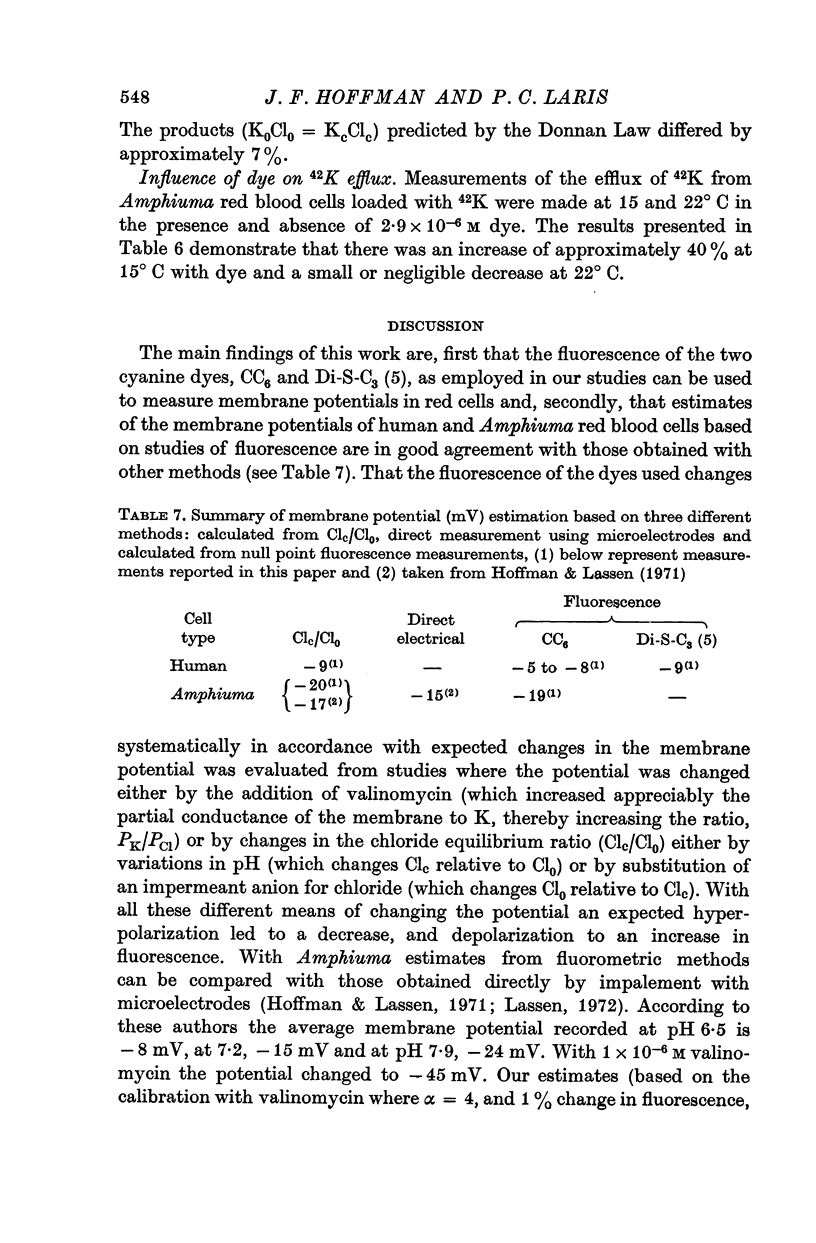
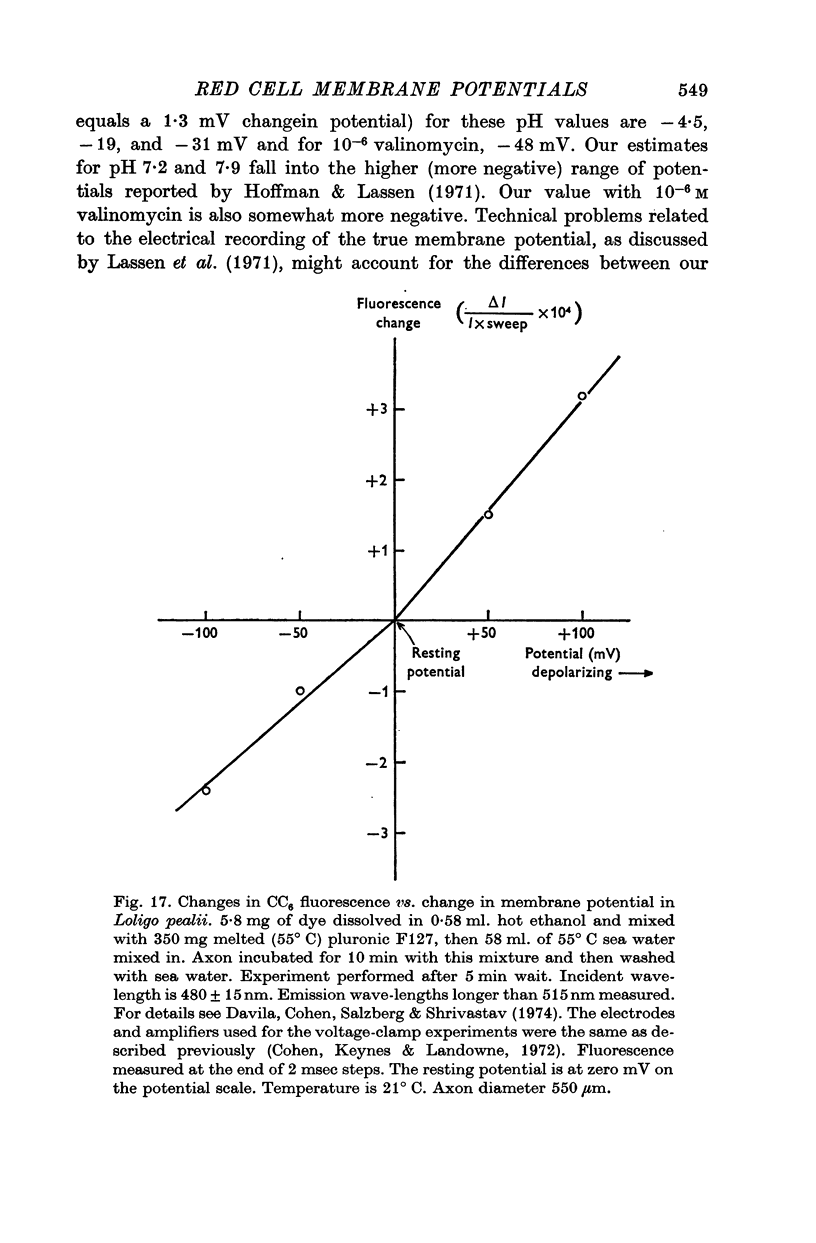
3. From values of cellular K and the corresponding external K concentrations for which there were no changes in fluorescence with valinomycin, estimations of membrane potentials