Abstract
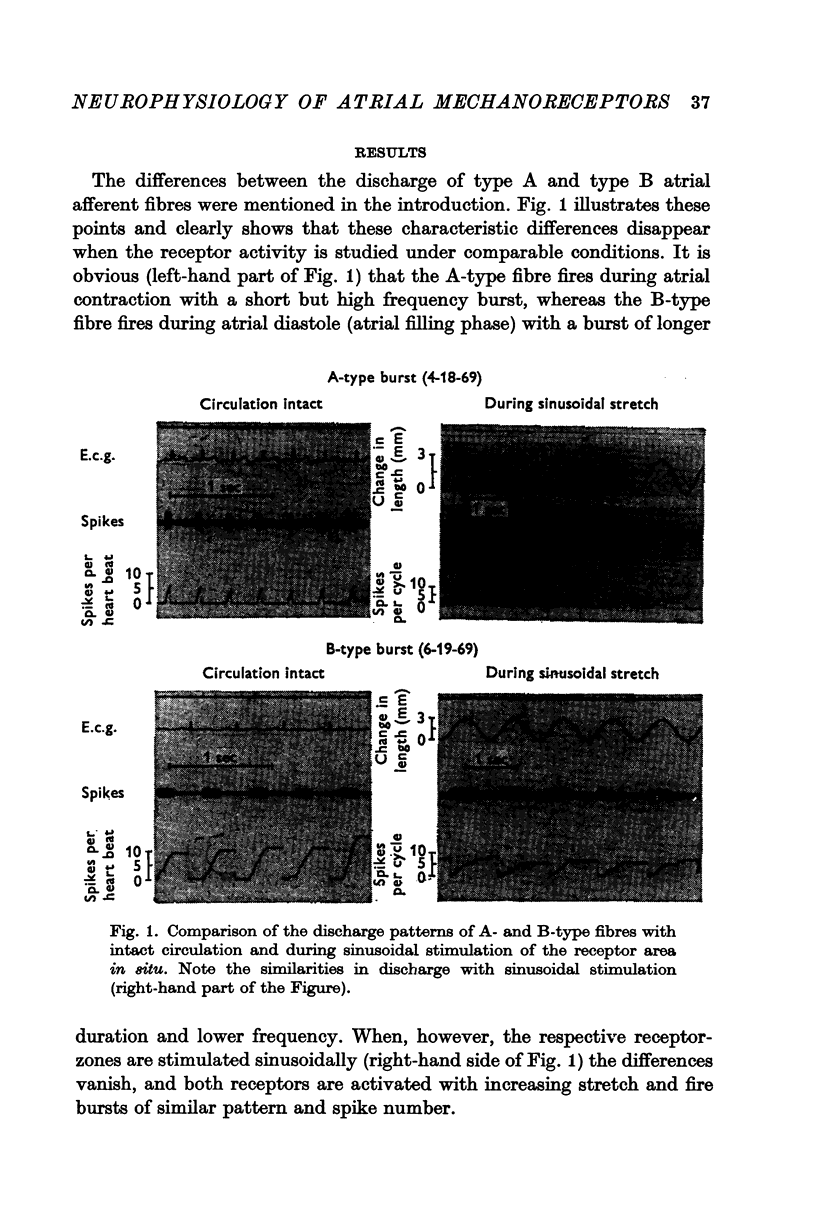
1. The differences in spike discharge from atrial mechanoreceptors in vivo suggest the existence of different receptor types. To test this possibility, and to see how atrial receptors compare with other mechanoreceptors, their response was studied under comparable stimulus conditions.
