Abstract
1. Progressive block of neuromuscular transmission in frog sartorius and gastrocnemius preparations by haemagglutinin-free crystalline Type A botulinum toxin (BTX) was investigated by in vitro application and by injection of the toxin into living animals.
2. Neuromuscular block was characterized by (a) decline in amplitude of evoked twitch contractions, (b) decline in amplitudes of end-plate potentials (e.p.p.s) and (c) changes in statistical characteristics of spontaneous miniature end-plate potentials (m.e.p.p.s).
3. Progress of the block was enhanced by nerve stimulation.
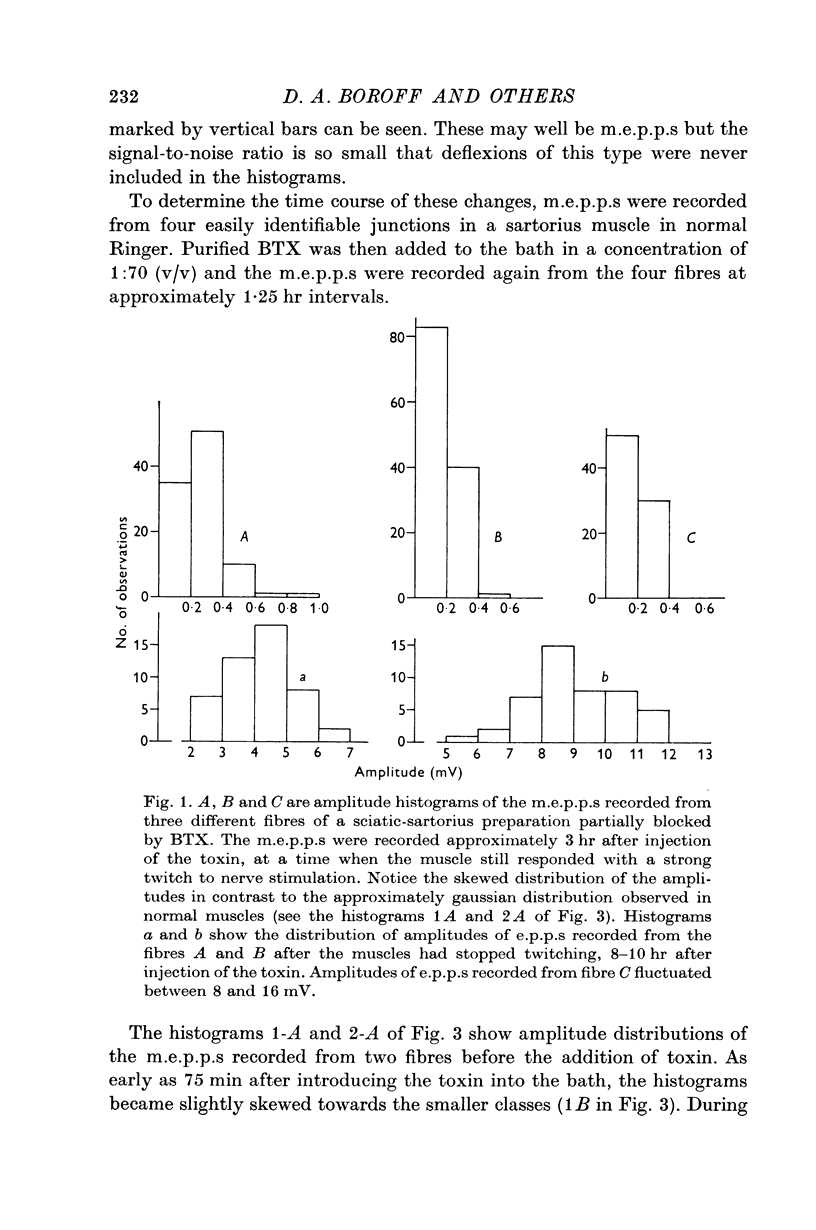
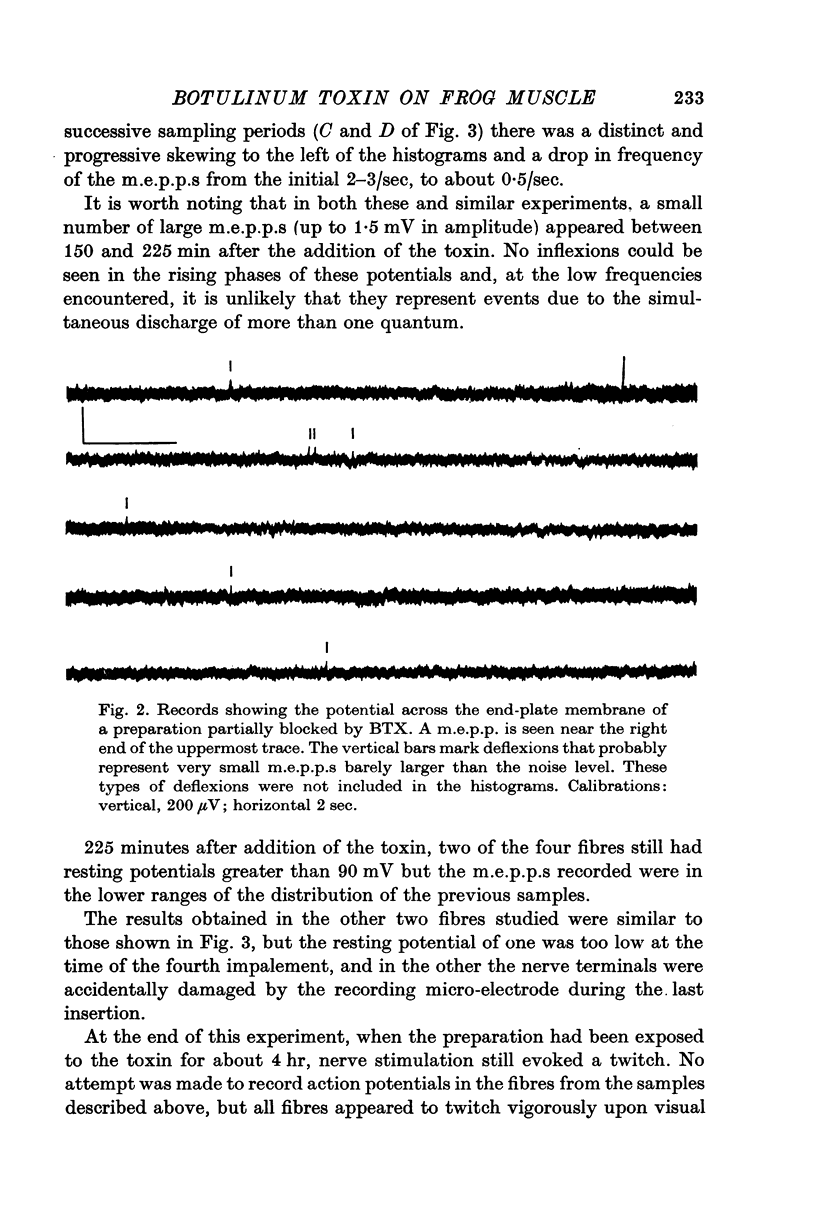