were made. The potential was -5 to -8 mV for the human red cell and -19 mV for Amphiuma. These values are in good agreement with the potentials estimated from the Cl ratios (-9 mV for human and -17 to -20 mV for Amphiuma) and from those obtained by direct electrical measurements (-15 mV for Amphiuma).
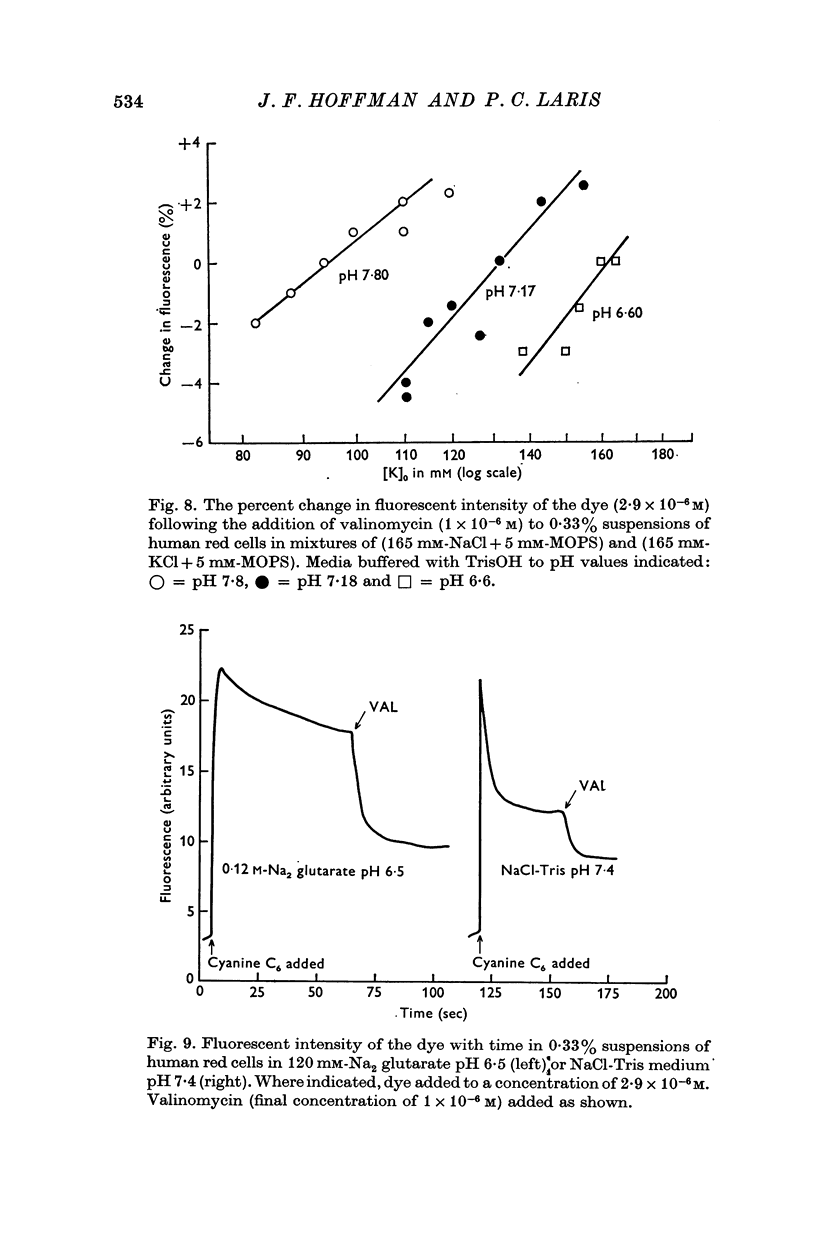
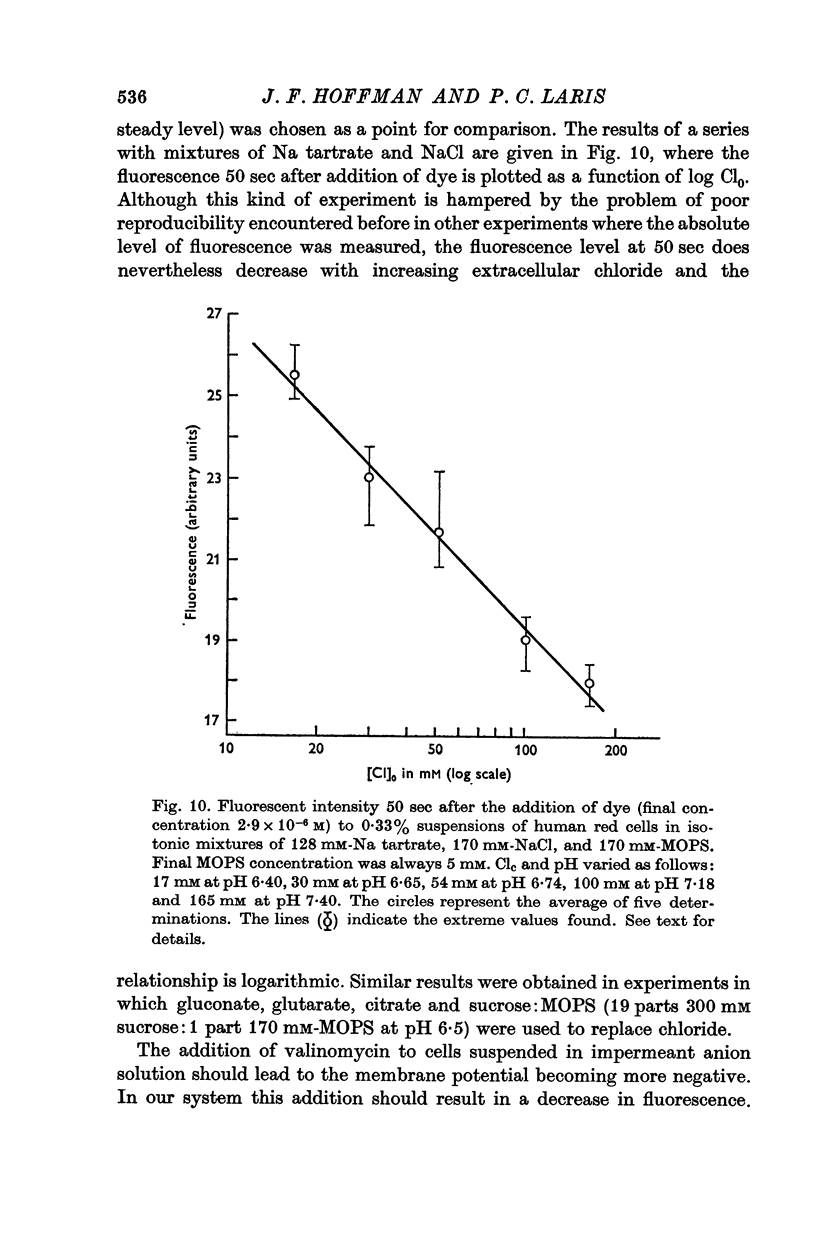
4. Fluorescent intensity of the dye in suspensions of human red cells was shown to be a linear function of the log Clc/Cl0.
