Abstract
1. Prostaglandin E1 increases sodium transport as measured by short circuit current (SCC) across isolated frog skin whereas calcium, added to the external Ringer fluid, decreases sodium transport. To help establish the site of action of prostaglandin the possible interaction of these two agents on sodium transport has been examined.
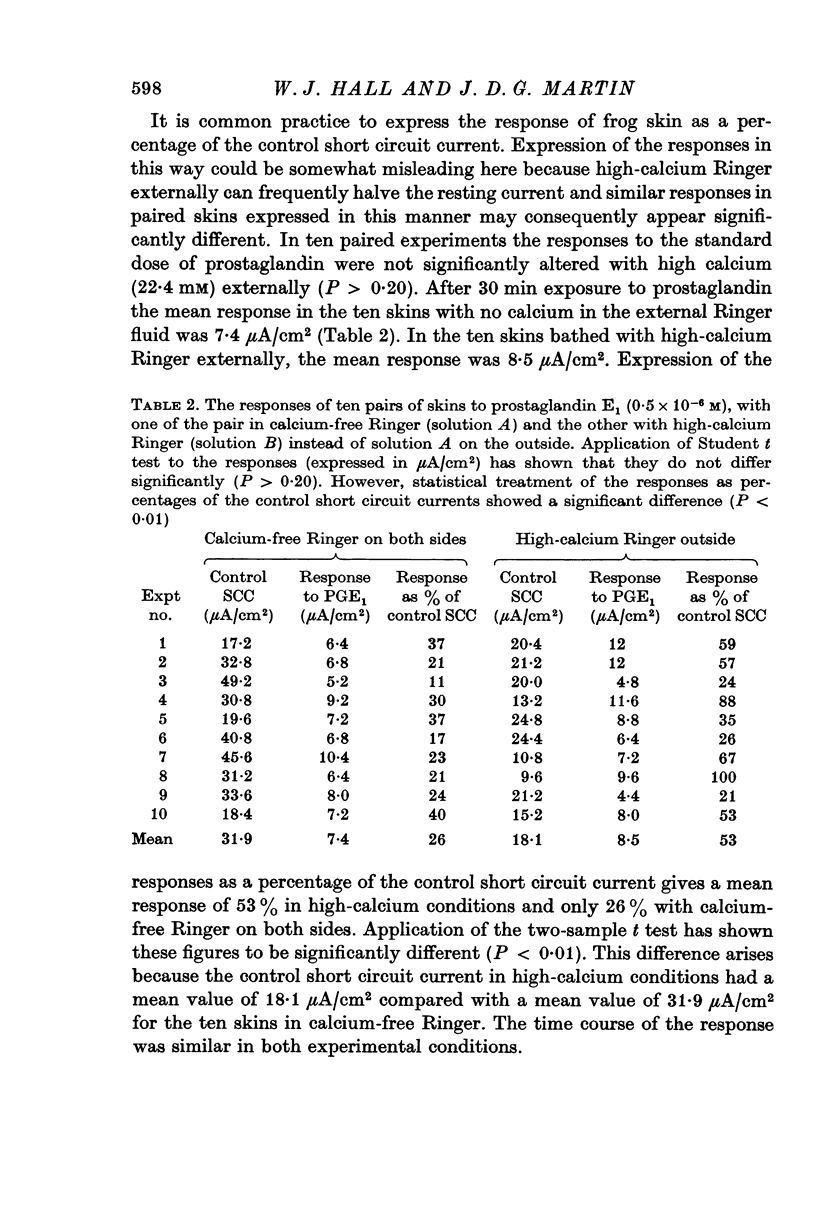
2. The effect of a standard dose of prostaglandin (0·5 × 10-6 M) on the short circuit current was tested on paired skins with either zero or high calcium (22·4 mM) in the external Ringer fluid. In ten experiments the responses to prostaglandin (expressed in μA/cm2) were not significantly affected by external calcium.
