Abstract
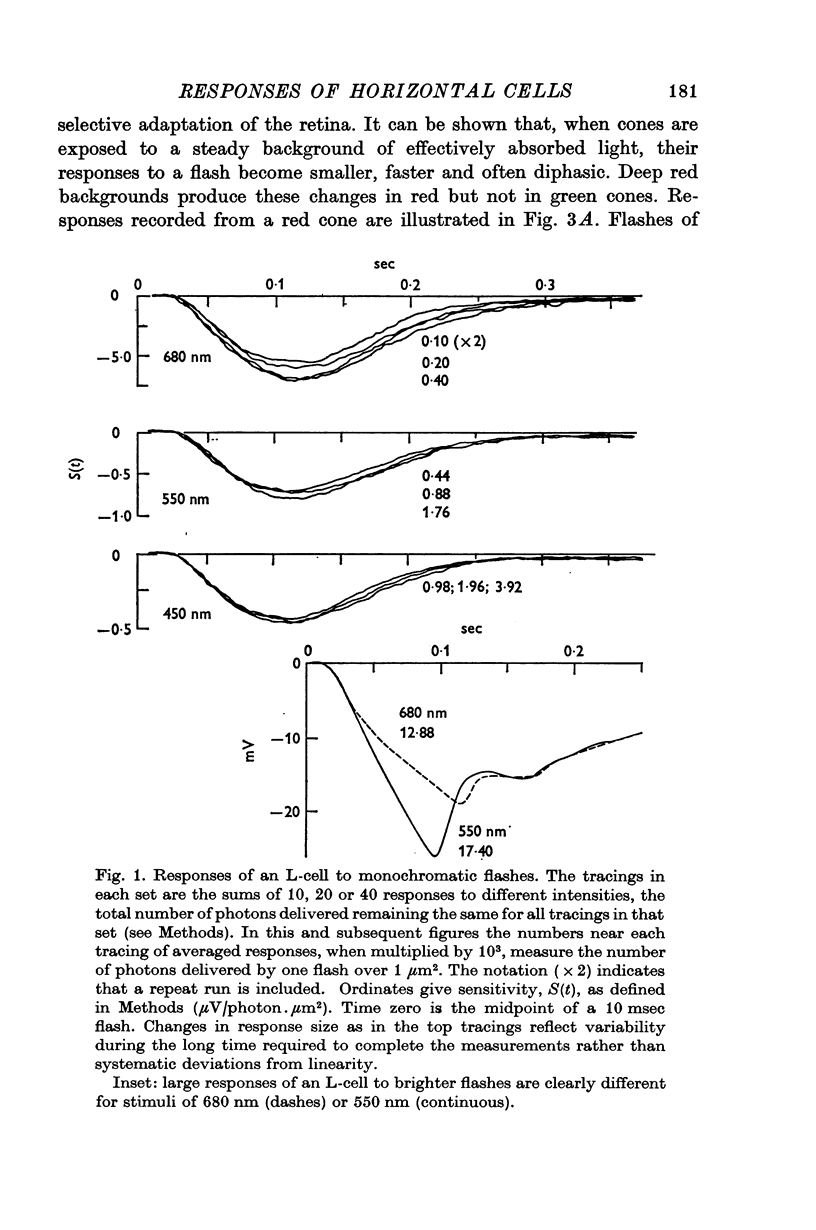
1. Small responses to large fields of dim monochromatic lights were recorded intracellularly from luminosity horizontal cells (L-cells), chromaticity horizontal cells (C-cells) and cones in the retinae of turtles, Pseudemys scripta elegans.
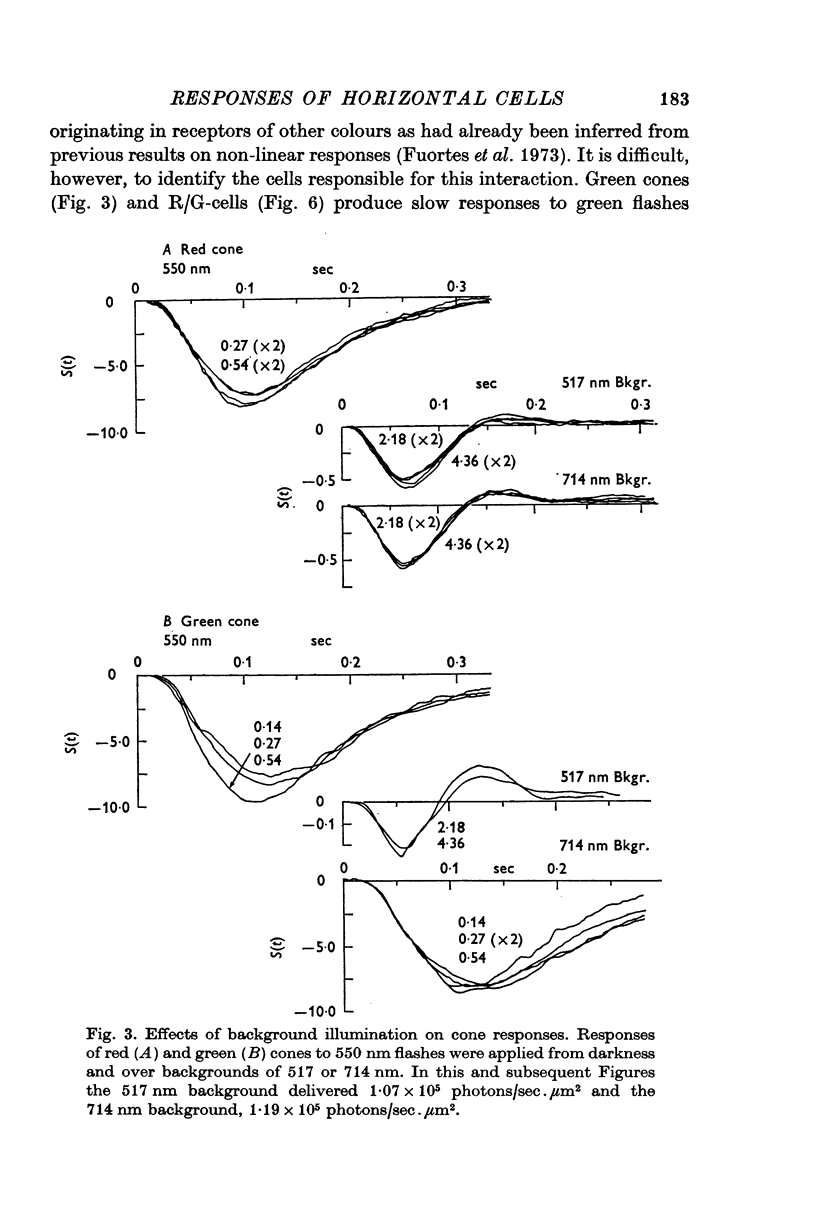
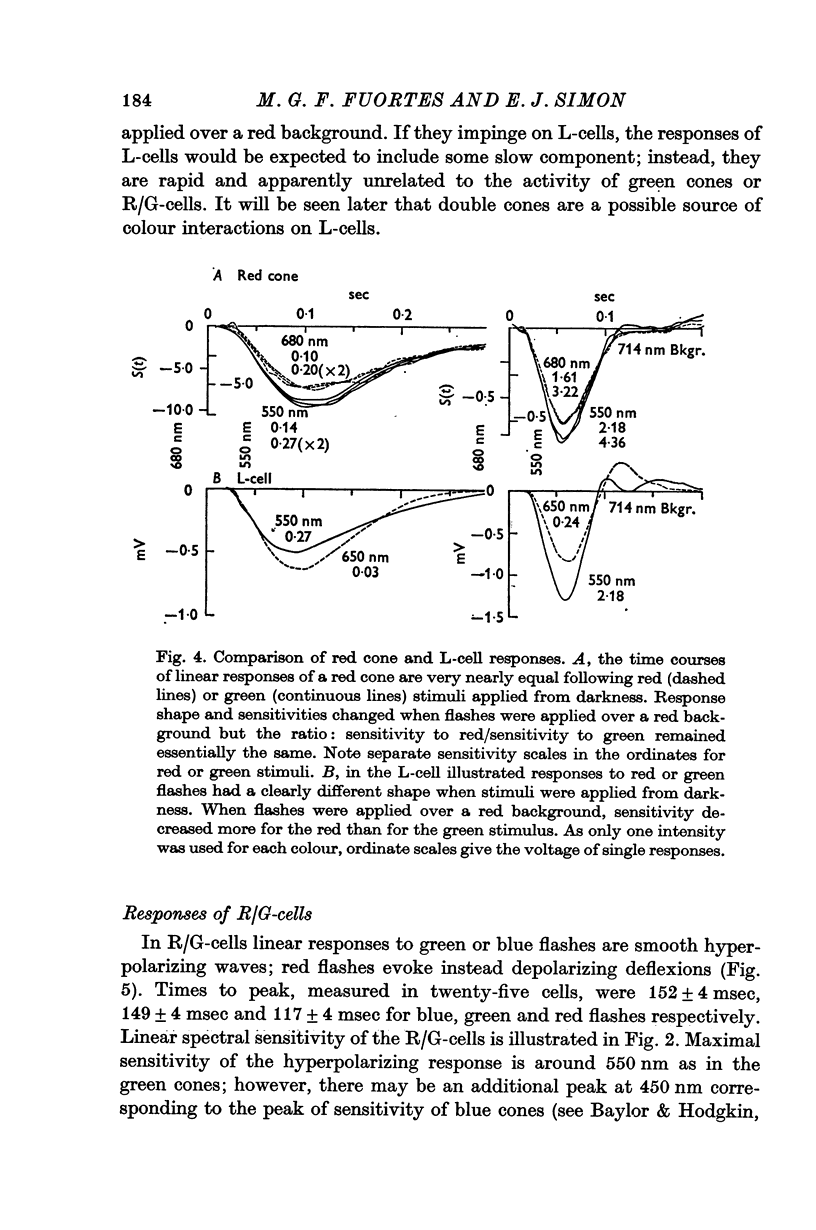
2. Responses of cones to brief flashes applied over steady backgrounds were studied in order to interpret the corresponding responses of horizontal cells. Steady red or green backgrounds make the responses of red-sensitive cones smaller, faster and often diphasic. Green backgrounds have similar effects on the responses of green-sensitive cones to green flashes, but red backgrounds do not change them appreciably. Responses of double cones have properties intermediate between those of red and green cones.
