Abstract
1. Electrical activity of longitudinal muscle from cat intestine was recorded in the double sucrose gap.
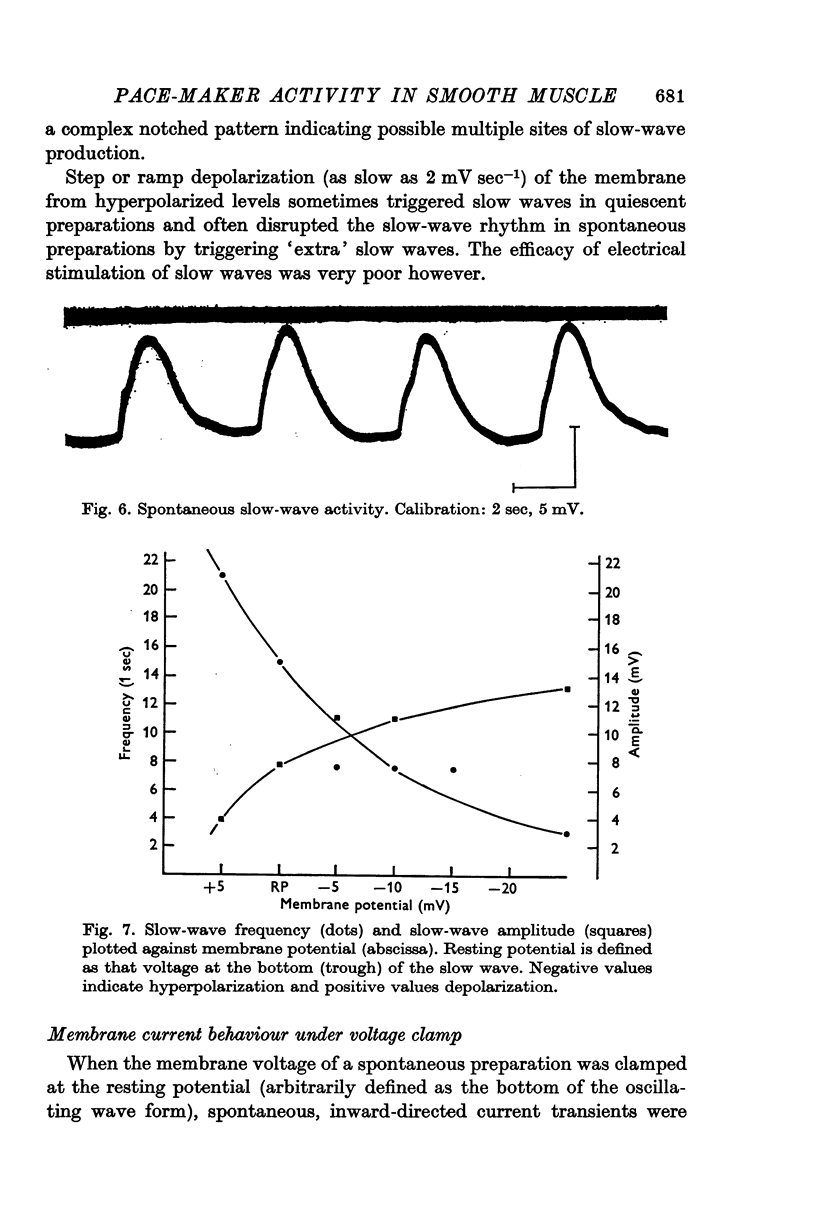
2. Approximately 20% of the preparations demonstrated slow, spontaneous fluctuations of membrane voltage, slow waves. This activity, although quite uniform in a given preparation, showed considerable inter-preparation variation with respect to amplitude, frequency and wave form.
3. Application of steady hyperpolarizing current decreased slow-wave frequency and increased slow-wave amplitude while depolarizing currents increased frequency and decreased amplitude.
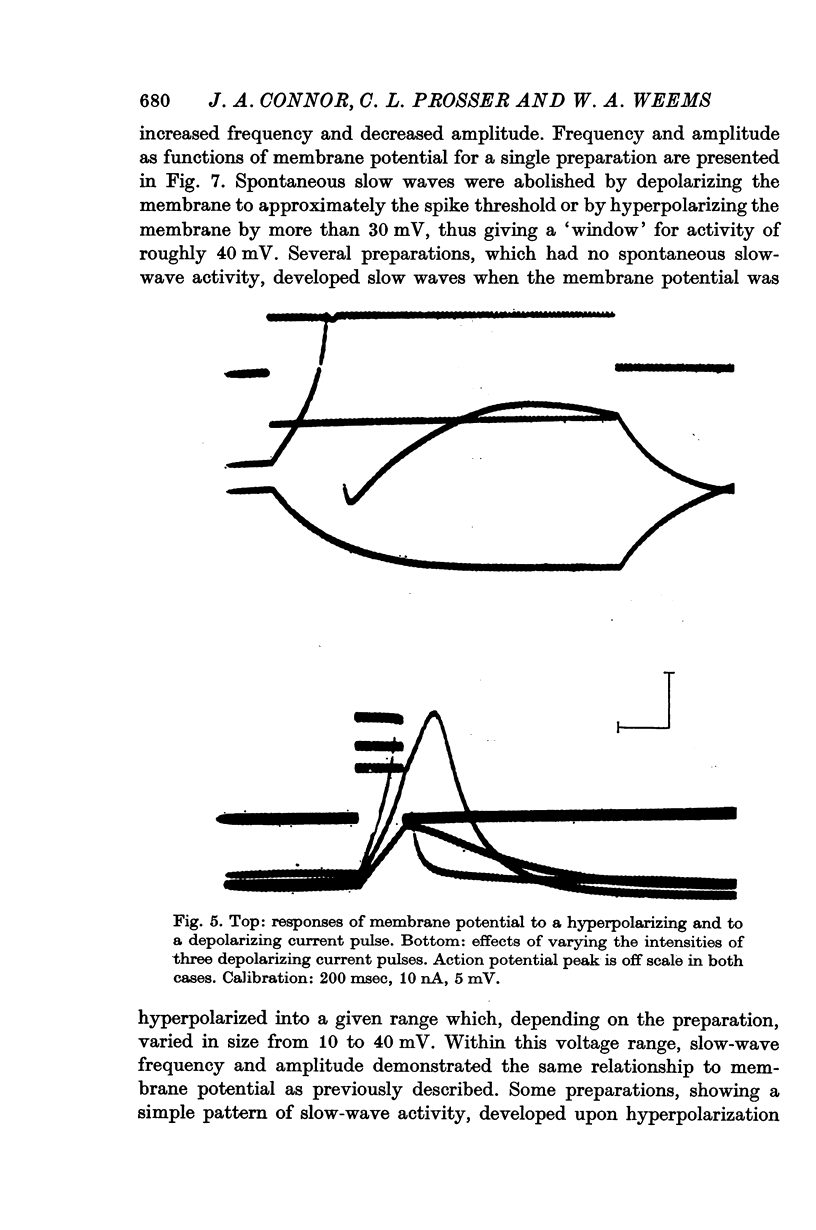
4. Some preparations with no spontaneous slow-wave activity developed slow waves when the membrane was hyperpolarized into a given range which, depending on the preparation, varied in size from 10 to 40 mV. Step or ramp depolarization of the membrane from hyperpolarized levels triggered slow waves in some preparations.