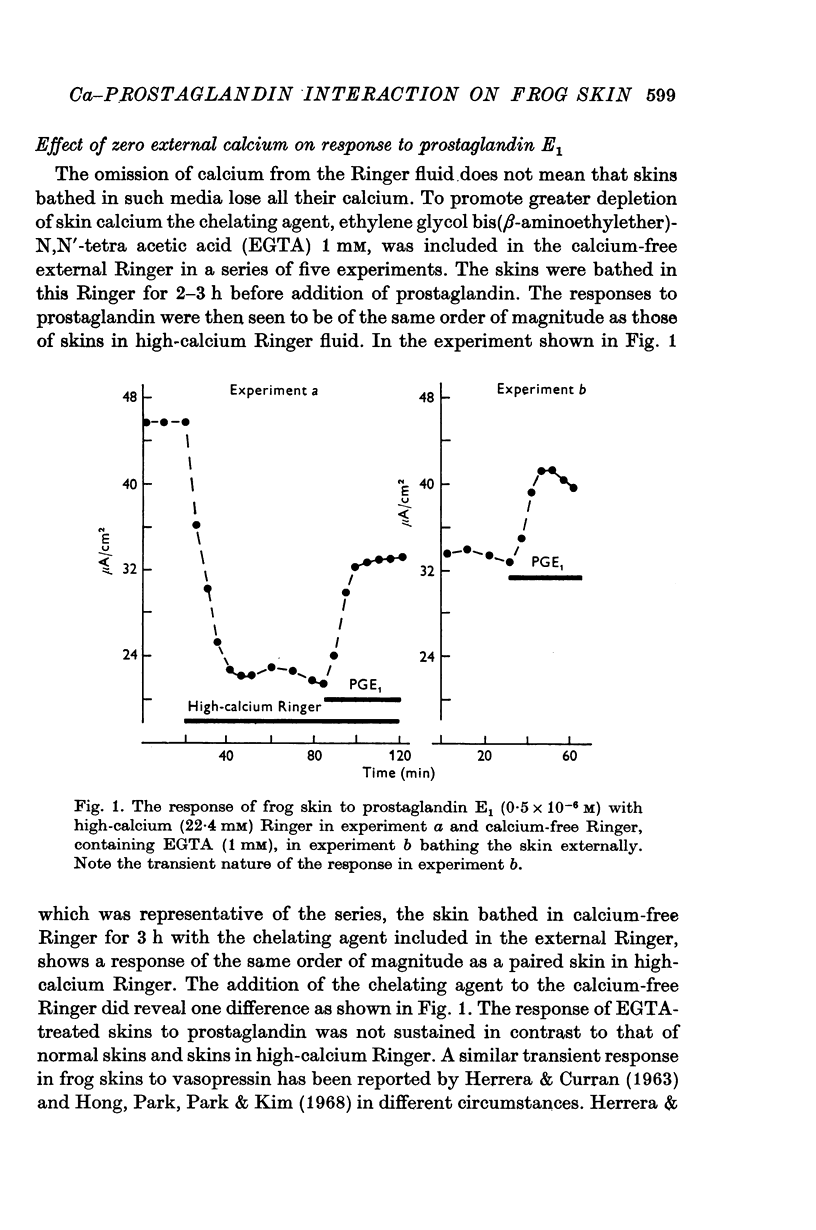
3. In another series of experiments the chelating agent, EGTA, was included in calcium-free external Ringer in order to promote greater depletion of skin calcium. The response of these skins to the standard dose of prostaglandin was of the same order of magnitude as that of control skins. The response was not sustained in contrast to that of normal skins and skins in high-calcium fluids.