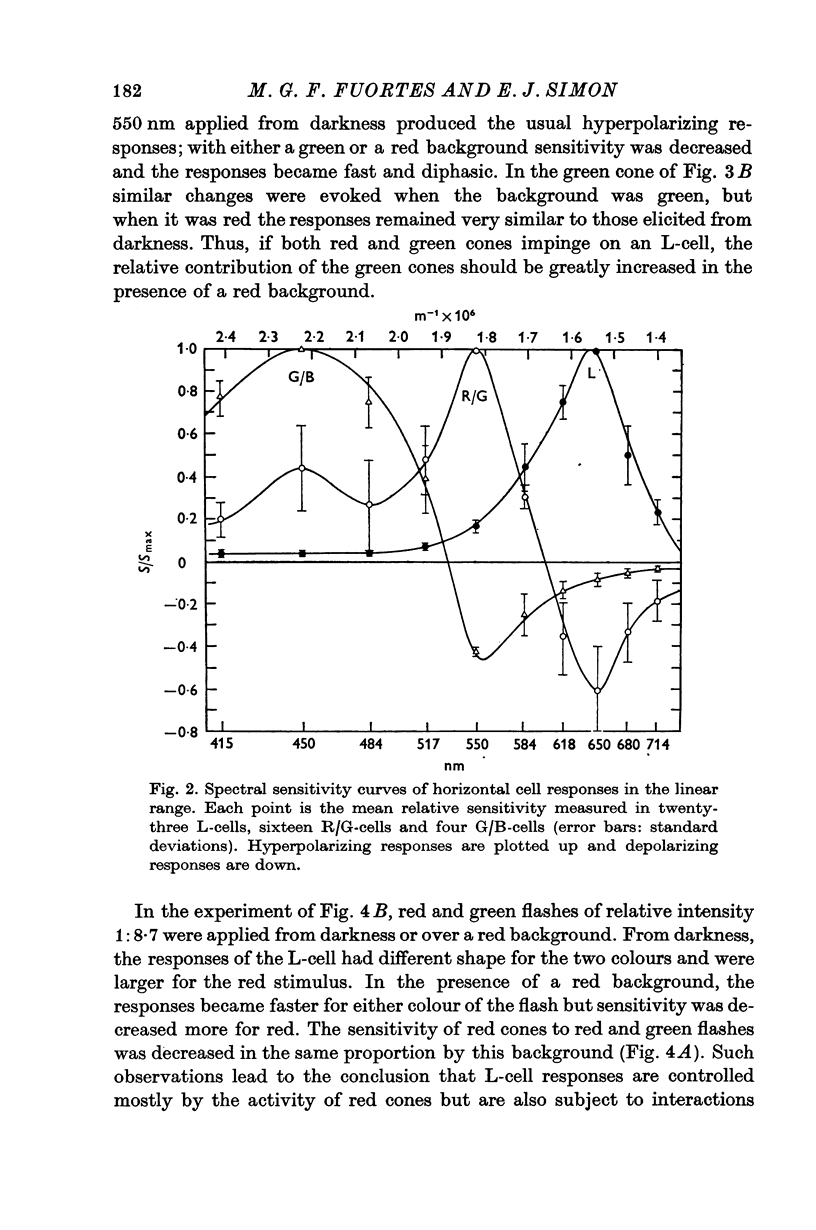
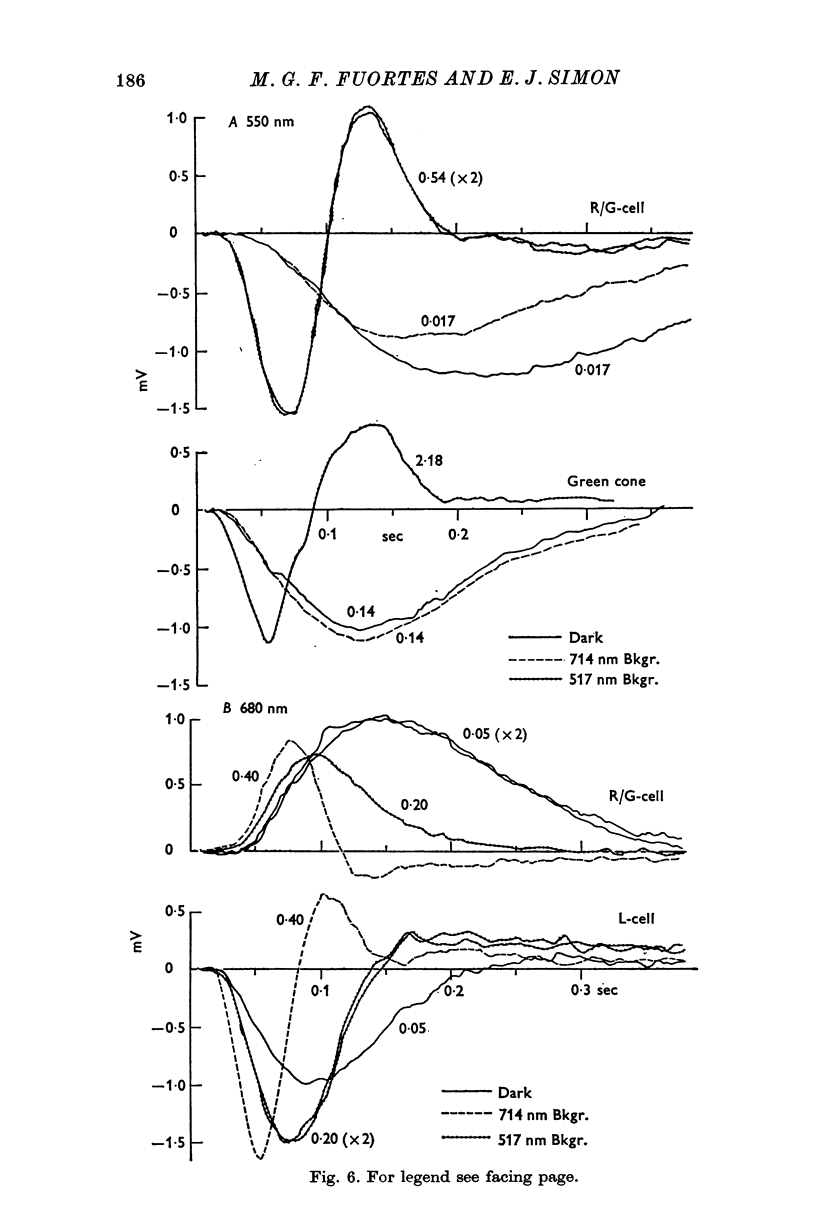
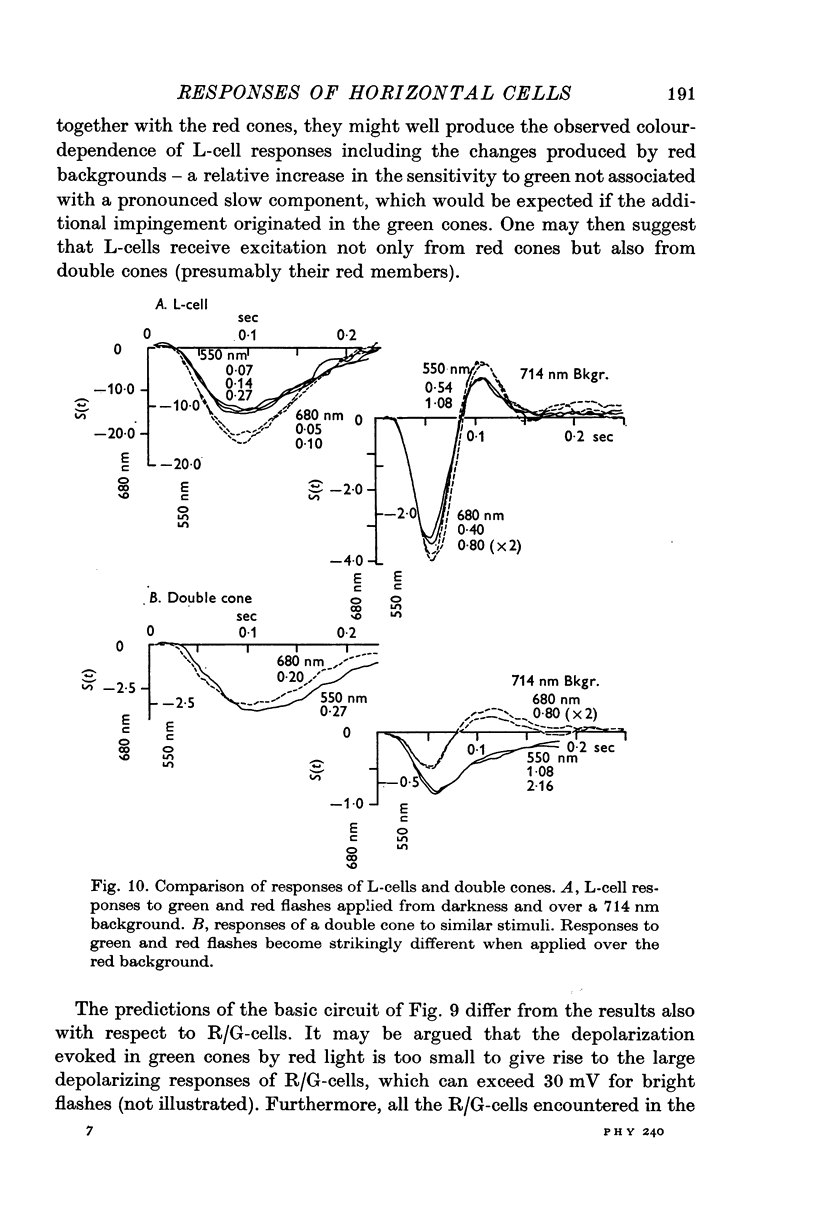
3. L-cells of both type I and type II are hyperpolarized by all visible wave-lengths, and their spectral sensitivity in the linear range resembles that of red cones. Their responses are not invariant with respect to colour, and their sensitivity to green relative to red stimuli increases during red backgrounds. These properties suggest that L-cells are activated mainly by red cones but also receive impingement from the red members of double cones.