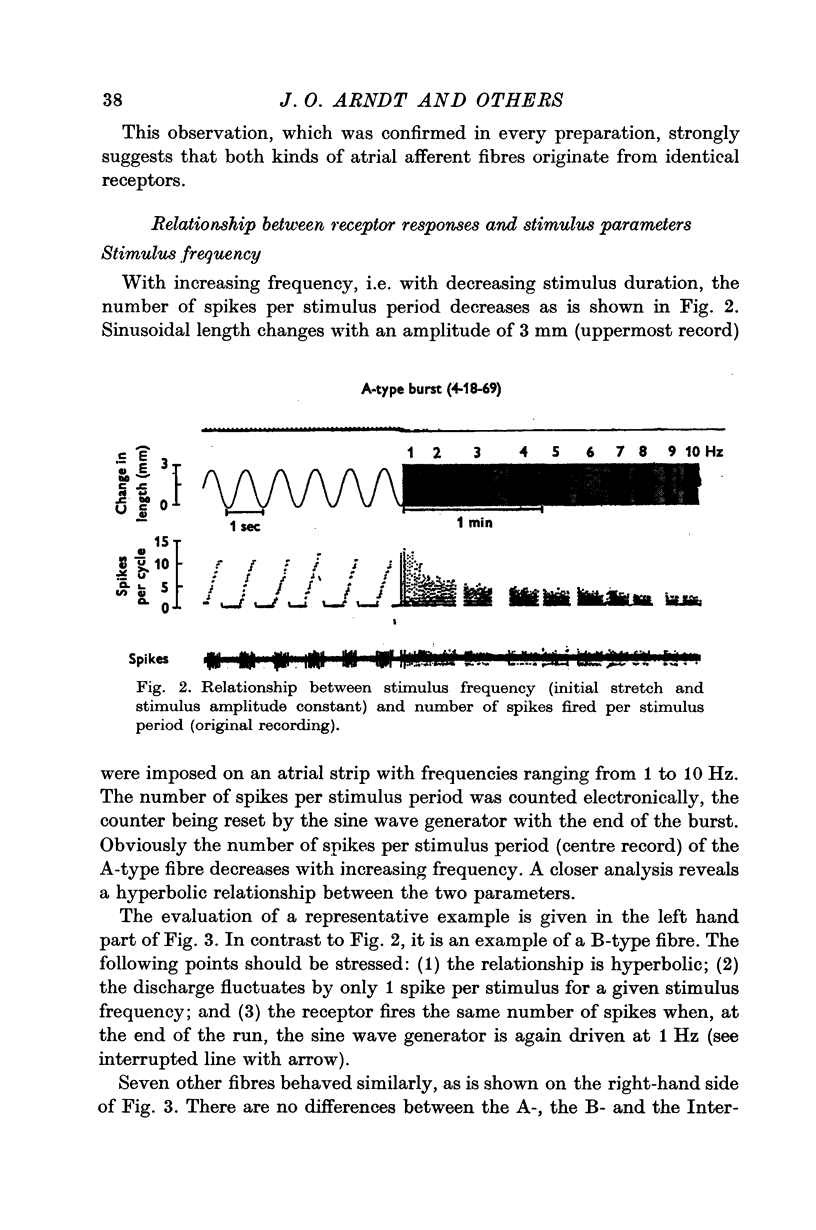
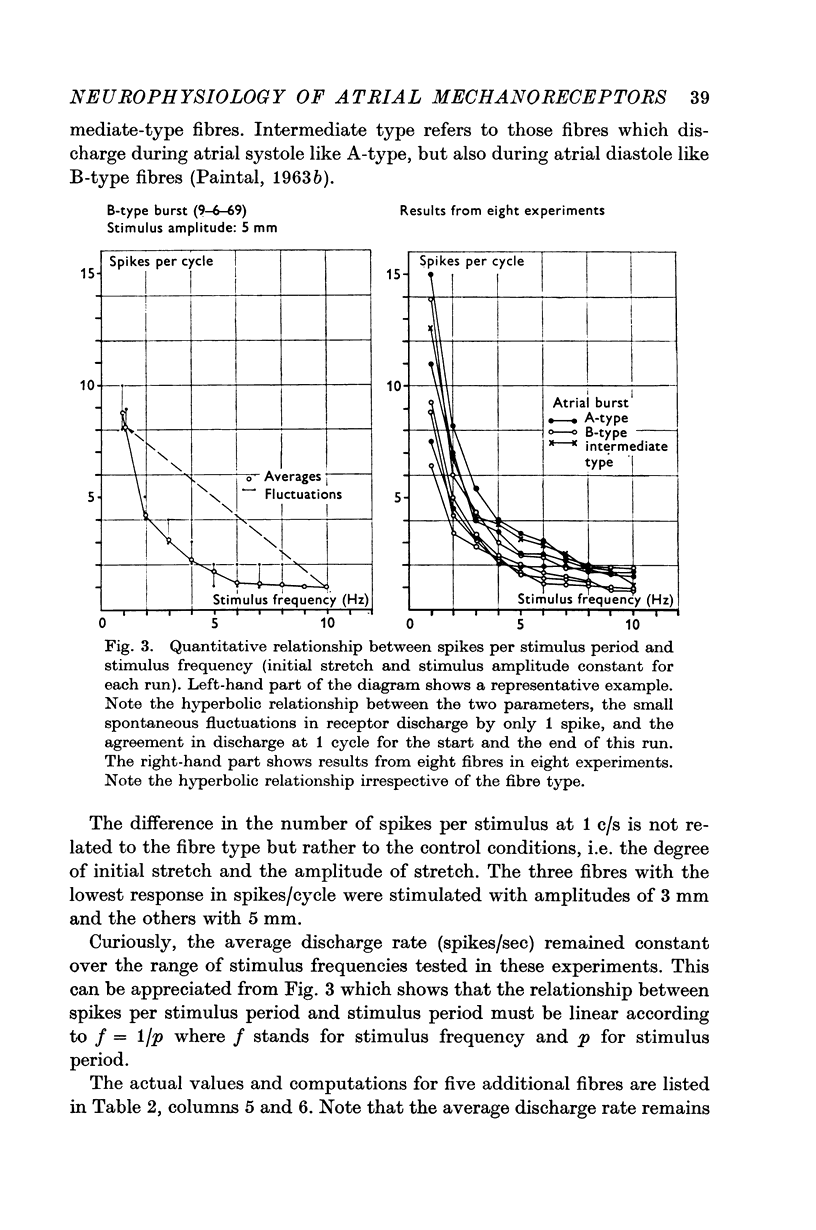
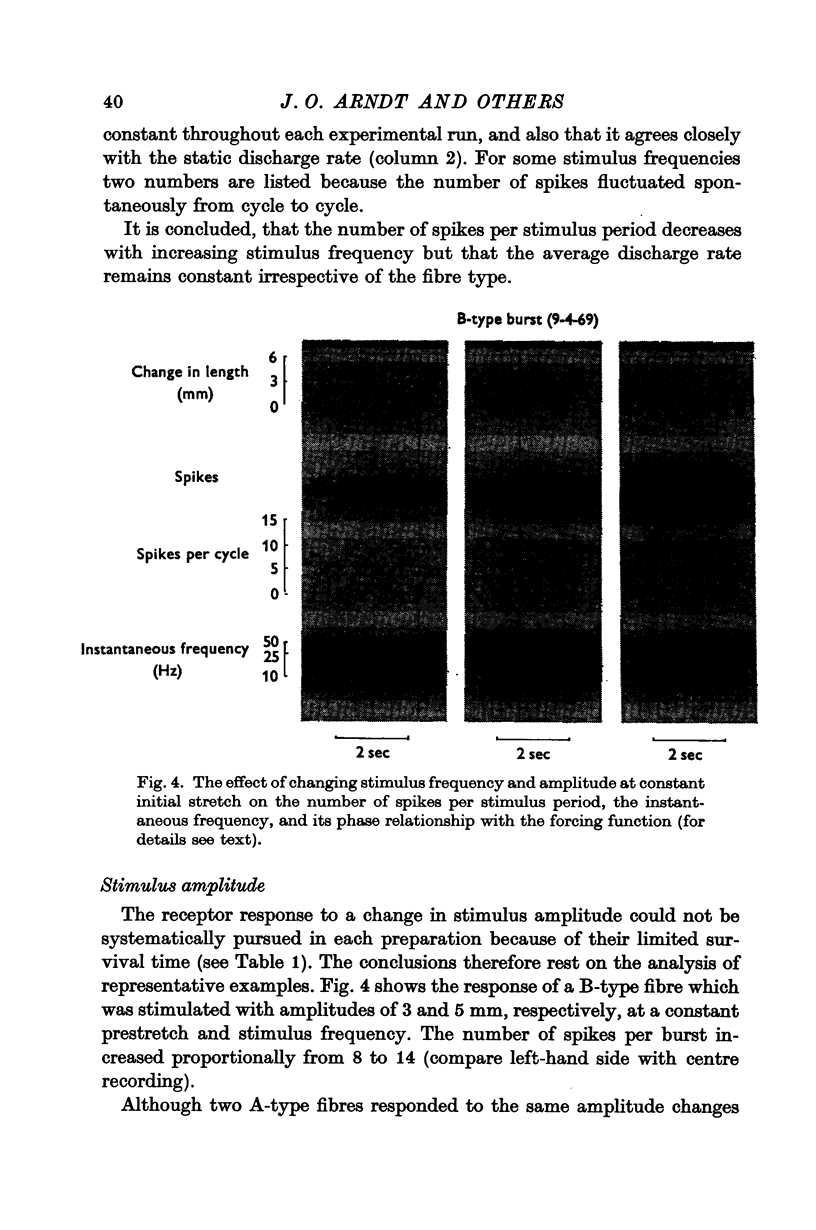
2. Sinusoidal length changes, with frequencies from 1 to 10 Hz and amplitudes