4. A decrease in frequency to less than 0·1/sec and decreased average amplitudes of m.e.p.p.s preceded observable impairment of neuromuscular transmission. These changes occurred as early as 3 hr after injection of the toxin into dorsal lymph sacs.
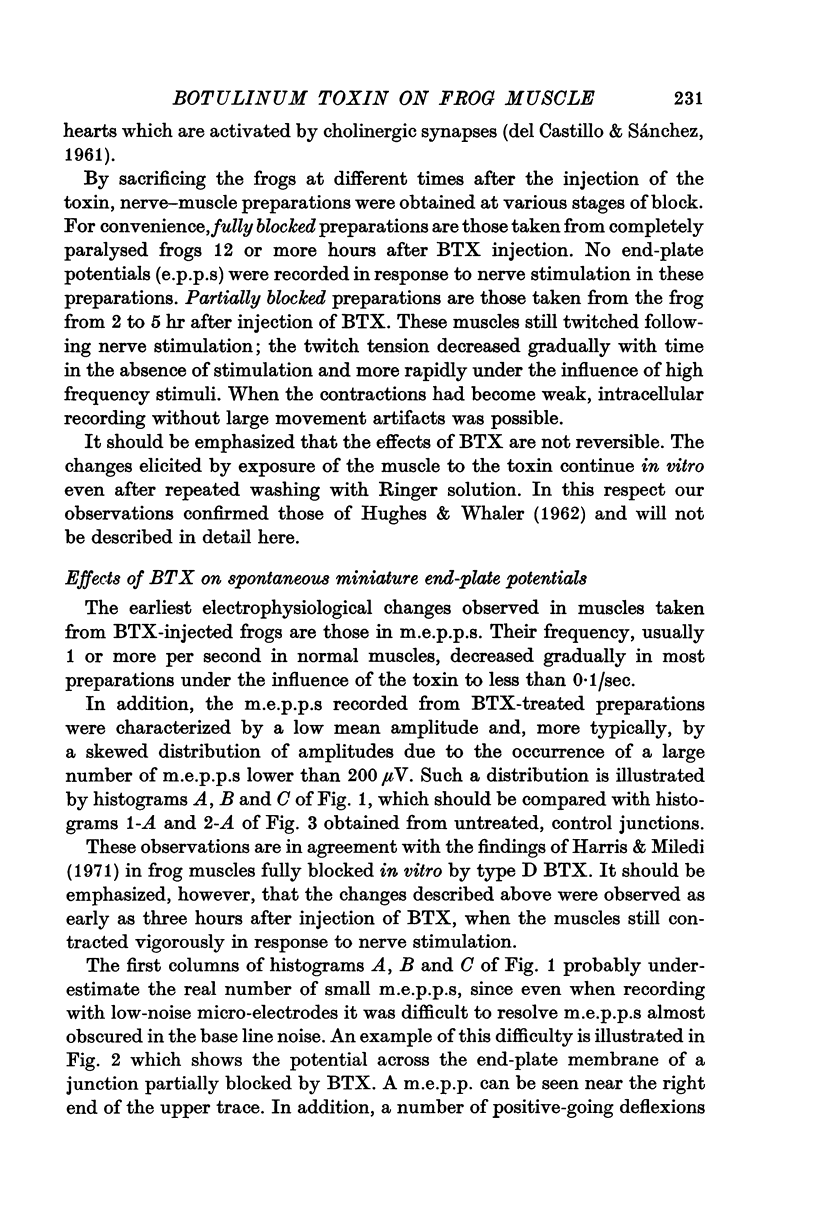
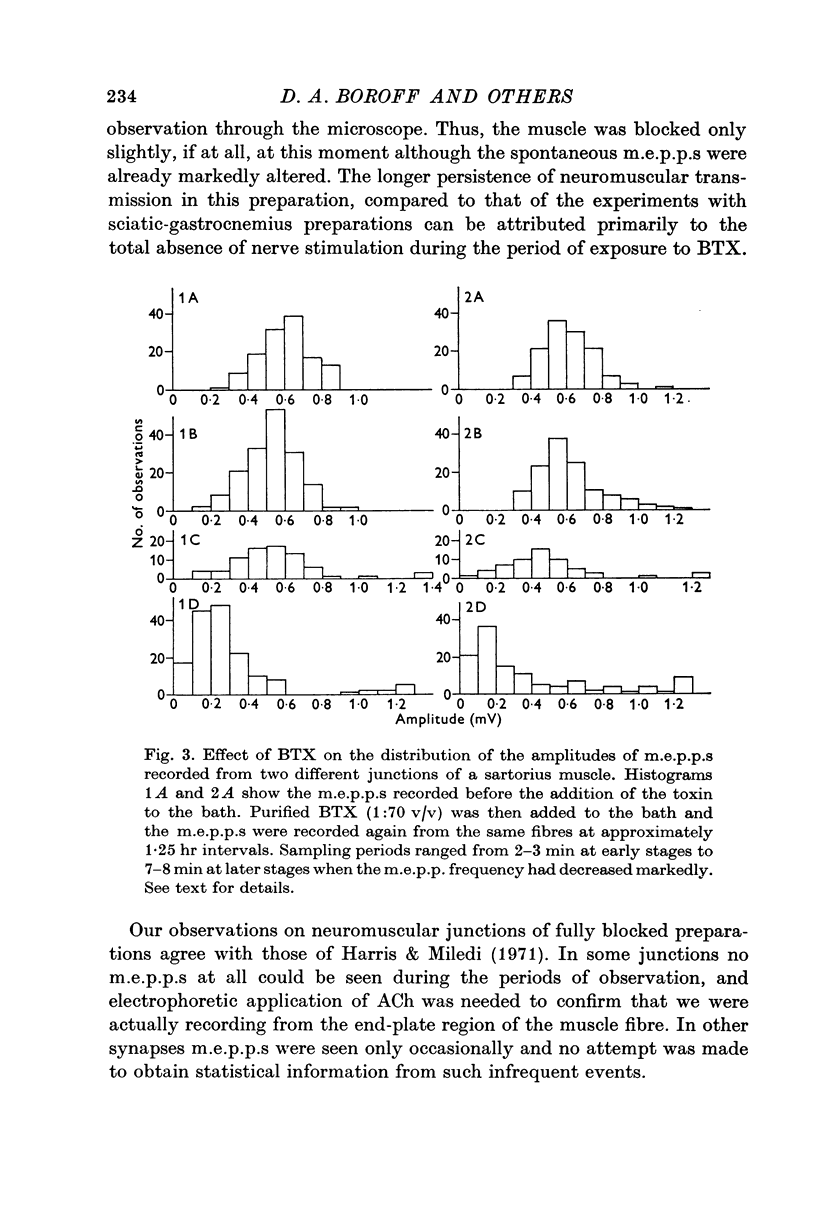