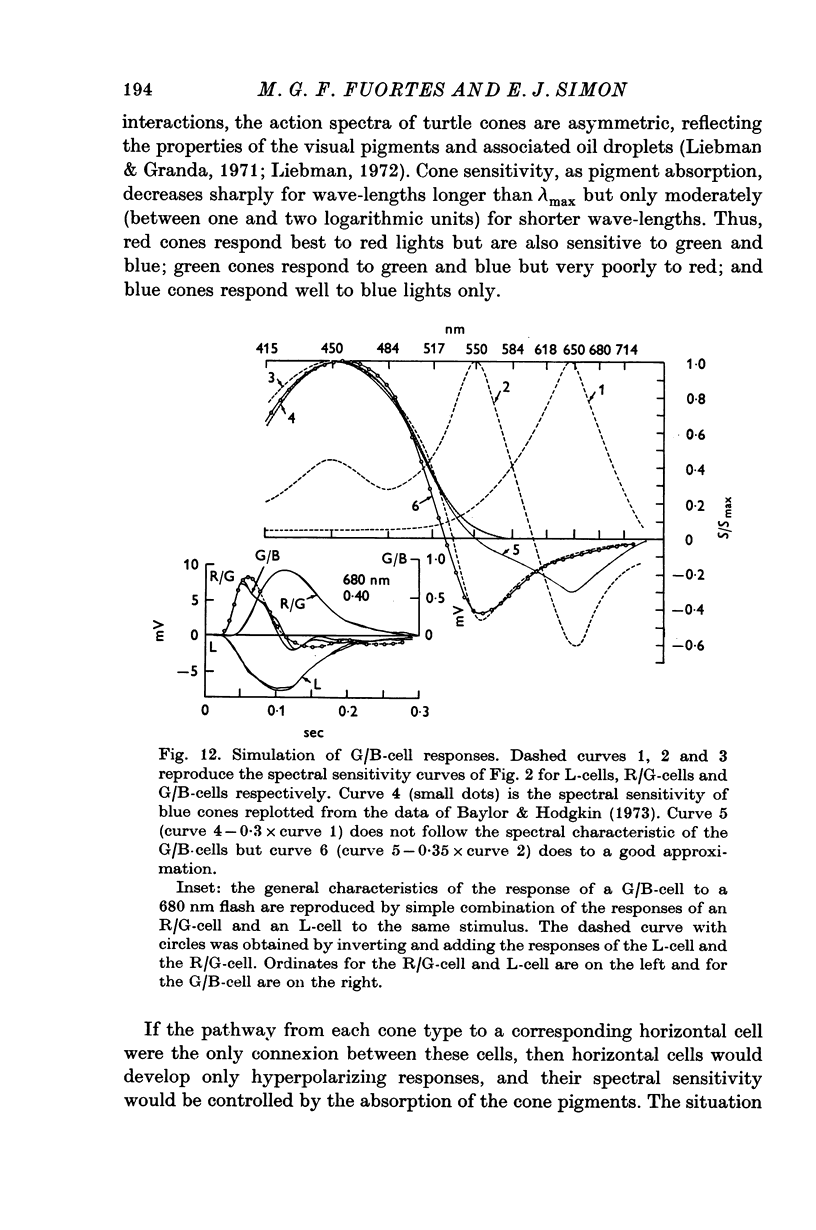
4. Spectral properties of red/green C-cells resemble those of green cones as modified by the recurrent action of L-cells. They can be explained assuming that red/green C-cells receive their principal impingement from green cones and subsidiary interactions from green/blue C-cells and the green members of double comes.
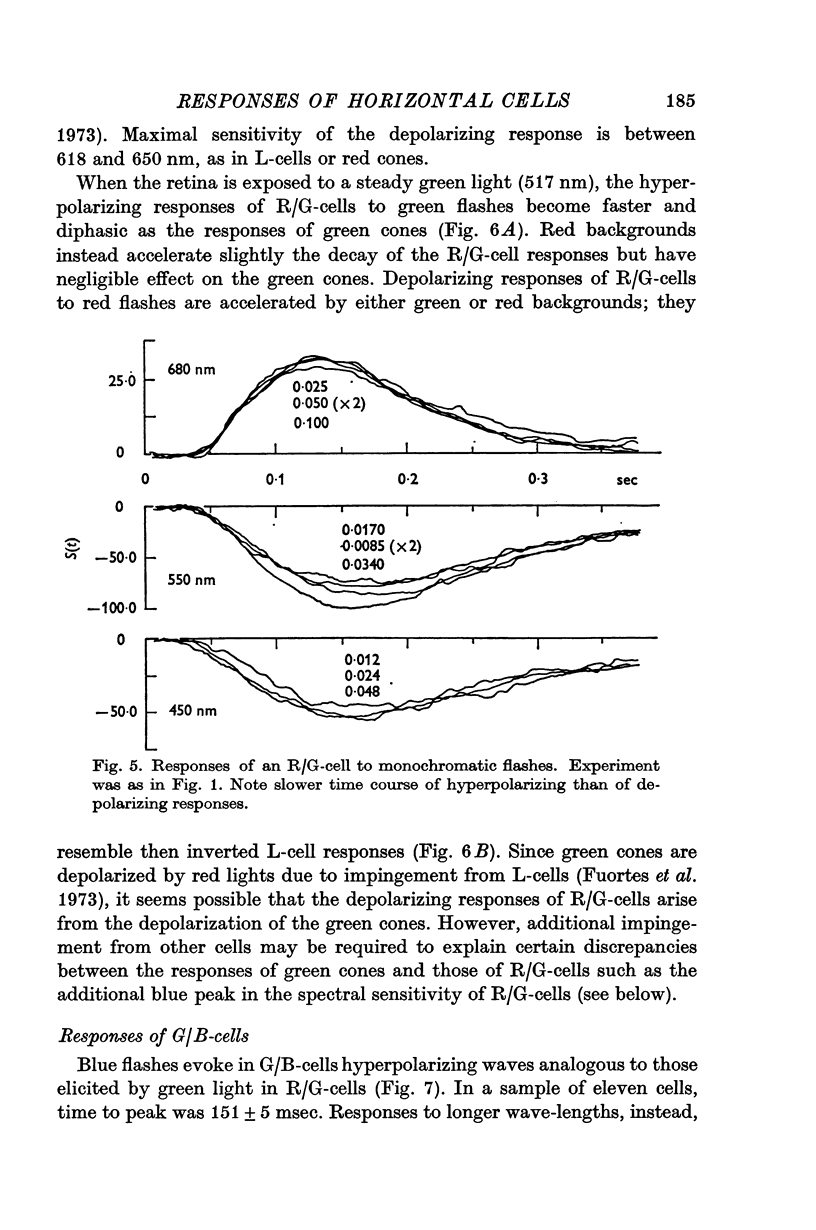
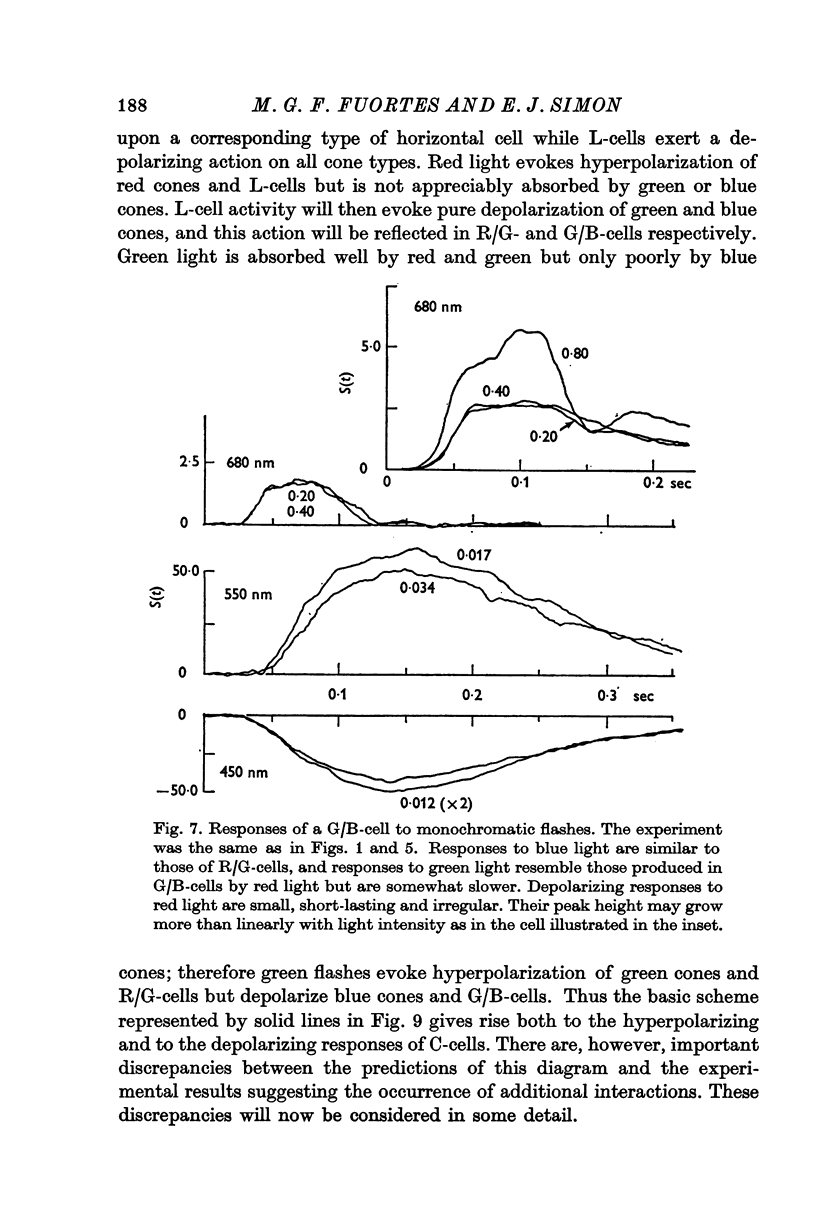
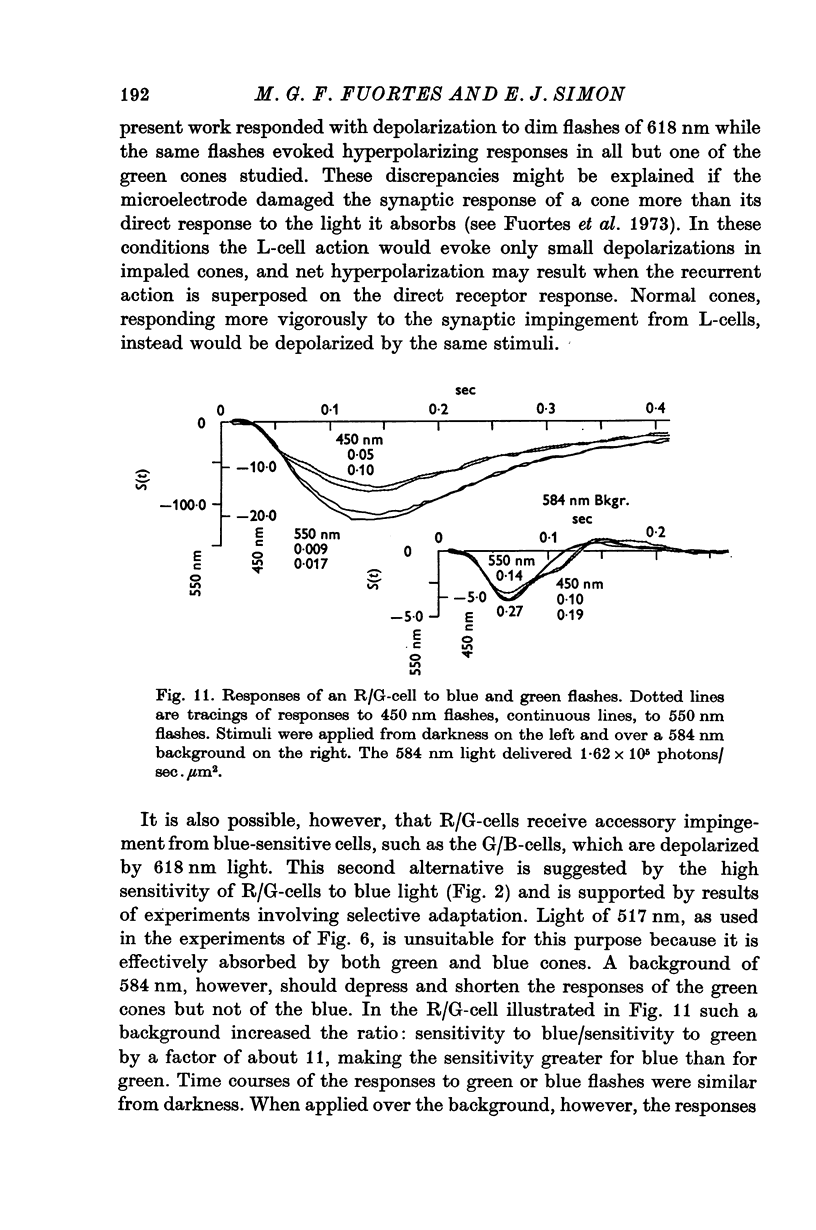
5. The spectral sensitivity of the hyperpolarizing responses of green/blue C-cells is ascribed to impingement from blue cones. Their depolarizing responses have complex properties which suggest that they are brought about by the activity of both L-cells (probably through the blue cones) and red/green C-cells.
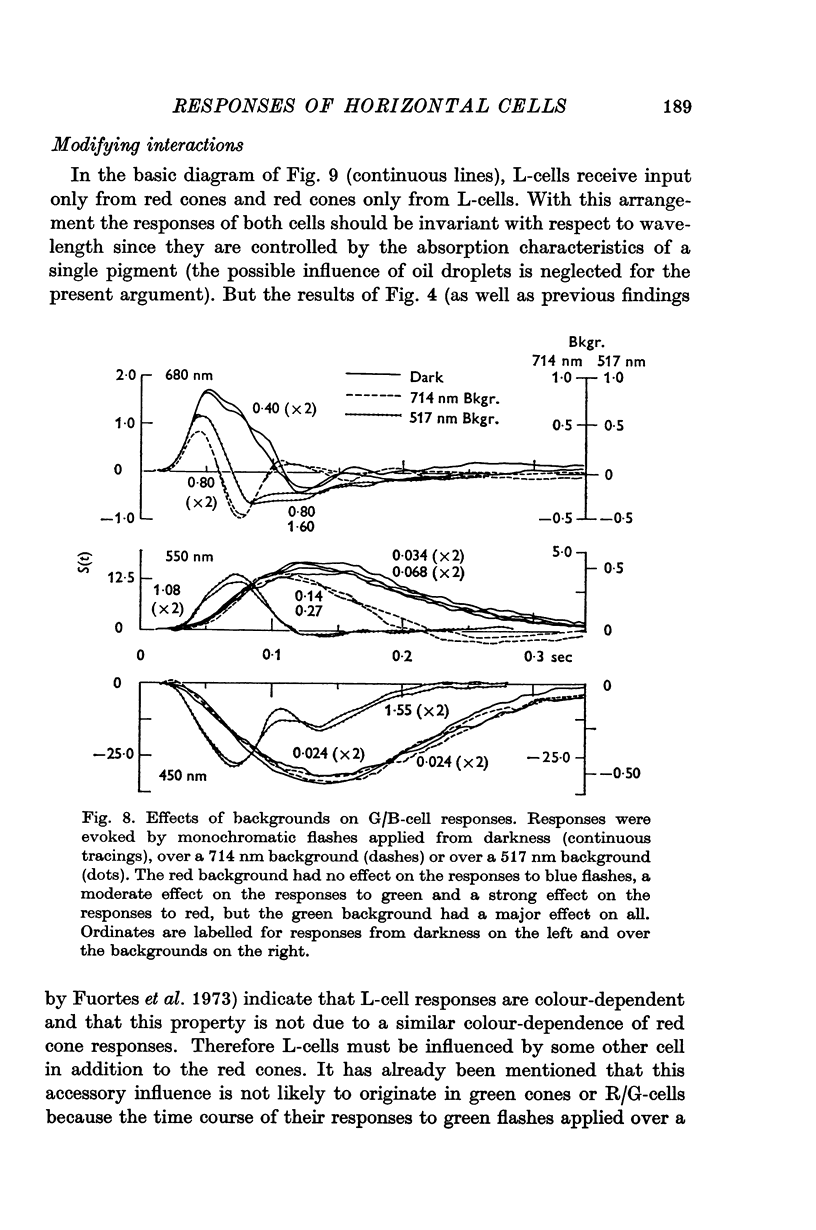