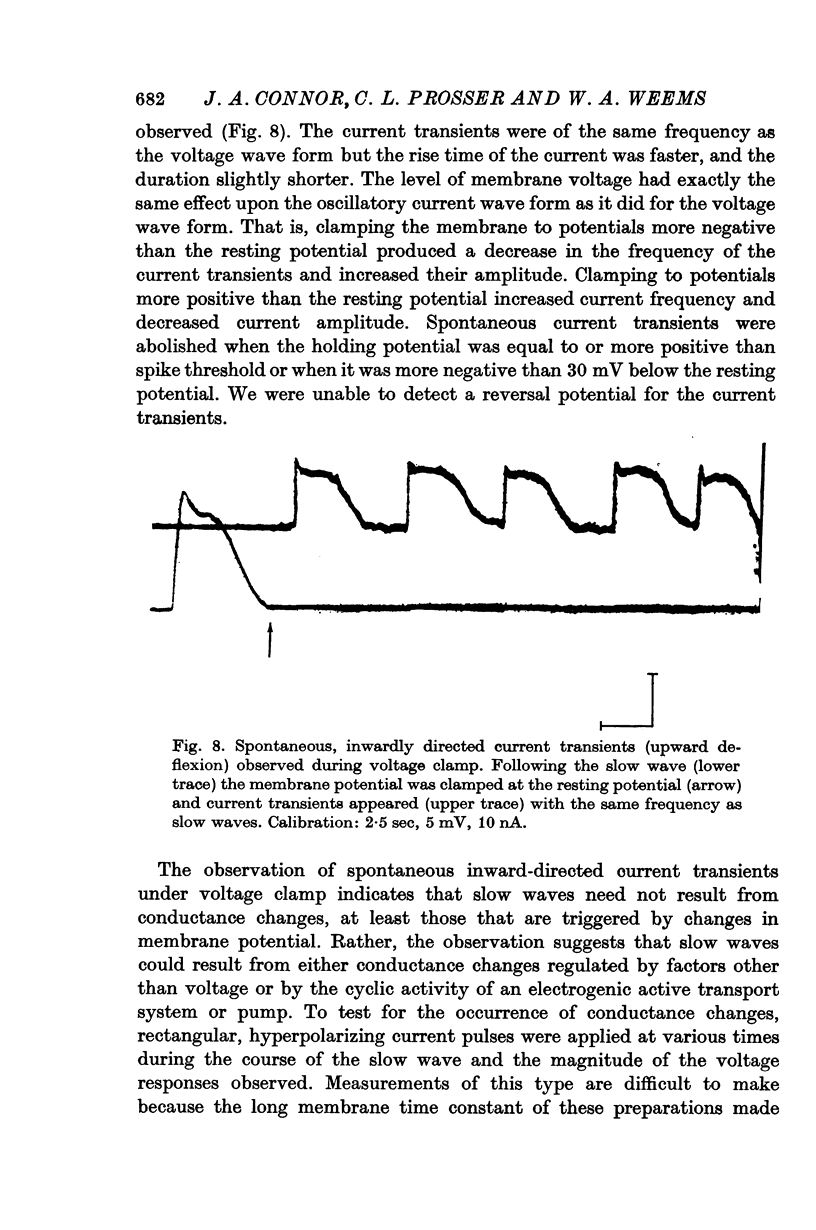
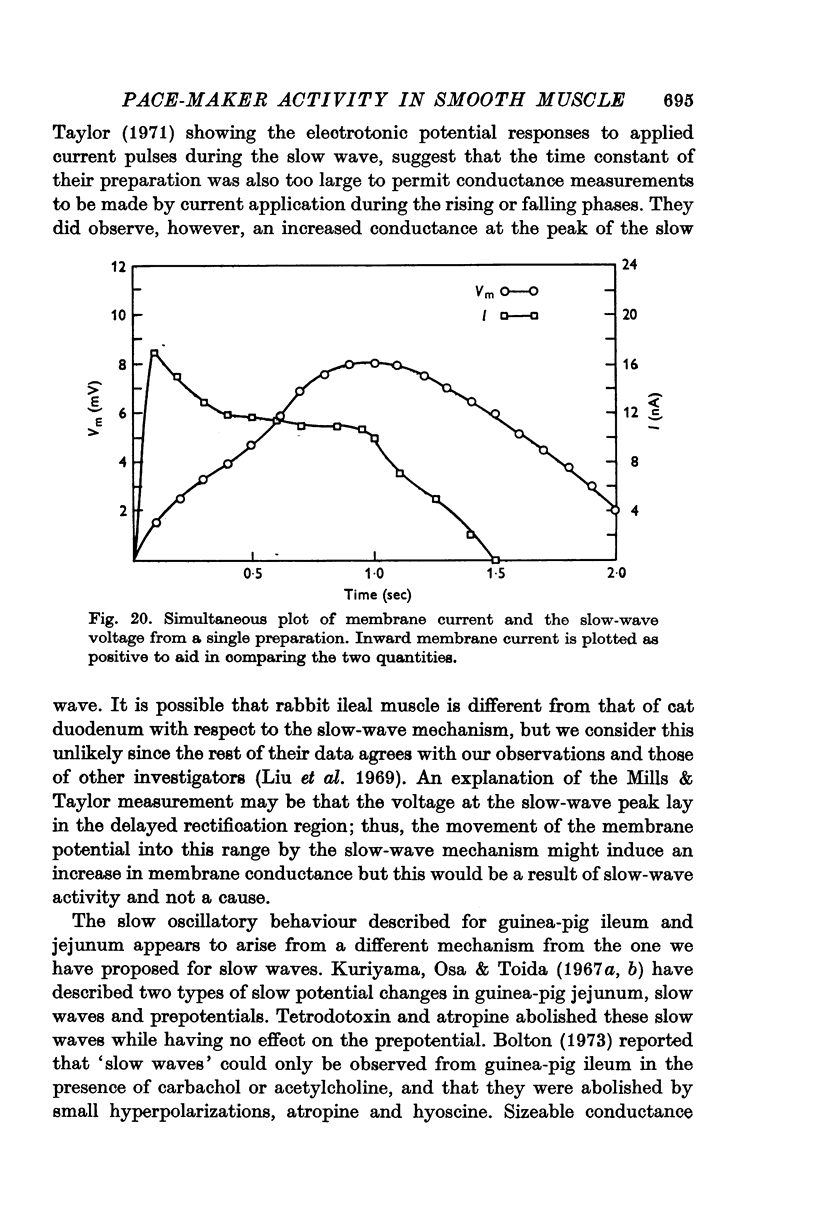
5. When the membrane potential of a slow-wave generating preparation was clamped at the resting potential, spontaneous inward-directed current transients were observed.