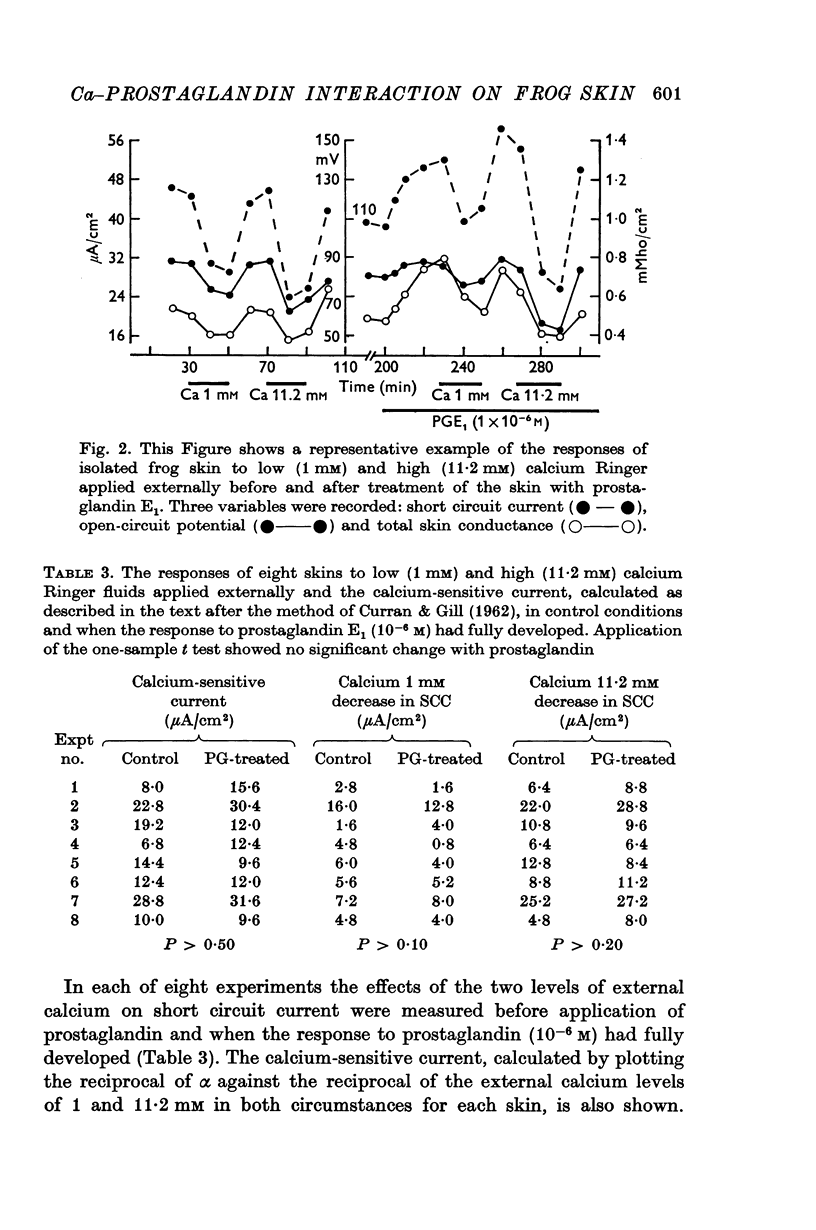
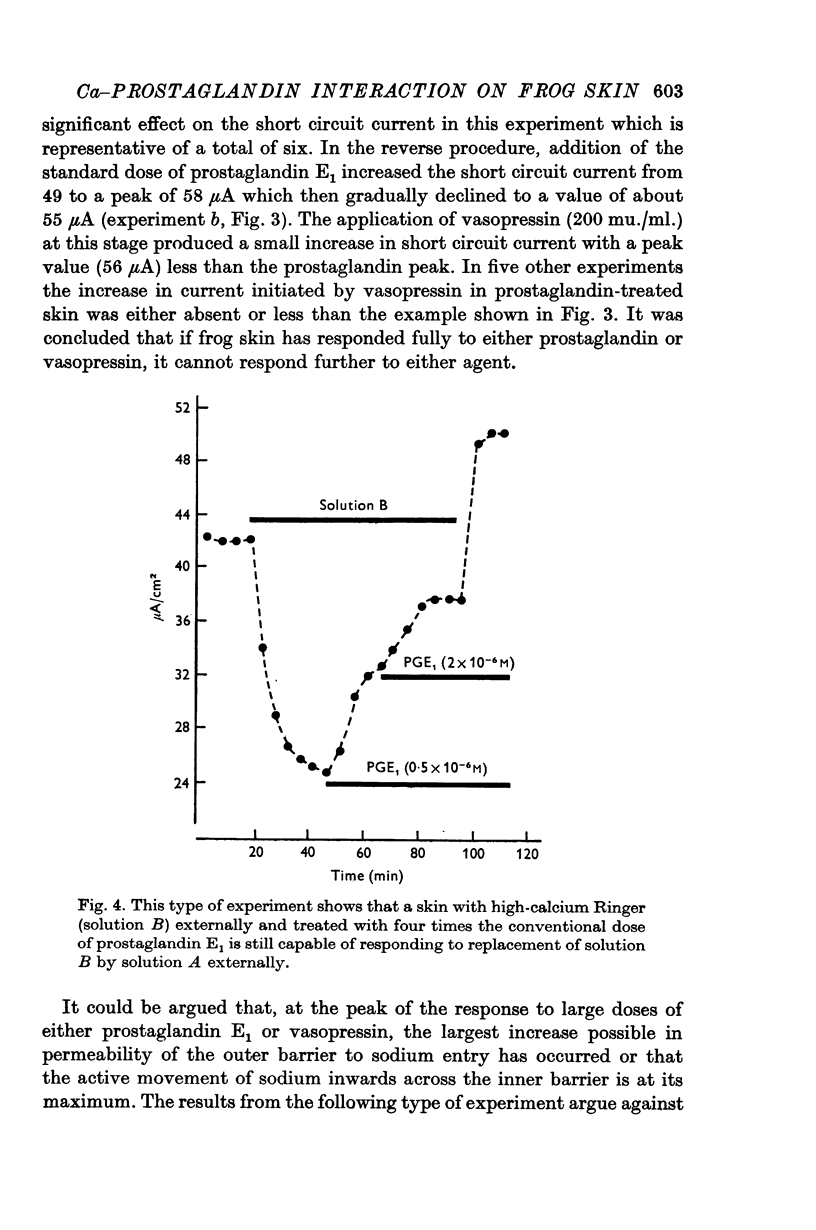
4. In a further series of experiments the reverse procedure was adopted whereby the response of the skin to low and high doses of calcium in the external Ringer was recorded in control conditions and when the skin had responded fully to twice the standard dose of prostaglandin. In addition, the calcium-sensitive current was calculated for each skin in both circumstances. The latter was unchanged on addition of prostaglandin, and graded doses of calcium caused the same degree of inhibition of the short circuit current.
5. The results show no interaction between external calcium and prostaglandin and also no need for external calcium in prostaglandin stimulation of sodium transport.
6. The findings do not support the concept of chelation by prostaglandin of calcium from critical sites on the skin as the primary mechanism of its action on sodium transport. The results closely parallel those of a similar type of study into the relationship between vasopressin and external calcium on frog skin also.