of 1·5-5·0 mm at a given static extension, were imposed in situ on strips of atrial receptor areas of cats.
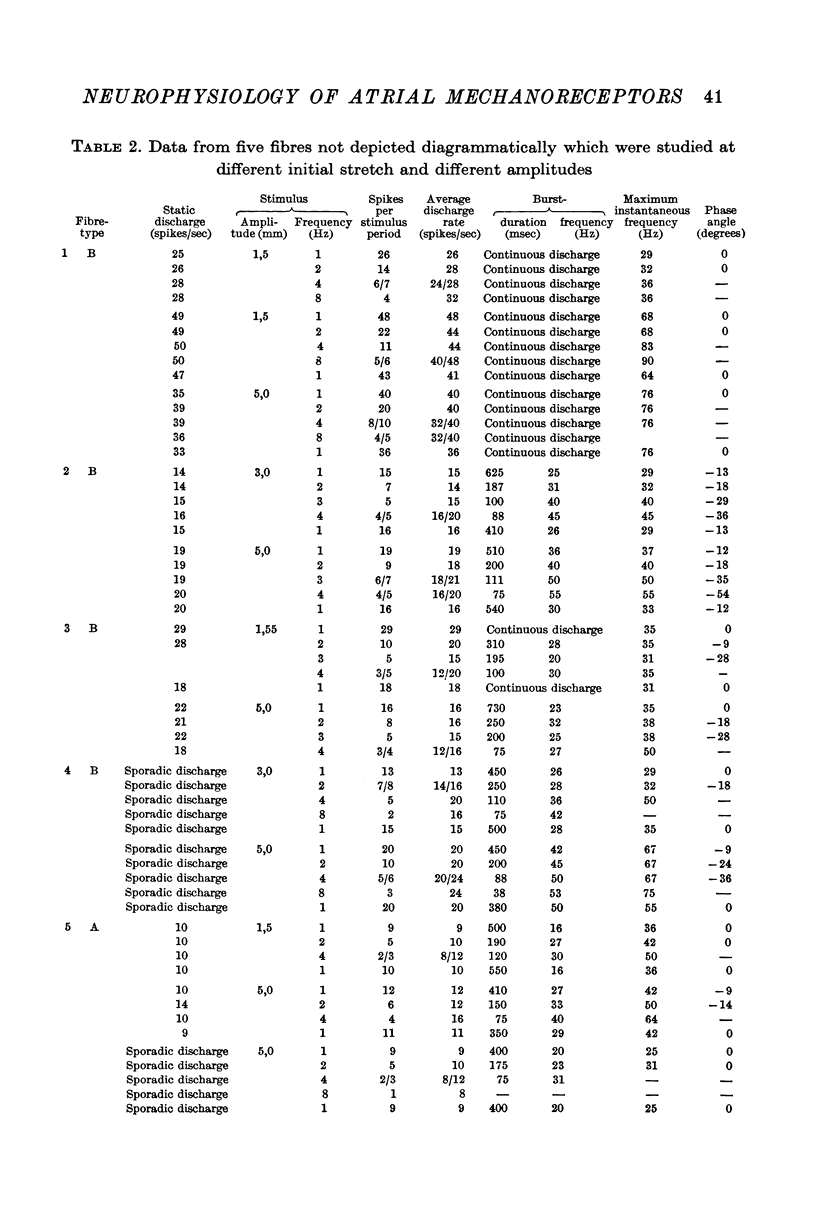
3. The receptor response was evaluated from functional single fibres in terms of number of spikes per stimulus period, average discharge rate, instantaneous spike frequency, and phase angle between forcing function and instantaneous frequency.