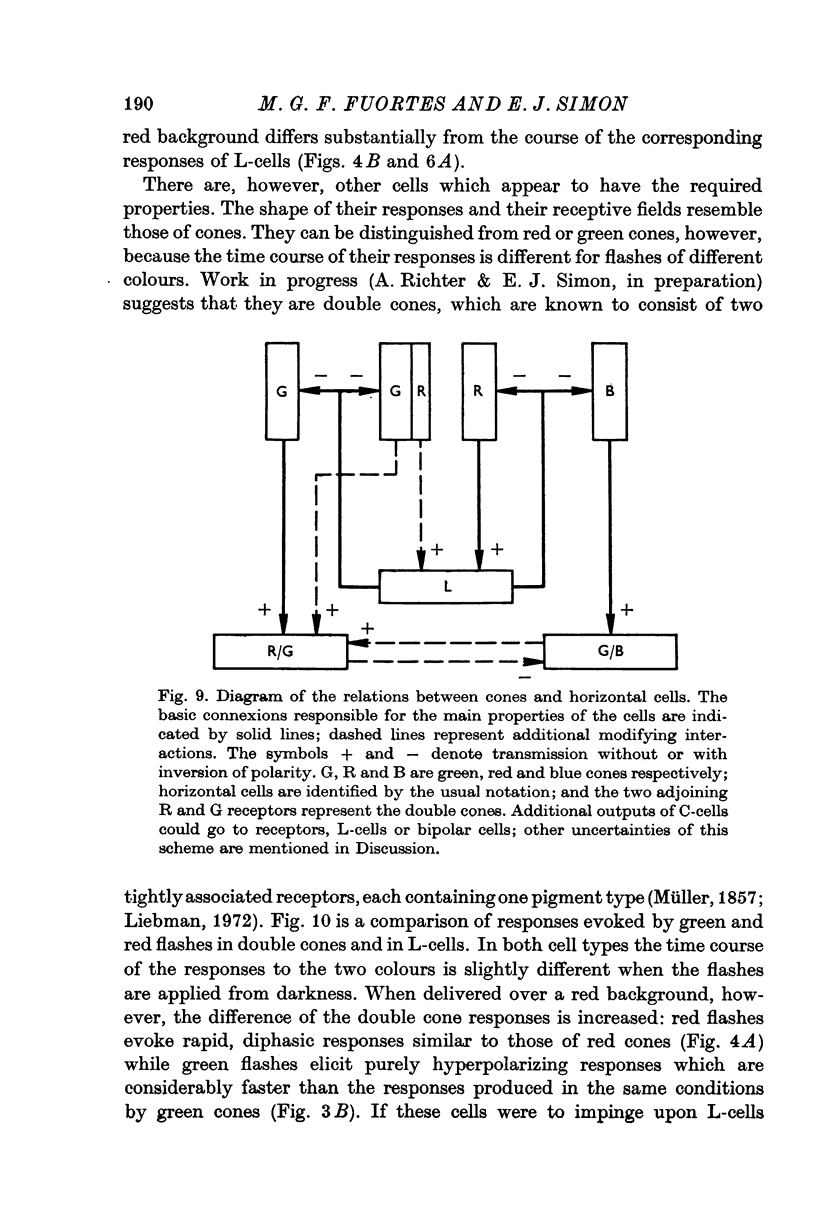
6. It is concluded that the main properties of the responses of the horizontal cells can be explained by a simple circuit in which each horizontal cell is connected to a corresponding type of cone and the L-cells have a recurrent impingement on all cones. The scheme is modified by additional interactions which operate on the responses of each horizontal cell type.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W. Neurophysiology of color vision. Physiol Rev. 1973 Jul;53(3):571–611. doi: 10.1152/physrev.1973.53.3.571. [DOI] [PubMed] [Google Scholar]
- Fuortes M. G., Schwartz E. A., Simon E. J. Colour-dependence of cone responses in the turtle retina. J Physiol. 1973 Oct;234(1):199–216. doi: 10.1113/jphysiol.1973.sp010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Yamada M. S-potentials in the dark-adapted retina of the carp. J Physiol. 