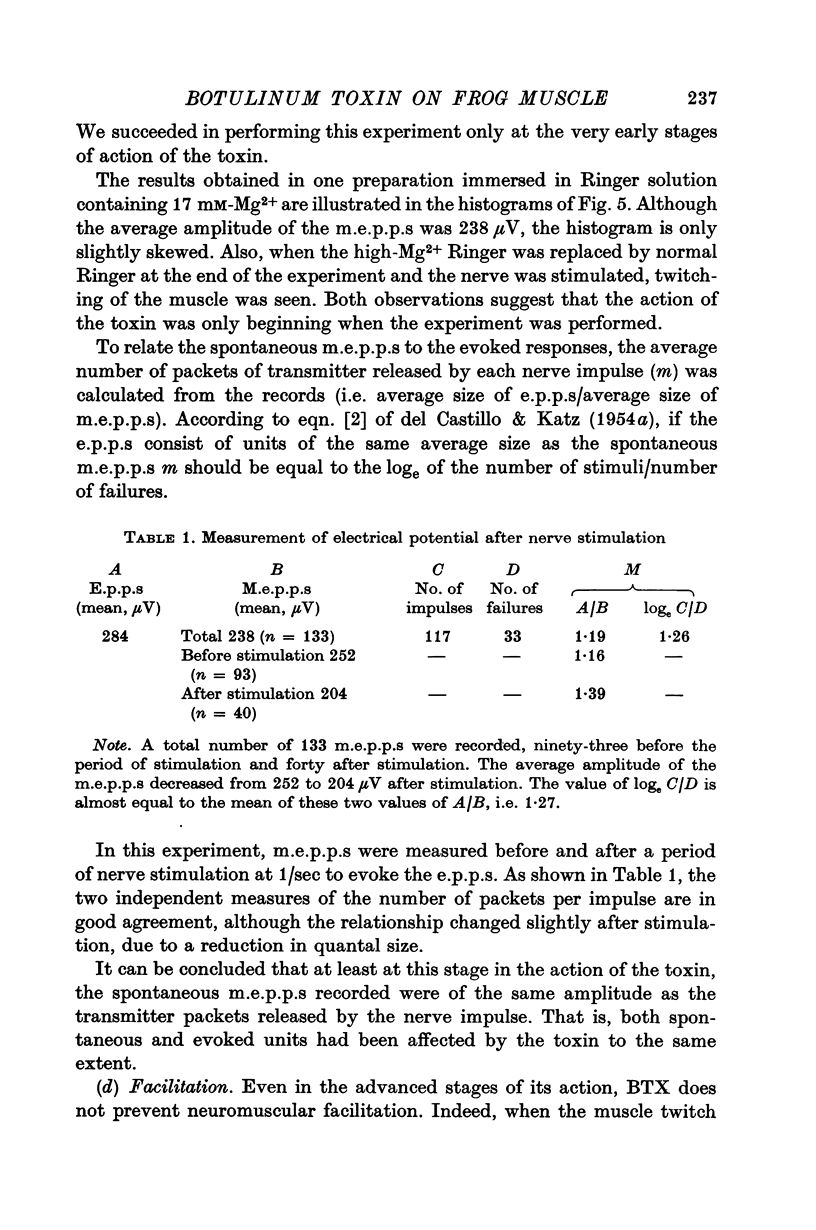
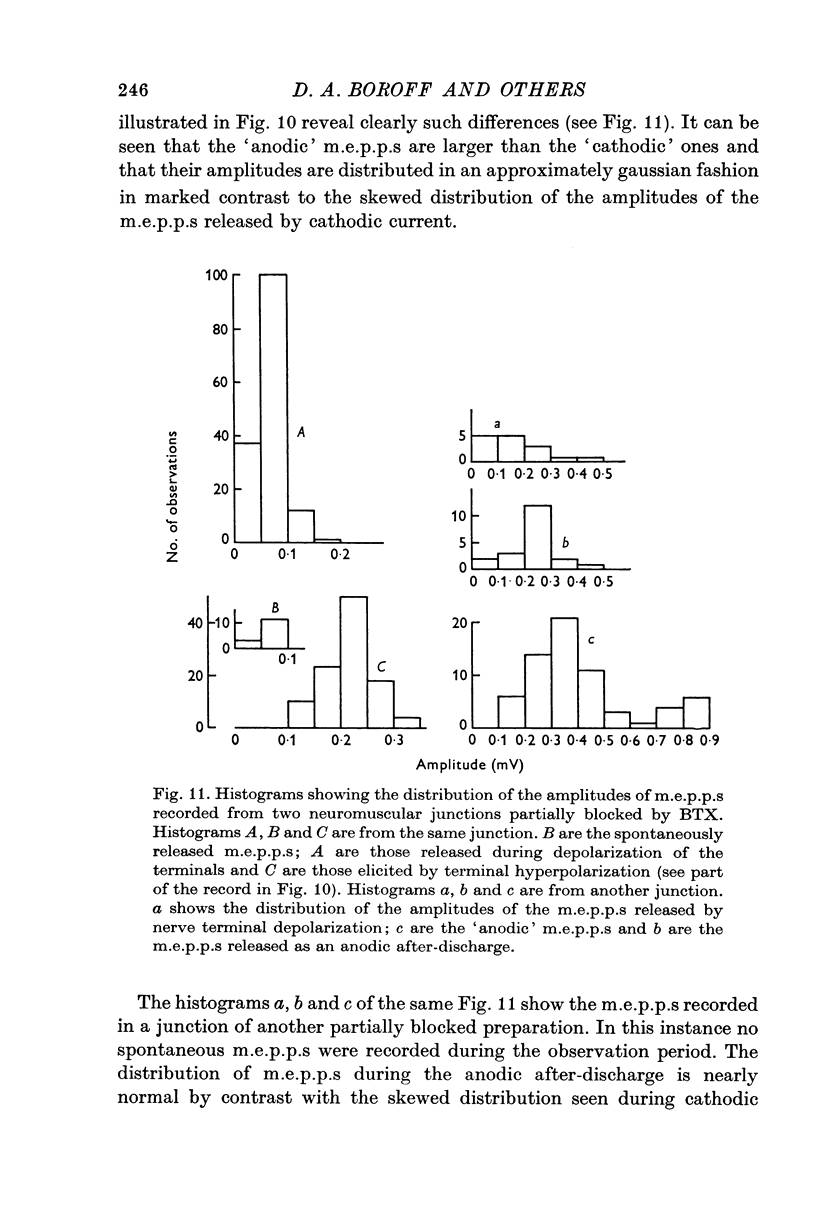
5. The amplitude distributions of m.e.p.p.s changed from a normal distribution to one that showed an increased skewness toward smaller amplitudes as the block progressed. These changes were first detectable as early as 75 min following addition of the toxin to the bath.