4. Irrespective of the fibre type, atrial fibres appear to originate from identical mechanoreceptors which sense length at low stimulus frequency and low stimulus amplitudes, but also sense velocity in the high frequency range and with large stimulus amplitudes.
5. The difference in the discharge pattern and in the functional behaviour of the atrial fibres in vivo can be explained on the basis of identical receptors.
6. The difference in the temporal occurrence of the atrial burst is still open to debate, but probably is related to the site of the receptor.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D. Afferent impulses in the vagus and their effect on respiration. J Physiol. 1933 Oct 6;79(3):332–358. doi: 10.1113/jphysiol.1933.sp003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angell James J. E. The effects of altering mean pressure, pulse pressure and pulse frequency on the impulse activity in baroreceptor fibres from the aortic arch and right subclavian artery in the rabbit. J Physiol. 1971 Apr;214(1):65–88. doi: 10.1113/jphysiol.1971.sp009419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt J. O., Brambring P., Hindorf K., Röhnelt M. The afferent impulse traffic from atrial A-type receptors in cats. Does the A-type receptor signal heart rate? Pflugers Arch. 1971;326(4):300–315. doi: 10.1007/BF00586994. [DOI] [PubMed] [Google Scholar]
- Arndt J. O. Die Beziehungen zwischen Umfang der Vorhöfe und Vorhofdrucken bei Volumenänderungen an narkotisierten Katzen. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;292(4):343–355. [PubMed] [Google Scholar]
- COLERIDGE H. M., COLERIDGE J. C., KIDD C. CARDIAC RECEPTORS IN THE DOG, WITH PARTICULAR REFERENCE TO TWO TYPES OF AFFERENT ENDING IN THE VENTRICULAR WALL. J Physiol. 1964 Nov;174:323–339. doi: 10.1113/jphysiol.1964.sp007490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. THE EFFECTS OF STIMULATION OF STATIC AND DYNAMIC FUSIMOTOR FIBRES ON THE RESPONSE TO STRETCHING OF THE PRIMARY ENDINGS OF MUSCLE SPINDLES. J Physiol. 1964 Oct;174:109–131. doi: 10.1113/jphysiol.1964.sp007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbert H., Grüsser O. J. Reaktionen primärer und sekundärer Muskelspindelafferenzen auf sinusförmige mechansiche Reizung. II. Anderung der statischen Vordehung. Pflugers Arch. 1968;304(3):258–270. doi: 10.1007/BF00592129. [DOI] [PubMed] [Google Scholar]
- Franz G. N. Nonlinear rate sensitivity of the carotid sinus reflex as a consequence of static and dynamic nonlinearities in baroreceptor behavior. Ann N Y Acad Sci. 1969 Apr 21;156(2):811–824. doi: 10.1111/j.1749-6632.1969.tb14016.x. [DOI] [PubMed] [Google Scholar]
- GAUER O. H., HENRY J. P. Circulatory basis of fluid volume control. Physiol Rev. 1963 Jul;43:423–481. doi: 10.1152/physrev.1963.43.3.423. [DOI] [PubMed] [Google Scholar]
- Gauer O. H., Henry J. P., Behn C. The regulation of extracellular fluid volume. Annu Rev Physiol. 1970;32:547–595. doi: 10.1146/annurev.ph.32.030170.002555. [DOI] [PubMed] [Google Scholar]
- Grüsser O. J., Thiele B. Reaktionen primärer und sekundärer Muskelspindelafferenzen auf sinusförmige mechanische Reizung. I. Variation der Sinusfrequenz. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968 Apr 23;300(3):161–184. [PubMed] [Google Scholar]