1972 Dec;227(1):261–273. doi: 10.1113/jphysiol.1972.sp010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Granda A. M. Microspectrophotometric measurements of visual pigments in two species of turtle, Pseudemys scripta and Chelonia mydas. Vision Res. 1971 Feb;11(2):105–114. doi: 10.1016/0042-6989(71)90227-6. [DOI] [PubMed] [Google Scholar]
- MACNICHOL E. J., SVAETICHIN G. Electric responses from the isolated retinas of fishes. Am J Ophthalmol. 1958 Sep;46(3 Pt 2):26–46. doi: 10.1016/0002-9394(58)90053-9. [DOI] [PubMed] [Google Scholar]
- Miller W. H., Hashimoto Y., Saito T., Tomita T. Physiological and morphological identification of L- and C-type S-potentials in the turtle retina. Vision Res. 1973 Feb;13(2):443–447. doi: 10.1016/0042-6989(73)90121-1. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryan P. M. Properties of the depolarizing synaptic potential evoked by peripheral illumination in cones of the turtle retina. J Physiol. 1973 Nov;235(1):207–223. doi: 10.1113/jphysiol.1973.sp010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVAETICHIN G., MACNICHOL E. F., Jr Retinal mechanisms for chromatic and achromatic vision. Ann N Y Acad Sci. 1959 Nov 12;74(2):385–404. doi: 10.1111/j.1749-6632.1958.tb39560.x. [DOI] [PubMed] [Google Scholar]
- Simon E. J. Two types of luminosity horizontal cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):199–211. doi: 10.1113/jphysiol.1973.sp010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. H. Rod and cone contributions to S-potentials from the cat retina. Vision Res. 1969 Nov;9(11):1319–1329. doi: 10.1016/0042-6989(69)90069-8. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]