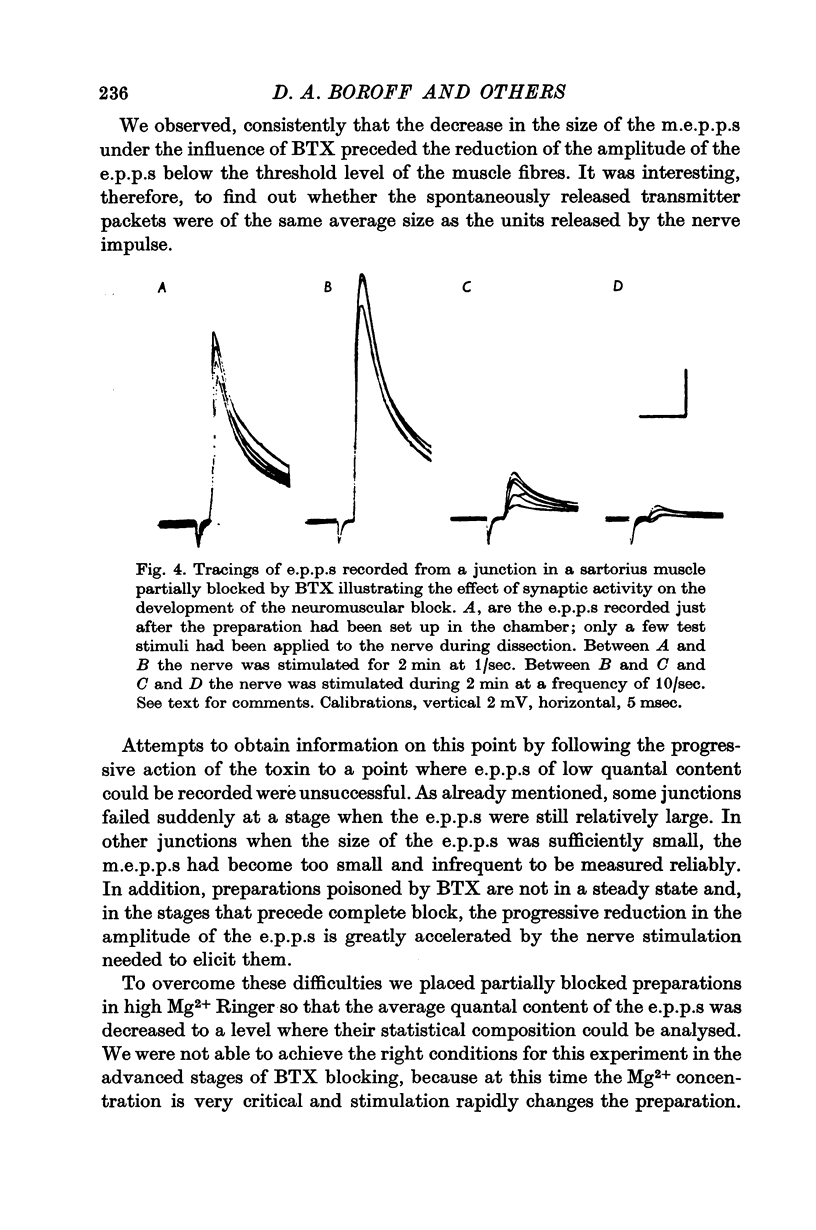
6. At later stages of toxin action, e.p.p.s began to decrease in amplitude and eventually failed altogether. E.p.p.s showed a normal quantal variation at very early stages in the block in Mg2+-treated preparations. At later stages of the block, it was not possible to test the quantal make-up of the e.p.p.