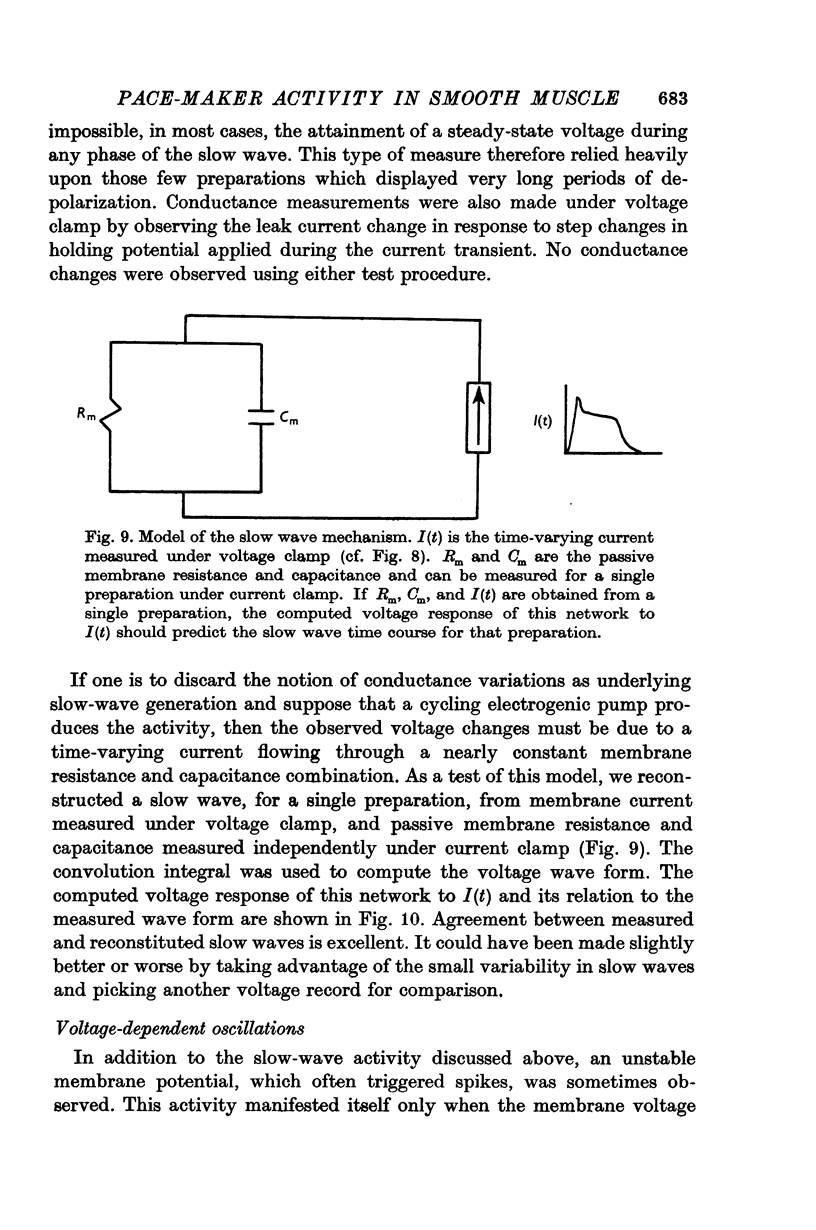
6. No changes in membrane conductance were observed during the course of a slow wave.
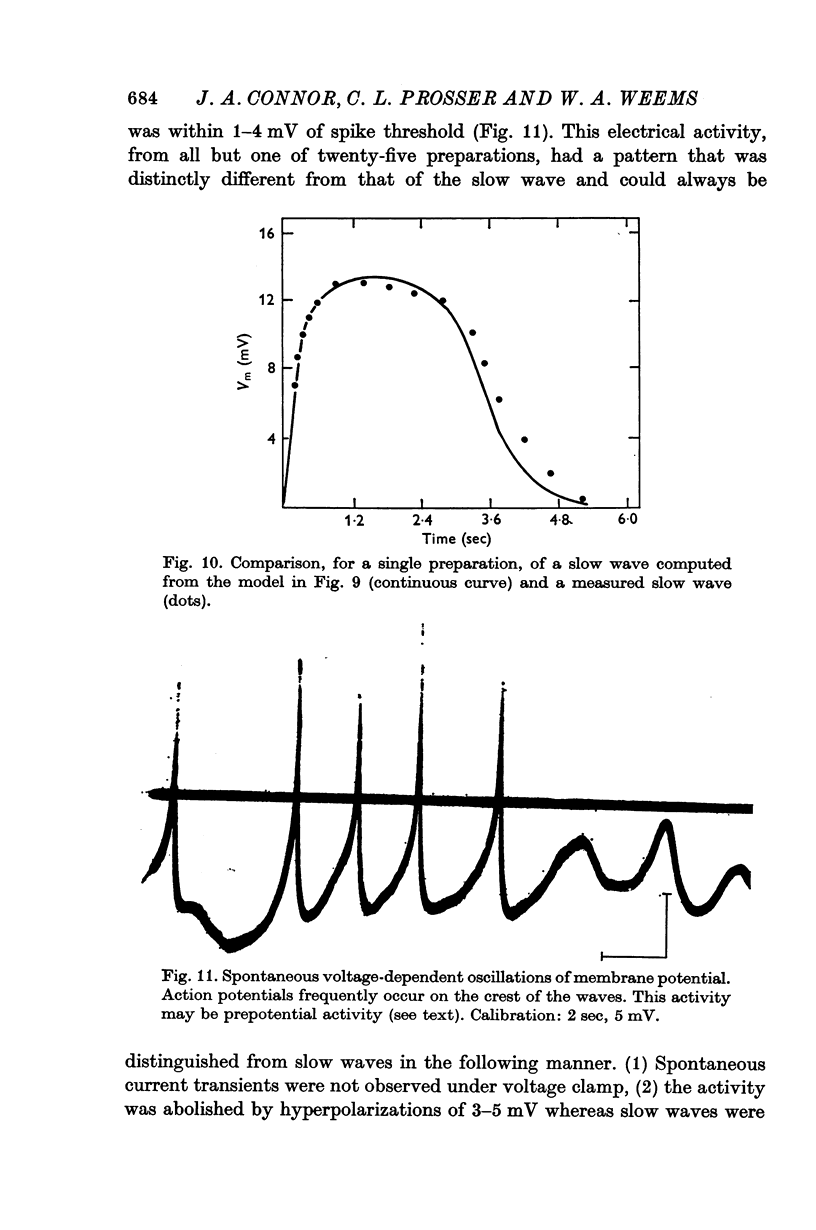
7. The slow-wave pattern was simulated for individual preparations by applying the membrane current measured under voltage clamp to the passive membrane resistance and capacitance measured independently under current clamp.