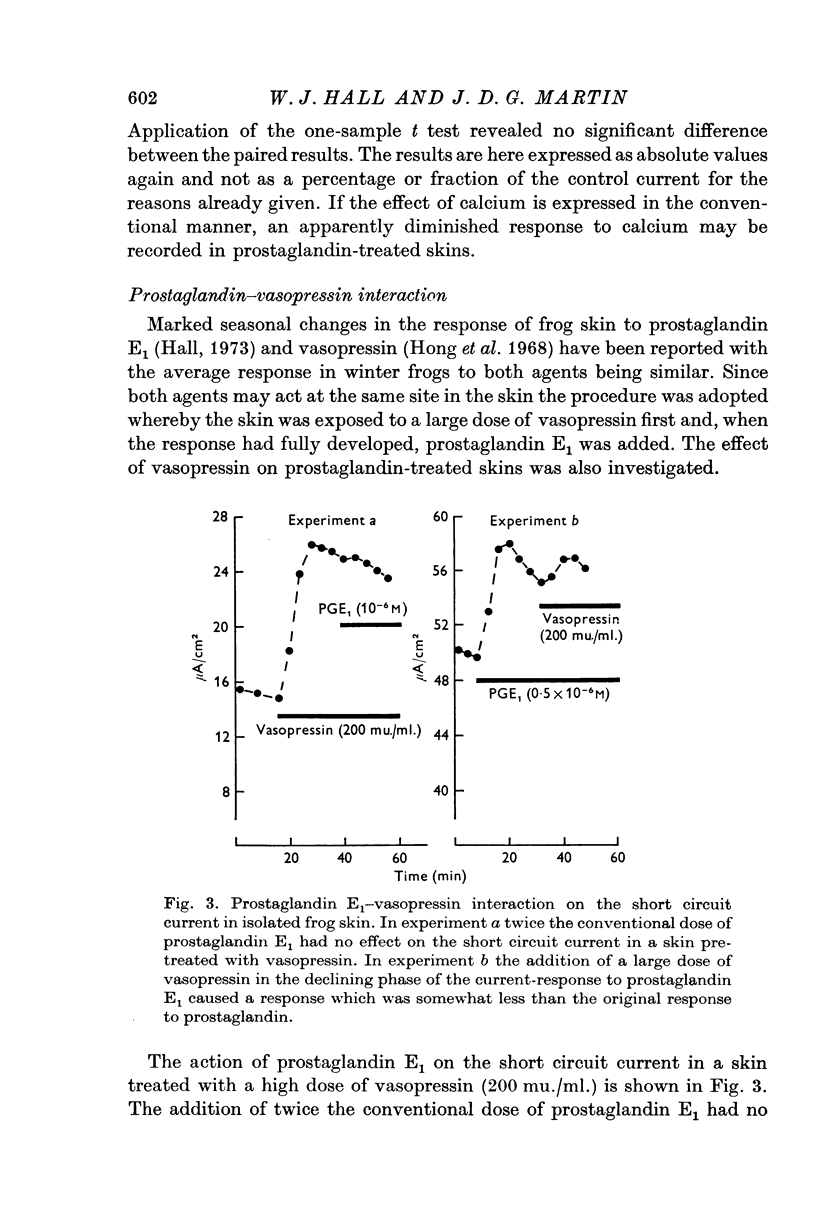
7. When frog skin has responded fully to either prostaglandin E1 or vasopressin, it shows no response to the other, although removal of calcium from the external Ringer fluid causes a further increase in short circuit current.
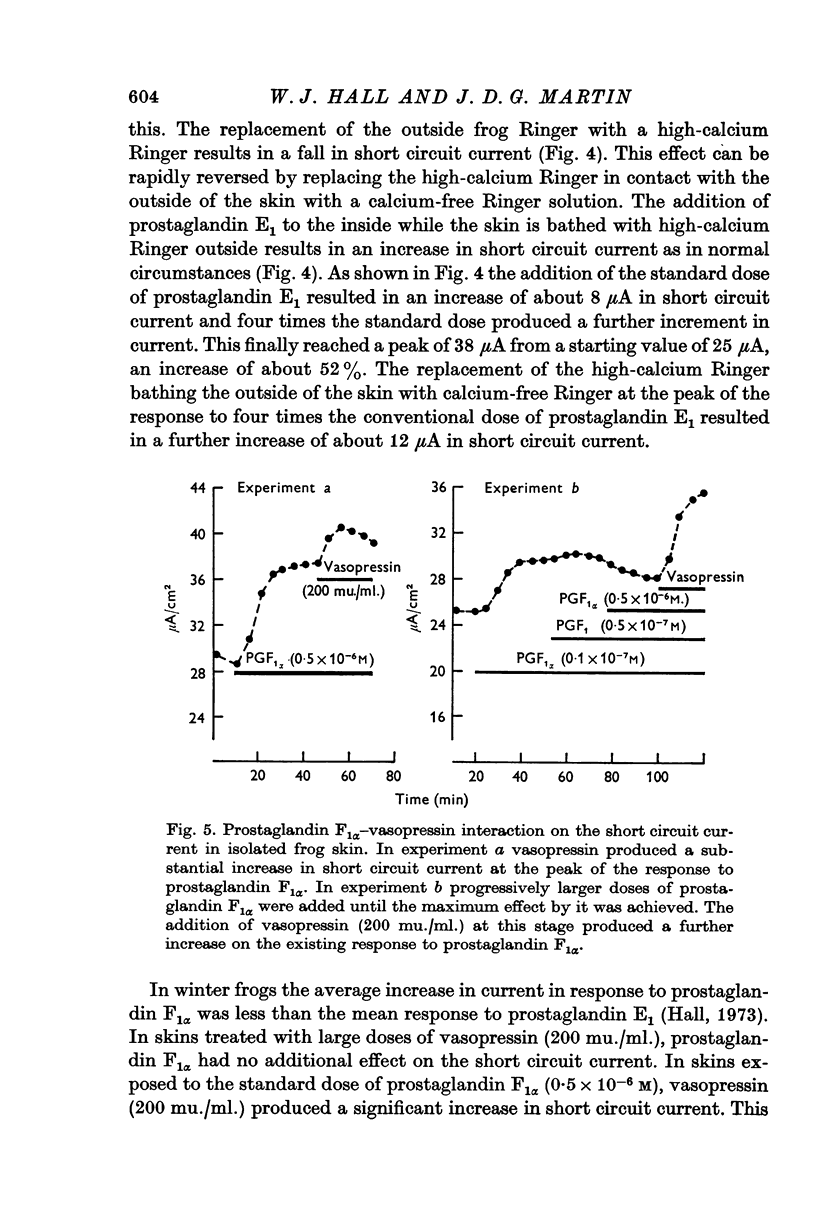
8. Vasopressin causes a further increase in short circuit current in skins treated with prostaglandin F1α. Prostaglandin F1α may be a weaker agonist on frog skin than either vasopressin or prostaglandin E1.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CURRAN P. F., GILL J. R., Jr The effect of calcium on sodium transport by frog skin. J Gen Physiol. 1962 Mar;45:625–641. doi: 10.1085/jgp.45.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN P. F., HERRERA F. C., FLANIGAN W. J. The effect of Ca and antidiuretic hormone on Na transport across frog skin. II. Sites and mechanisms of action. J Gen Physiol. 1963 May;46:1011–1027. doi: 10.1085/jgp.46.