- HENRY J. P., PEARCE J. W. The possible role of cardiac atrial stretch receptors in the induction of changes in urine flow. J Physiol. 1956 Mar 28;131(3):572–585. doi: 10.1113/jphysiol.1956.sp005483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk J., Simon W. Responses of Golgi tendon organs to forces applied to muscle tendon. J Neurophysiol. 1967 Nov;30(6):1466–1481. doi: 10.1152/jn.1967.30.6.1466. [DOI] [PubMed] [Google Scholar]
- Husmark I., Ottoson D. Relation between tension and sensory response of the isolated frog muscle spindle during stretch. Acta Physiol Scand. 1970 Jul;79(3):321–334. doi: 10.1111/j.1748-1716.1970.tb04732.x. [DOI] [PubMed] [Google Scholar]
- LANGREHR D., KRAMER K. [Relationships of median impulse frequency of the auricular receptors to the thoracic blood volume]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;271:797–807. [PubMed] [Google Scholar]
- LANGREHR D. [The discharge pattern and general excitation conditions of the auricular receptors in the dog and cat]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;271:257–269. [PubMed] [Google Scholar]
- Lennerstrand G. Position and velocity sensitivity of muscle spindles in the cat. I. Primary and secondary endings deprived of fusimotor activation. Acta Physiol Scand. 1968 Jul;73(3):281–299. doi: 10.1111/j.1748-1716.1968.tb04106.x. [DOI] [PubMed] [Google Scholar]
- Matthews P. B., Stein R. B. The sensitivity of muscle spindle afferents to small sinusoidal changes of length. J Physiol. 1969 Feb;200(3):723–743. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIL E., JOELS N. The impulse activity in cardiac afferent vagal fibres. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1961;240:453–460. doi: 10.1007/BF00244938. [DOI] [PubMed] [Google Scholar]
- PAINTAL A. S. A study of gastric stretch receptors; their role in the peripheral mechanism of satiation of hunger and thirst. J Physiol. 1954 Nov 29;126(2):255–270. doi: 10.1113/jphysiol.1954.sp005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. A study of right and left atrial receptors. J Physiol. 1953 Jun 29;120(4):596–610. doi: 10.1113/jphysiol.1953.sp004920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. NATURAL STIMULATION OF TYPE B ATRIAL RECEPTORS. J Physiol. 1963 Nov;169:116–136. doi: 10.1113/jphysiol.1963.sp007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. VAGAL AFFERENT FIBRES. Ergeb Physiol. 1963;52:74–156. [PubMed] [Google Scholar]
- STAMPFLI R. Bau und Funktion isolierter markhaltiger Nervenfasern. Ergeb Physiol. 1952;47:70–165. [PubMed] [Google Scholar]
- STRUPPLER A. Afferente vagale Herznervenimpulse und ihre Beziehung zur Hämodynamik. Z Biol. 1955 Apr;107(6):416–428. [PubMed] [Google Scholar]
- Schäfer S. S., Henatsch H. D. Dehnungsantworten der primären Muskelspindel-Afferenz bei elektrischer Reizung und natürlicher Innervation der beiden fusimotorischen Fasertypen. Exp Brain Res. 1968;4(4):275–291. doi: 10.1007/BF00235696. [DOI] [PubMed] [Google Scholar]
- Toyama K. An analysis of impulse discharges from the spindle receptor. Jpn J Physiol. 1966 Apr 15;16(2):113–125. doi: 10.2170/jjphysiol.16.113. [DOI] [PubMed] [Google Scholar]
- Whitteridge D. Afferent nerve fibres from the heart and lungs in the cervical vagus. J Physiol. 1948 Sep 30;107(4):496–512. doi: 10.1113/jphysiol.1948.sp004294. [DOI] [PMC free article] [PubMed] [Google Scholar]