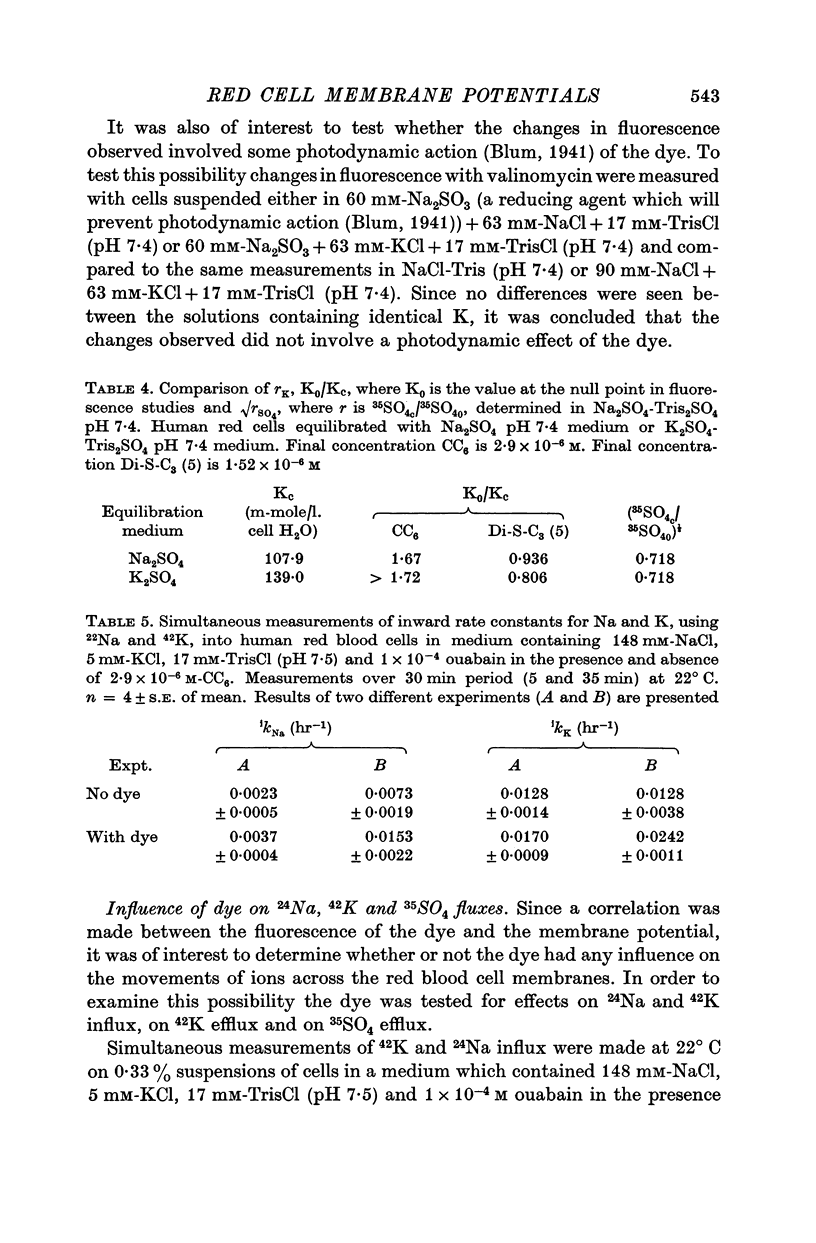
5. The dye (2·9 × 10-6 M) increased the inward rate constants for 24Na (3-4-fold) and 42K (0·5-2-fold) for human red cells. In addition, the dye was found to be haemolytic (5-6% in 1 hr) at 22° C.