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN P. F., ZADUNAISKY J., GILL J. R., Jr The effect of ethylenediaminetetraacetate on ion permeability of the isolated frog skin. Biochim Biophys Acta. 1961 Sep 16;52:392–395. doi: 10.1016/0006-3002(61)90694-1. [DOI] [PubMed] [Google Scholar]
- Clegg P. C., Hall W. J., Pickles V. R. The action of ketonic prostaglandins on the guinea-pig myometrium. J Physiol. 1966 Mar;183(1):123–144. doi: 10.1113/jphysiol.1966.sp007855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINKELSTEIN A. ELECTRICAL EXCITABILITY OF ISOLATED FROG SKIN AND TOAD BLADDER. J Gen Physiol. 1964 Jan;47:545–565. doi: 10.1085/jgp.47.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassina G., Carpenedo F., Santi R. Effect of prostaglandin E1 on isolated short-circuited frog skin. Life Sci. 1969 Feb 1;8(3):181–187. doi: 10.1016/0024-3205(69)90092-7. [DOI] [PubMed] [Google Scholar]
- HERRERA F. C., CURRAN P. F. The effect of Ca and antidiuretic hormone on Na transport across frog skin. I. Examination of interrelationships between Ca and hormone. J Gen Physiol. 1963 May;46:999–1010. doi: 10.1085/jgp.46.