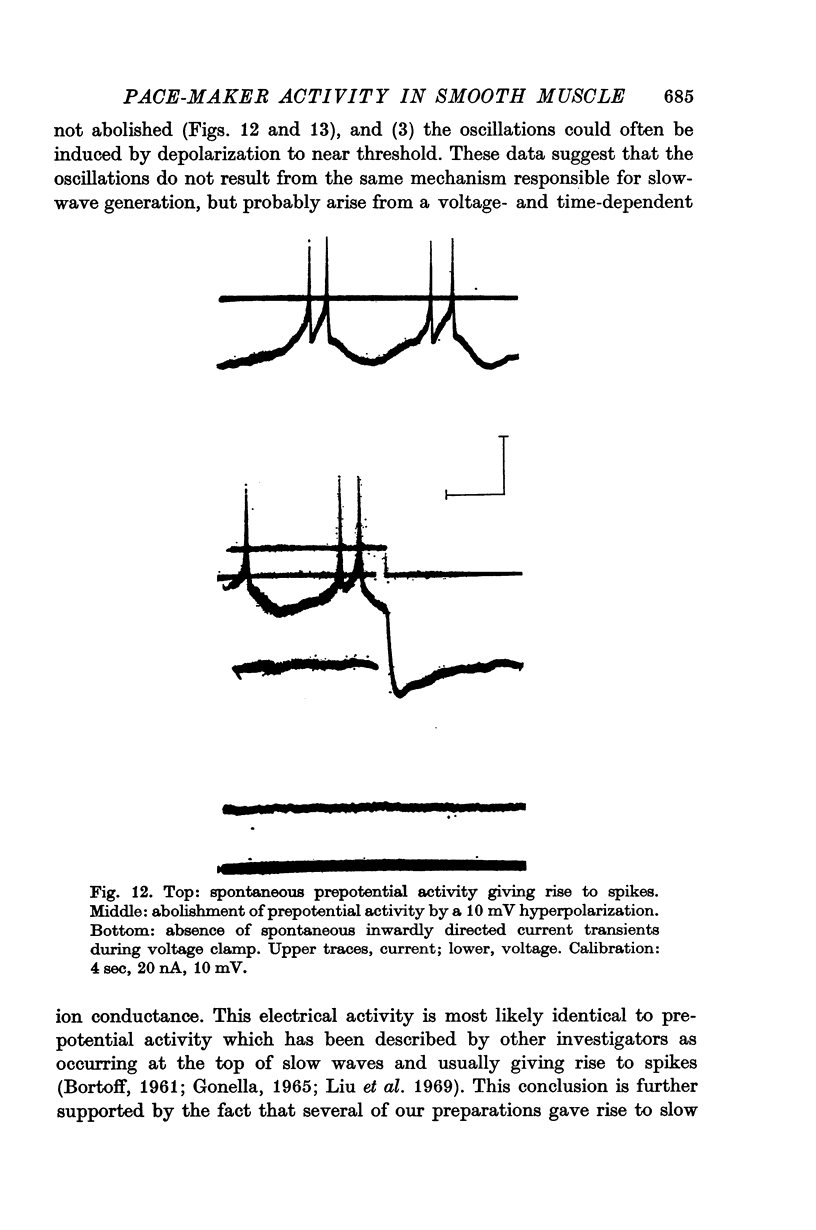
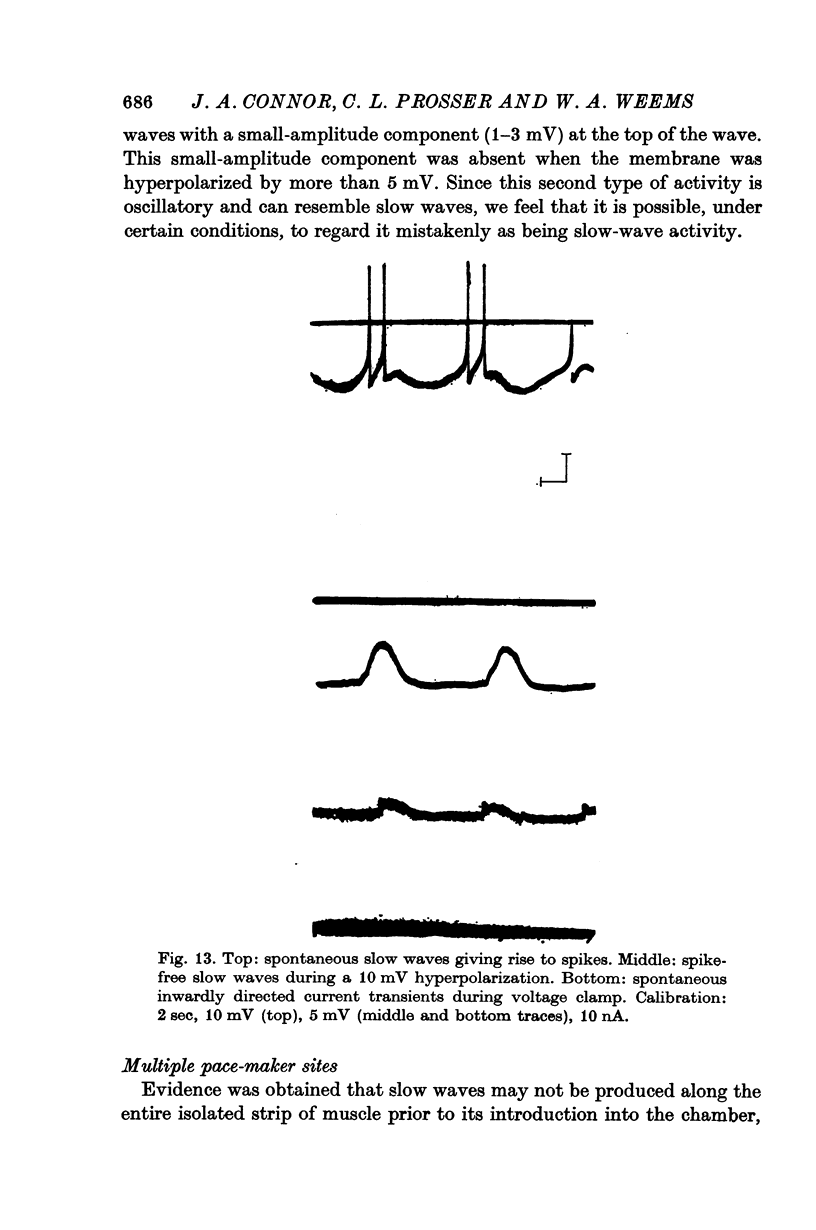
8. In addition to the defined slow-wave activity, voltage-dependent oscillations in membrane potential were sometimes observed.