6. In contrast, the dye did not alter the rate of 35SO4 efflux at 37° C from human red cells previously equilibrated with a Cl-free SO4 medium.
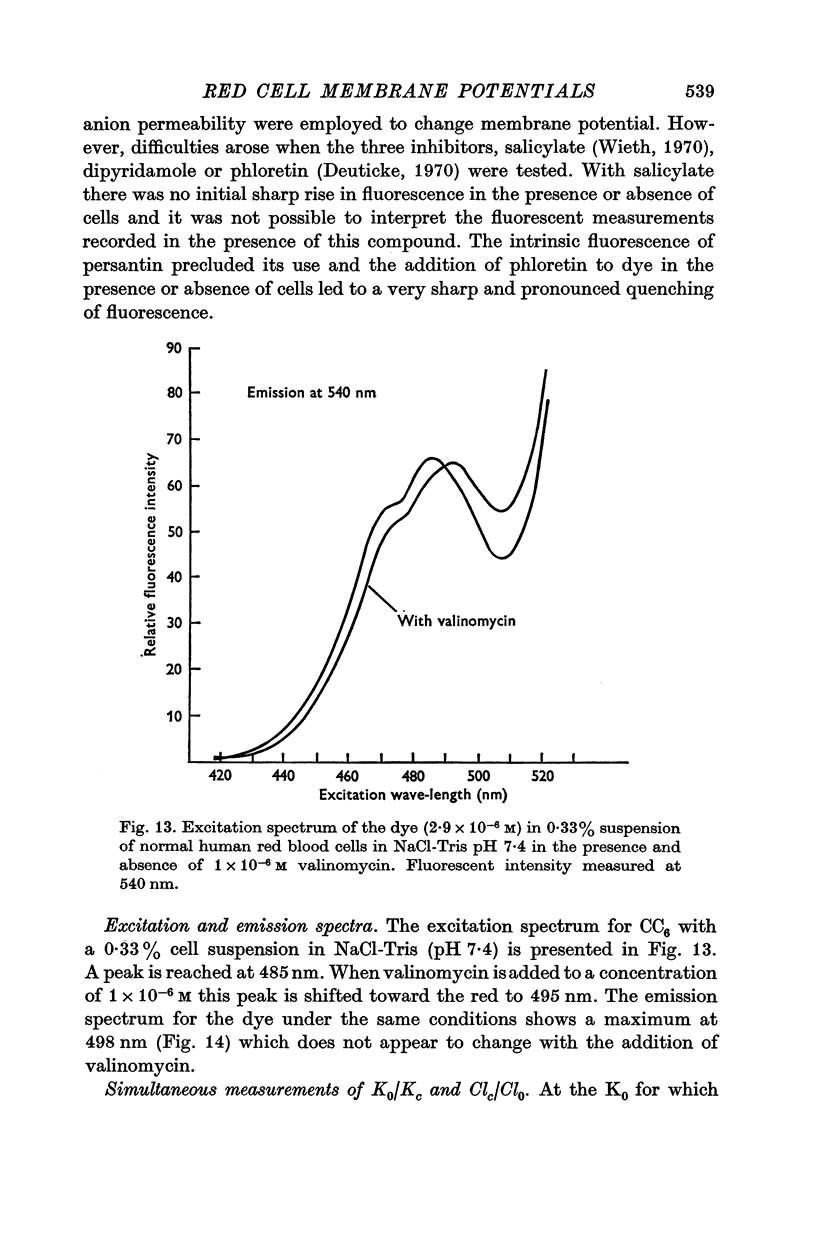
7. The dye was also seen to interact with certain impermeant anions and other compounds, e.g. inhibitors of anion permeability, of interest. These interactions and other limitations of the use of this dye are discussed.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodemann H., Passow H. Factors controlling the resealing of the membrane of human erythrocyte ghosts after hypotonic hemolysis. J Membr Biol. 1972;8(1):1–26. doi: 10.1007/BF01868092. [DOI] [PubMed] [Google Scholar]
- Cohen L. B. Changes in neuron structure during action potential propagation and synaptic transmission. Physiol Rev. 1973 Apr;53(2):373–418. doi: 10.1152/physrev.1973.53.2.373. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Landowne D. Changes in light scattering that accompany the action potential in squid giant axons: potential-dependent components. J Physiol. 1972 Aug;224(3):701–725. doi: 10.1113/jphysiol.1972.sp009919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Davila H. V., Salzberg B. M., Cohen L. B., Waggoner A. S. A large change in axon fluorescence that provides a promising method for measuring membrane potential. Nat New Biol. 1973 Jan 31;241(109):159–160. doi: 10.1038/newbio241159a0. [DOI] [PubMed] [Google Scholar]
- Deuticke B. Anion permeability of the red blood cell. Naturwissenschaften. 1970 Apr;57(4):172–179. doi: 10.1007/BF00592968. [DOI] [PubMed] [Google Scholar]
- Garrahan P. J., Rega A. F. Cation loading of red blood cells. J Physiol. 1967 Nov;193(2):459–466. doi: 10.1113/jphysiol.1967.sp008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEINZ E., HOFFMAN J. F. PHOSPHATE INCORPORATION AND NA, K-ATPASE ACTIVITY IN HUMAN RED BLOOD CELL GHOSTS. J Cell Physiol. 1965 Feb;65:31–43. doi: 10.1002/jcp.1030650106. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN J. F. The active transport of sodium by ghosts of human red blood cells. J Gen Physiol. 1962 May;45:837–859. doi: 10.1085/jgp.45.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., Pressman B. C. Obligate cation exchanges in red cells. Nature. 1967 Dec 2;216(5118):918–920. doi: 10.1038/216918a0. [DOI] [PubMed] [Google Scholar]