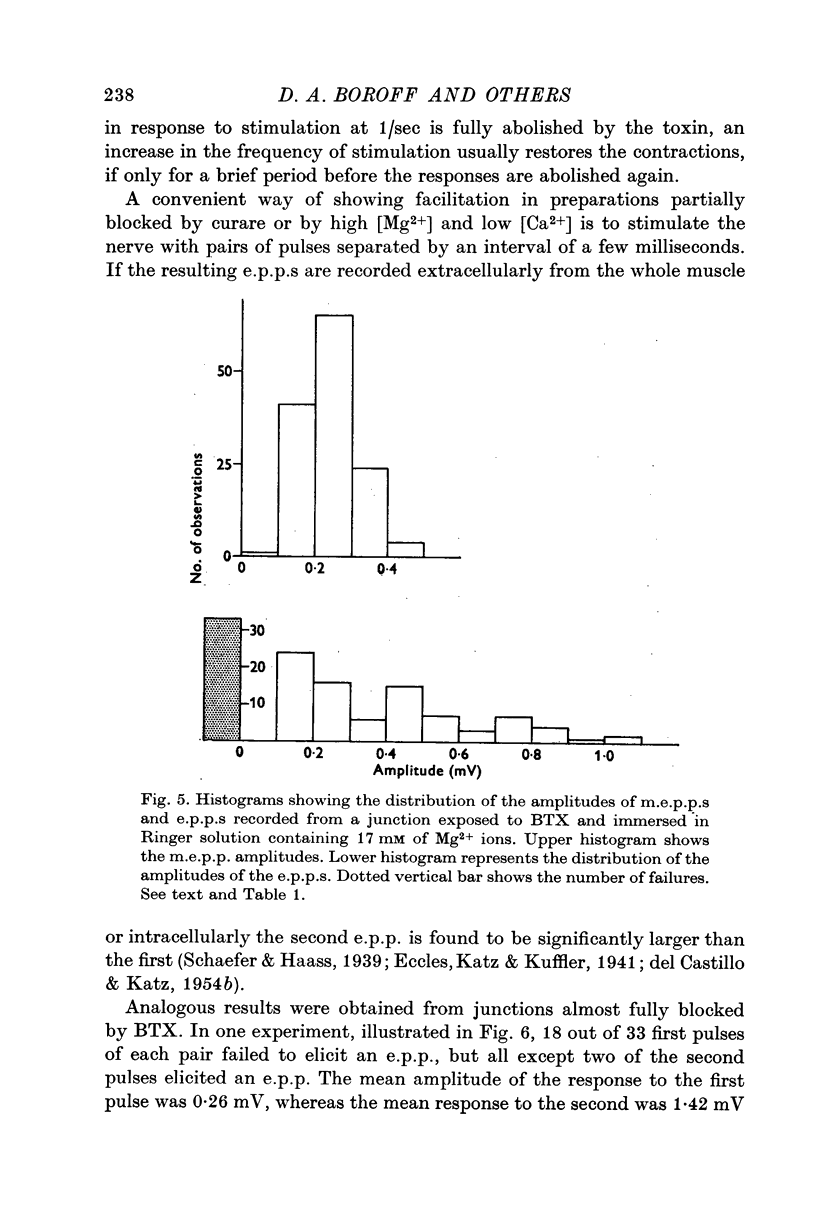
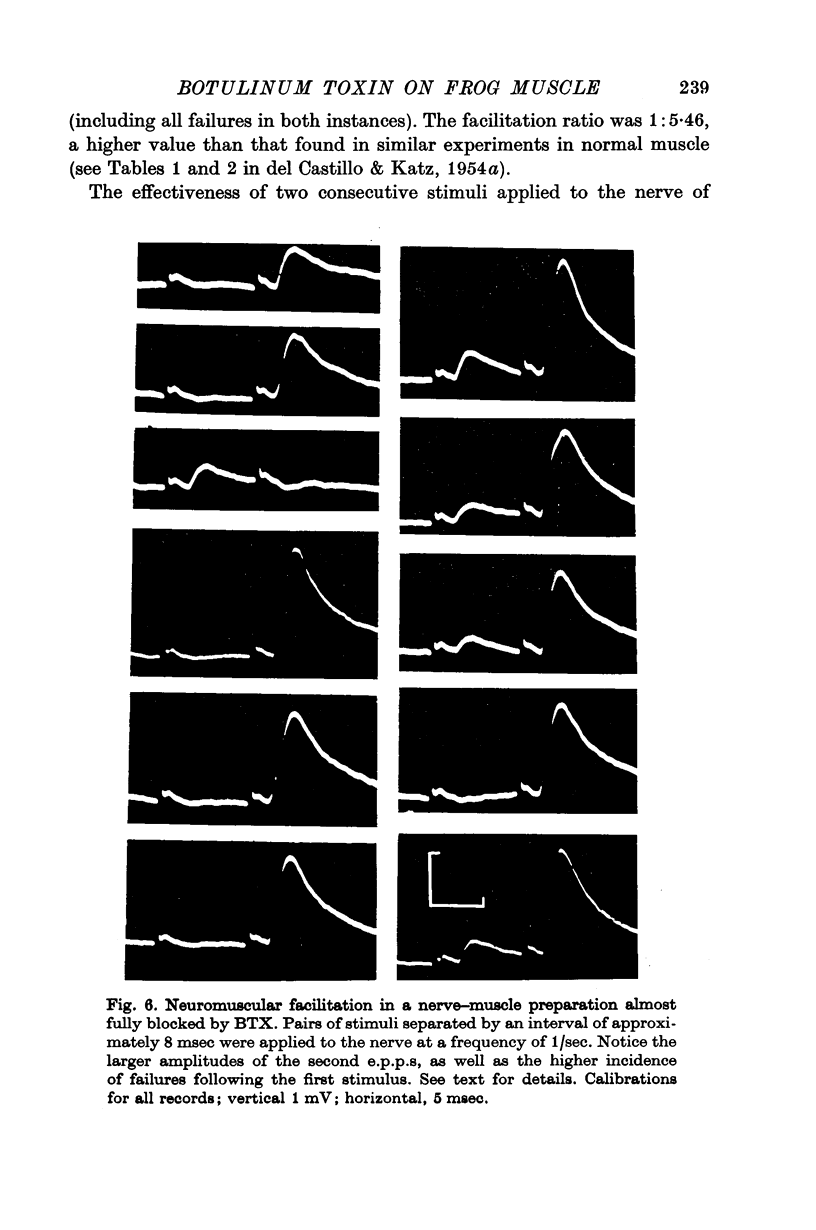
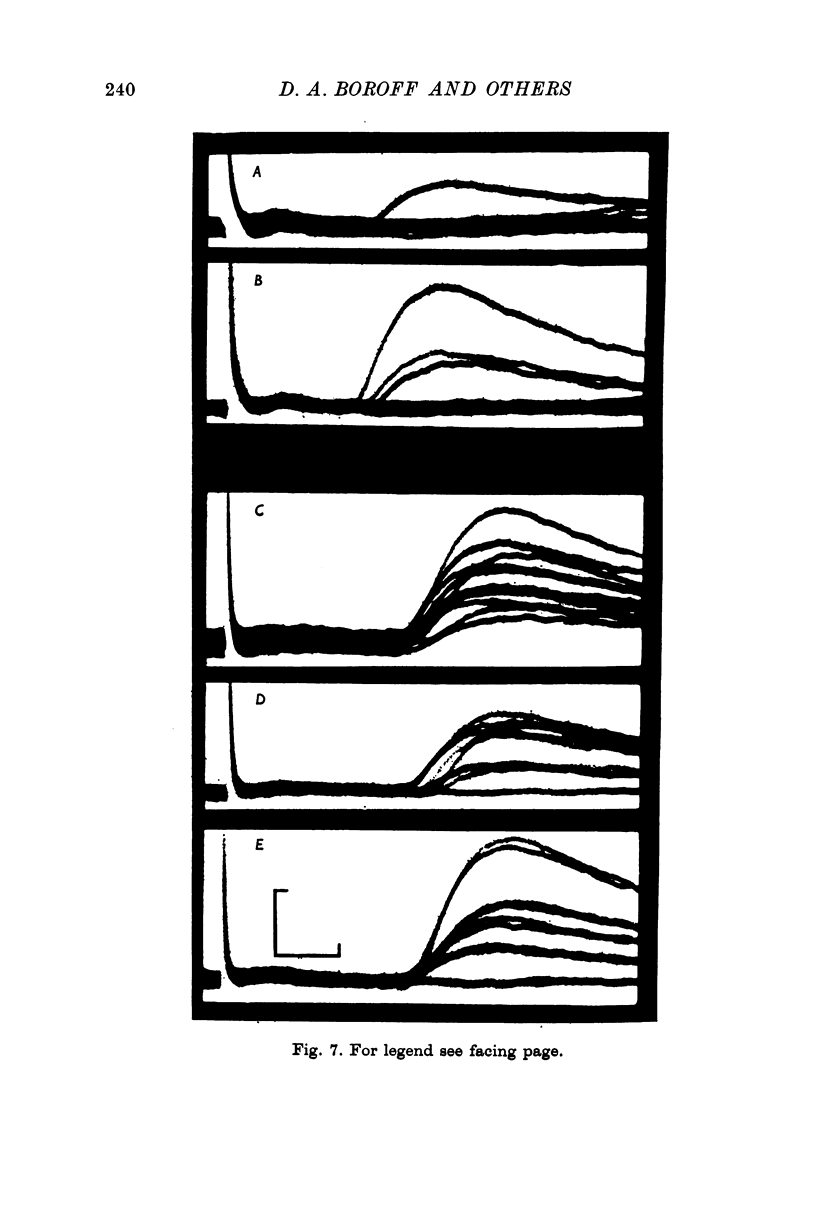
7. At all stages before complete failure it was possible to obtain normal or greater than normal degrees of synaptic facilitation with paired stimuli to the nerve. This aspect of the coupling of nerve terminal depolarization to transmitter release appears to be relatively unaffected by BTX.