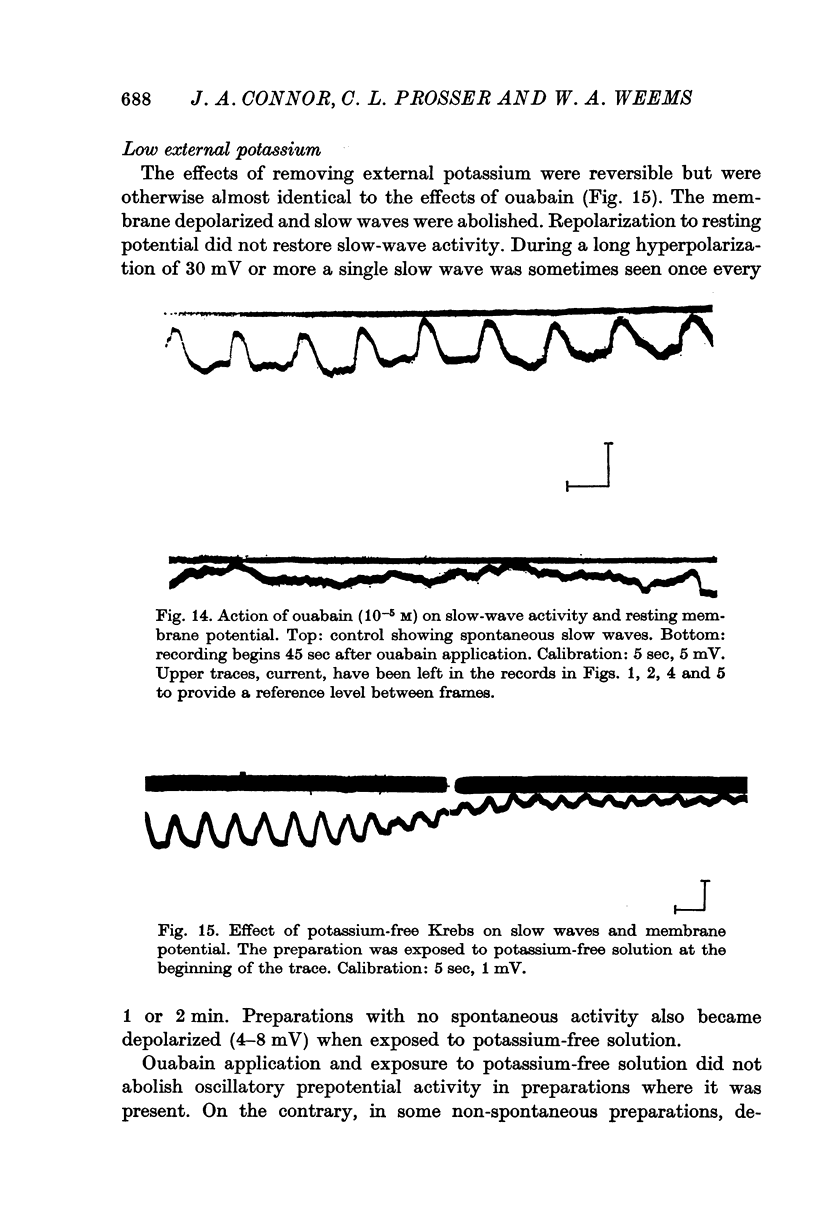
9. Application of 10-5 M ouabain irreversibly blocked slow waves and produced a membrane depolarization equal to or slightly greater than the slow wave crest. Repolarization of the membrane to the resting potential, or hyperpolarization, failed to restore slow-wave activity.