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. J. Seasonal changes in the sensitivity of frog skin to prostaglandin and the effect of external sodium and chloride on the response. Ir J Med Sci. 1973 Sep;142(5):230–243. doi: 10.1007/BF02950017. [DOI] [PubMed] [Google Scholar]
- Hong S. K., Park C. S., Park Y. S., Kim J. K. Seasonal changes of antidiuretic hormone action on sodium transport across frog skin. Am J Physiol. 1968 Aug;215(2):439–443. doi: 10.1152/ajplegacy.1968.215.2.439. [DOI] [PubMed] [Google Scholar]
- Lindemann B., Thorns U. Fast potential spike of frog skin generated at the outer surface of the epithelium. Science. 1967 Dec 15;158(3807):1473–1477. doi: 10.1126/science.158.3807.1473. [DOI] [PubMed] [Google Scholar]
- Lipson L. C., Sharp G. W. Effect of prostaglandin E1 on sodium transport and osmotic water flow in the toad bladder. Am J Physiol. 1971 Apr;220(4):1046–1052. doi: 10.1152/ajplegacy.1971.220.4.1046. [DOI] [PubMed] [Google Scholar]
- Ramwell P. W., Shaw J. E. Biological significance of the prostaglandins. Recent Prog Horm Res. 1970;26:139–187. doi: 10.1016/b978-0-12-571126-5.50008-x. [DOI] [PubMed] [Google Scholar]