- Jay A. W., Burton A. C. Direct measurement of potential difference across the human red blood cell membrane. Biophys J. 1969 Feb;9(2):115–121. doi: 10.1016/S0006-3495(69)86372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf P. A., Rothstein A. Chemical modification of membranes. I. Effects of sulfhydryl and amino reactive reagents on anion and cation permeability of the human red blood cell. J Gen Physiol. 1971 Aug;58(2):190–210. doi: 10.1085/jgp.58.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lassen U. V., Sten-Knudsen O. Direct measurements of membrane potential and membrane resistance of human red cells. J Physiol. 1968 Apr;195(3):681–696. doi: 10.1113/jphysiol.1968.sp008482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepke S., Passow H. The effect of pH at hemolysis on the reconstitution of low cation permeability in human erythrocyte ghosts. Biochim Biophys Acta. 1972 Feb 11;255(2):696–702. doi: 10.1016/0005-2736(72)90174-5. [DOI] [PubMed] [Google Scholar]
- Sachs J. R. Recoupling the Na-K pump. J Clin Invest. 1972 Dec;51(12):3244–3247. doi: 10.1172/JCI107151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R., Welt L. G. The concentration dependence of active potassium transport in the human red blood cell. J Clin Invest. 1967 Jan;46(1):65–76. doi: 10.1172/JCI105512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A., Cecchetto A., Azzone G. F. Permeability of erythrocytes to anions and the regulation of cell volume. Nature. 1968 Aug 3;219(5153):529–531. doi: 10.1038/219529a0. [DOI] [PubMed] [Google Scholar]
- Scarpa A., Cecchetto A., Azzone G. F. The mechanism of anion translocation and pH equilibration in erythrocytes. Biochim Biophys Acta. 1970;219(1):179–188. doi: 10.1016/0005-2736(70)90073-8. [DOI] [PubMed] [Google Scholar]
- Warburg E. J. Studies on Carbonic Acid Compounds and Hydrogen Ion Activities in Blood and Salt Solutions. A Contribution to the Theory of the Equation of Lawrence J. Henderson and K. A. Hasselbach: Introduction. Biochem J. 1922;16(2):153–154. doi: 10.1042/bj0160153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieth J. O. Effect of some monovalent anions on chloride and sulphate permeability of human red cells. J Physiol. 1970 May;207(3):581–609. doi: 10.1113/jphysiol.1970.sp009082. [DOI] [PMC free article] [PubMed] [Google Scholar]