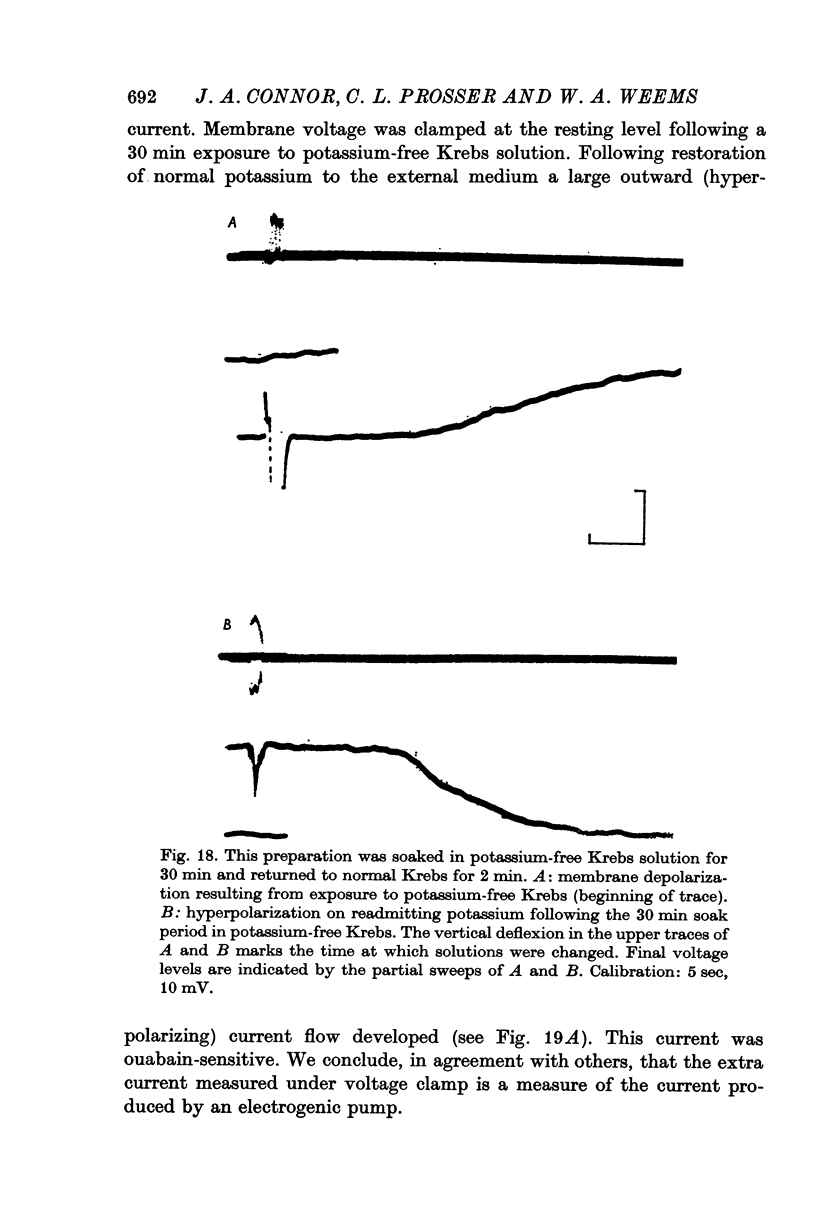
10. Removal of external potassium produced a reversible sequence of events almost identical to those following ouabain application.
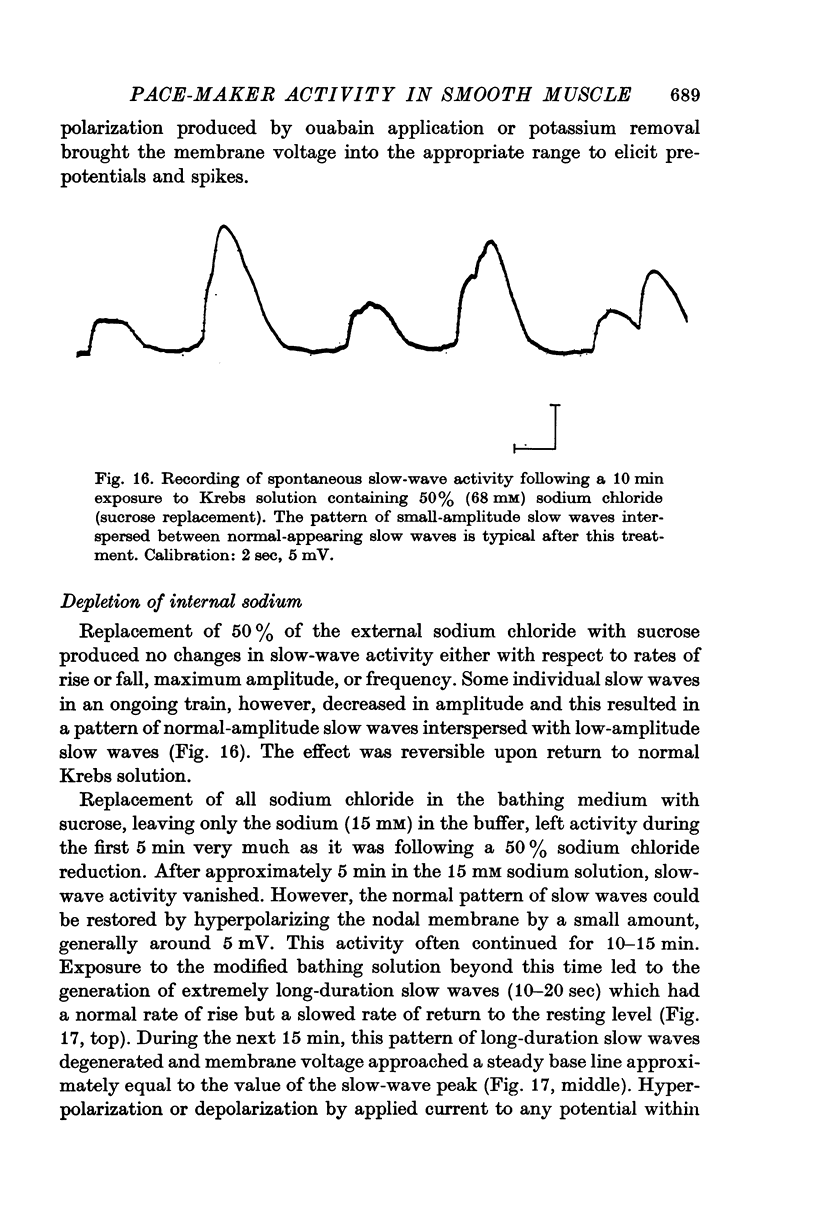
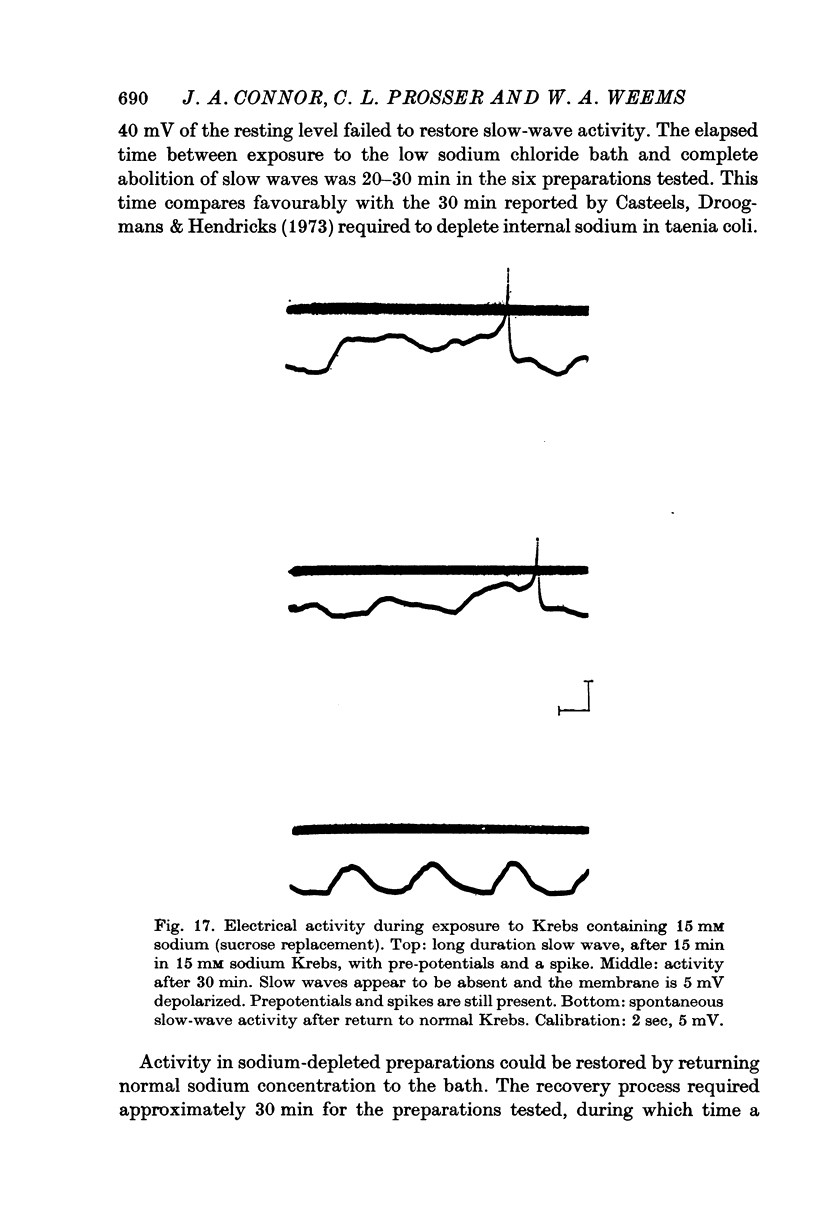
11. Replacement of 50% of the external sodium chloride with sucrose produced no changes in slow-wave activity with respect to rates of rise or fall, maximum amplitude or frequency. Sucrose replacement of all external sodium chloride eliminated slow waves after 5 min; however, activity could be restored by a slight hyperpolarization. Longer exposures to the modified bath abolished activity.
12. Following a conditioning exposure to potassium-free Krebs solution, readmission of potassium at normal concentration produced a mean hyperpolarization of 20·5 mV and in spontaneous preparations an arrest of activity.
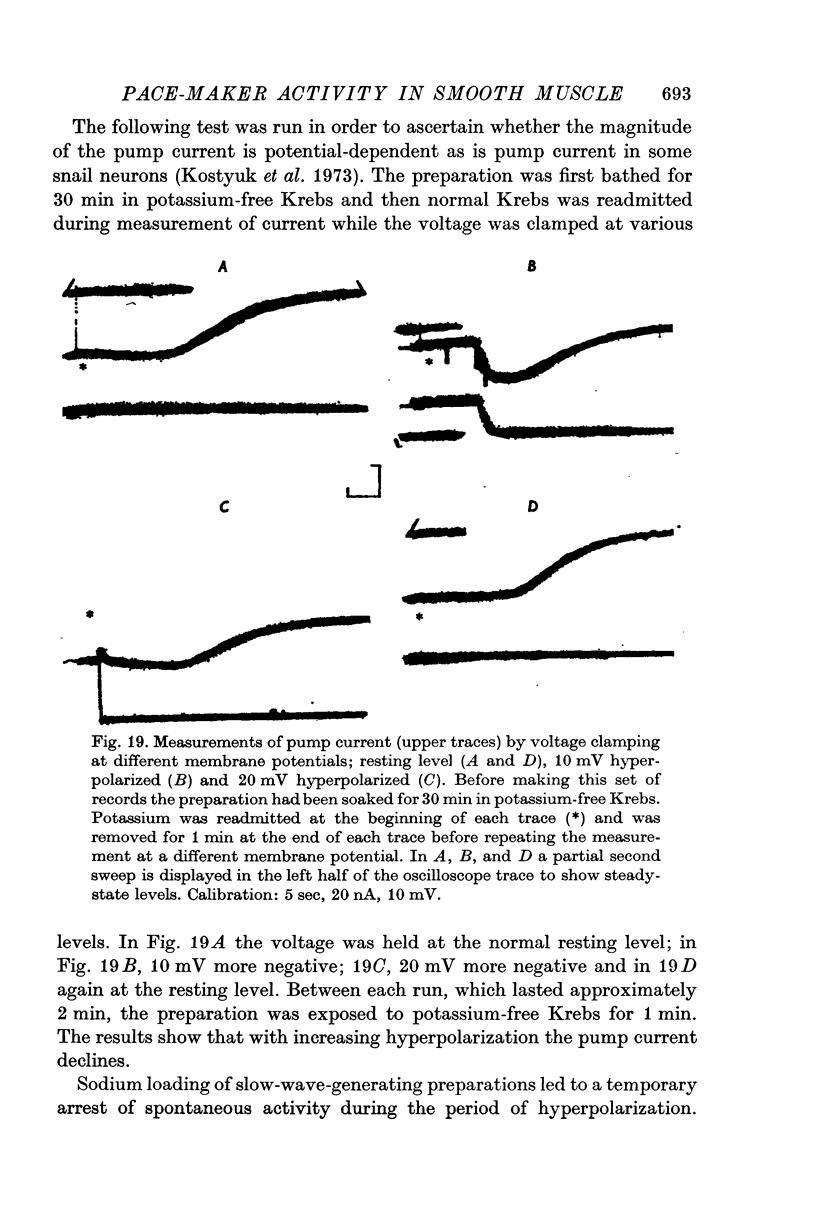
13. Pump current in sodium-loaded, non-spontaneously active preparations was measured by voltage clamp and was observed to be voltage-dependent.
14. The results of this study indicate that an electrogenic pump is present in longitudinal muscle of cat duodenum, and that oscillations in the level of pump current produce slow waves.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Slayman C. L. Membrane potential and conductance during transport of sodium, potassium and rubidium in frog muscle. J Physiol. 1966 Jun;184(4):970–1014. doi: 10.1113/jphysiol.1966.sp007961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. C., Jr Voltage-clamp studies on uterine smooth muscle. J Gen Physiol. 1969 Aug;54(2):145–165. doi: 10.1085/jgp.54.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASS P., CODE C. F., LAMBERT E. H. Motor and electric activity of the duodenum. Am J Physiol. 1961 Aug;201:287–291. doi: 10.1152/ajplegacy.1961.201.2.287. [DOI] [PubMed] [Google Scholar]
- BULBRING E., BURNSTOCK G., HOLMAN M. E. Excitation and conduction in the smooth muscle of the isolated taenia coli of the guinea-pig. J Physiol. 1958 Aug 6;142(3):420–437. doi: 10.1113/jphysiol.1958.sp006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr L., Berger W., Dewey M. M. Electrical transmission at the nexus between smooth muscle cells. J Gen Physiol. 1968 Mar;51(3):347–368. doi: 10.1085/jgp.51.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger W., Barr L. Use of rubber membranes to improve sucrose-gap and other electrical recording techniques. J Appl Physiol. 1969 Mar;26(3):378–382. doi: 10.1152/jappl.1969.26.3.378. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. Effects of electrogenic sodium pumping on the membrane potential of longitudinal smooth muscle from terminal ileum of guinea-pig. J Physiol. 1973 Feb;228(3):693–712. doi: 10.1113/jphysiol.1973.sp010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. On the nature of the oscillations of the membrane potential (slow waves) produced by acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1971 Jul;216(2):403–418. doi: 10.1113/jphysiol.1971.sp009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoff A. Electrical transmission of slow waves from longitudinal to circular intestinal muscle. Am J Physiol. 1965 Dec;209(6):1254–1260. doi: 10.1152/ajplegacy.1965.209.6.1254. [DOI] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Effect of sodium and sodium-substitutes on the active ion transport and on the membrane potential of smooth muscle cells. J Physiol. 1973 Feb;228(3):733–748. doi: 10.1113/jphysiol.1973.sp010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Inward and delayed outward membrane currents in isolated neural somata under voltage clamp. J Physiol. 1971 Feb;213(1):1–19. doi: 10.1113/jphysiol.1971.sp009364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. B., Keynes R. D., Rybová R. The coupling of sodium efflux and potassium influx in frog muscle. J Physiol. 1965 Dec;181(4):865–880. doi: 10.1113/jphysiol.1965.sp007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANIEL E. E. EFFECTS OF INTRA-ARTERIAL PERFUSIONS ON ELECTRICAL ACTIVITY AND ELECTROLYTE CONTENTS OF DOG SMALL INTESTINE. Can J Physiol Pharmacol. 1965 Jul;43:551–577. doi: 10.1139/y65-056. [DOI] [PubMed] [Google Scholar]
- DANIEL E. E., HONOUR A. J., BOGOCH A. Electrical activity of the longitudinal muscle of dog small intestine studied in vivo using microelectrodes. Am J Physiol. 1960 Jan;198:113–118. doi: 10.1152/ajplegacy.1960.198.1.113. [DOI] [PubMed] [Google Scholar]
- De Weer P., Geduldig D. Electrogenic sodium pump in squid giant axon. Science. 1973 Mar 30;179(4080):1326–1328. doi: 10.1126/science.179.4080.1326. [DOI] [PubMed] [Google Scholar]
- Gonella J. Variation de l'activité électrique spontanée du duodénum de Lapin avec le lieu de dérivation. C R Acad Sci Hebd Seances Acad Sci D. 1965 May 17;260(20):5362–5365. [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Membrane potentials of the lobster giant axon obtained by use of the sucrose-gap technique. J Gen Physiol. 1962 Jul;45:1195–1216. doi: 10.1085/jgp.45.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D. D. Effect of antibiotics and selective inhibitors of ATP on intestinal slow waves. Am J Physiol. 1971 Feb;220(2):299–306. doi: 10.1152/ajplegacy.1971.220.2.299. [DOI] [PubMed] [Google Scholar]
- Job D. D. Ionic basis of intestinal electrical activity. Am J Physiol. 1969 Nov;217(5):1534–1541. doi: 10.1152/ajplegacy.1969.217.5.1534. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- KERKUT G. A., THOMAS R. C. AN ELECTROGENIC SODIUM PUMP IN SNAIL NERVE CELLS. Comp Biochem Physiol. 1965 Jan;14:167–183. doi: 10.1016/0010-406x(65)90017-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Nagai T., Prosser C. L. Electrical interaction between muscle layers of cat intestine. Am J Physiol. 1966 Dec;211(6):1281–1291. doi: 10.1152/ajplegacy.1966.211.6.1281. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Prosser C. L., Nagai T. Electrical properties of intestinal muscle as measured intracellularly and extracellularly. Am J Physiol. 1967 Jul;213(1):275–286. doi: 10.1152/ajplegacy.1967.213.1.275. [DOI] [PubMed] [Google Scholar]
- Kootsey J. M., Johnson E. A. Voltage clamp of cardiac muscle. A theoretical analysis of early currents in the single sucrose gap. Biophys J. 1972 Nov;12(11):1496–1508. doi: 10.1016/S0006-3495(72)86177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto M., Horn L. Voltage clamping of smooth muscle from Taenia coli. Microvasc Res. 1970 Apr;2(2):188–201. doi: 10.1016/0026-2862(70)90007-5. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Toida N. Electrophysiological study of the intestinal smooth muscle of the guinea-pig. J Physiol. 1967 Jul;191(2):239–255. doi: 10.1113/jphysiol.1967.sp008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Toida N. Nervous factors influencing the membrane activity of intestinal smooth muscle. J Physiol. 1967 Jul;191(2):257–270. doi: 10.1113/jphysiol.1967.sp008249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Prosser C. L., Job D. D. Ionic dependence of slow waves and spikes in intestinal muscle. Am J Physiol. 1969 Nov;217(5):1542–1547. doi: 10.1152/ajplegacy.1969.217.5.1542. [DOI] [PubMed] [Google Scholar]
- Mills R. G., Taylor G. S. Studies of intestinal slow wave activity with a double sucrose gap apparatus. Life Sci I. 1971 Mar 15;10(6):347–353. doi: 10.1016/0024-3205(71)90134-2. [DOI] [PubMed] [Google Scholar]
- Taylor G. S., Paton D. M., Daniel E. E. Characteristics of electrogenic sodium pumping in rat myometrium. J Gen Physiol. 1970 Sep;56(3):360–375. doi: 10.1085/jgp.56.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T., Watanabe H. Factors controlling myogenic activity in smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):73–85. doi: 10.1098/rstb.1973.0010. [DOI] [PubMed] [Google Scholar]