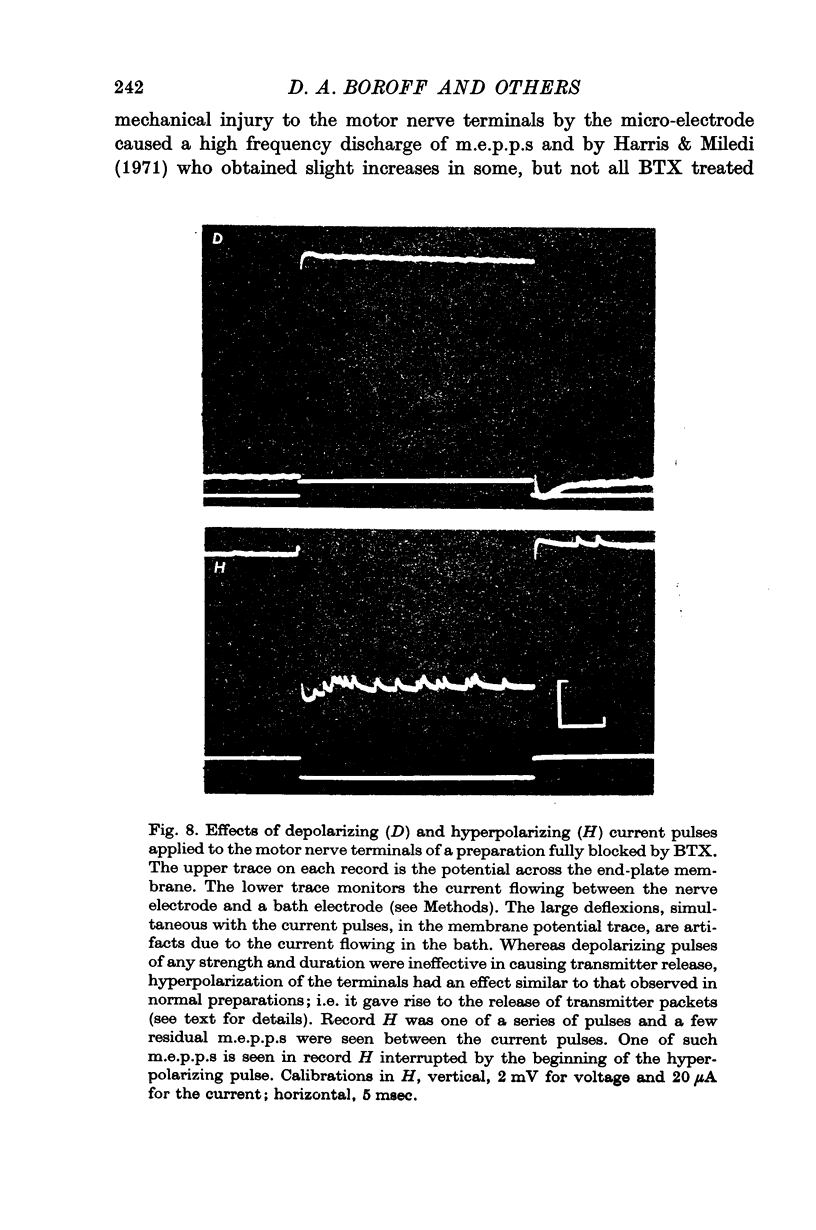
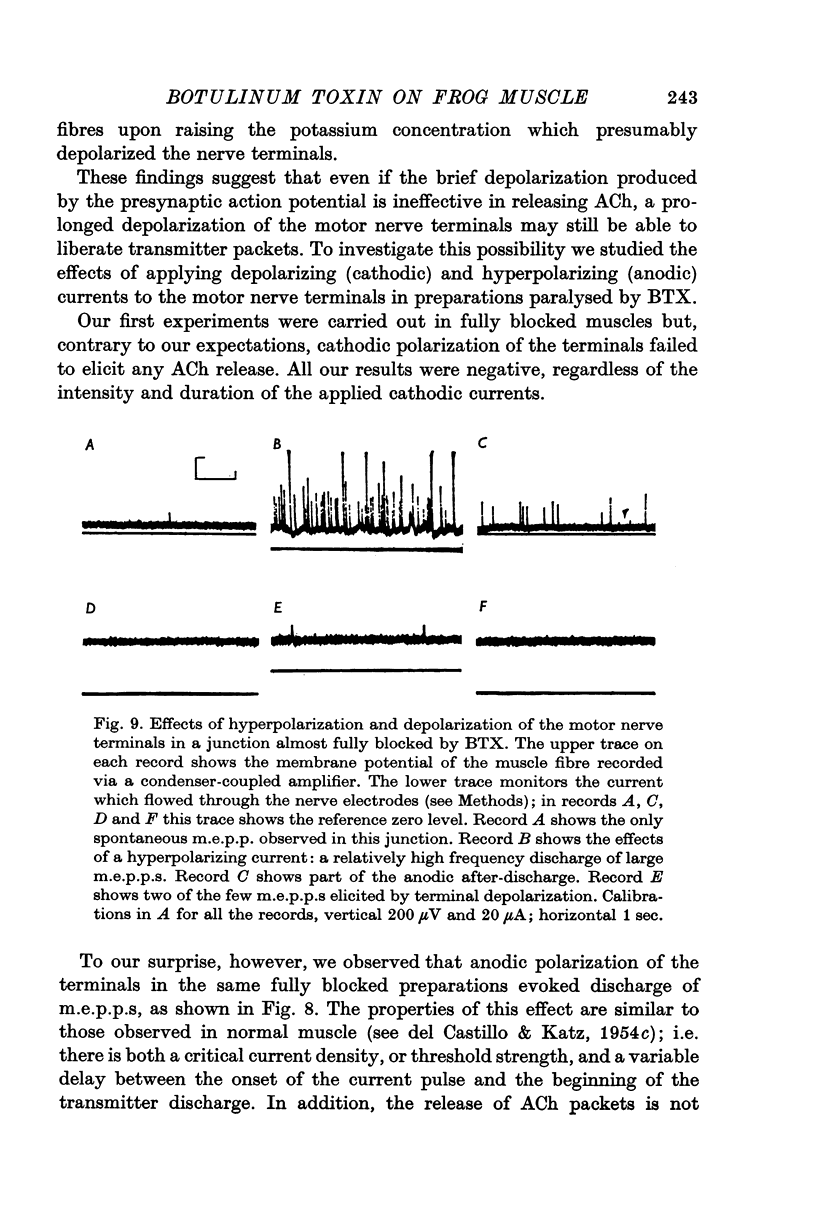
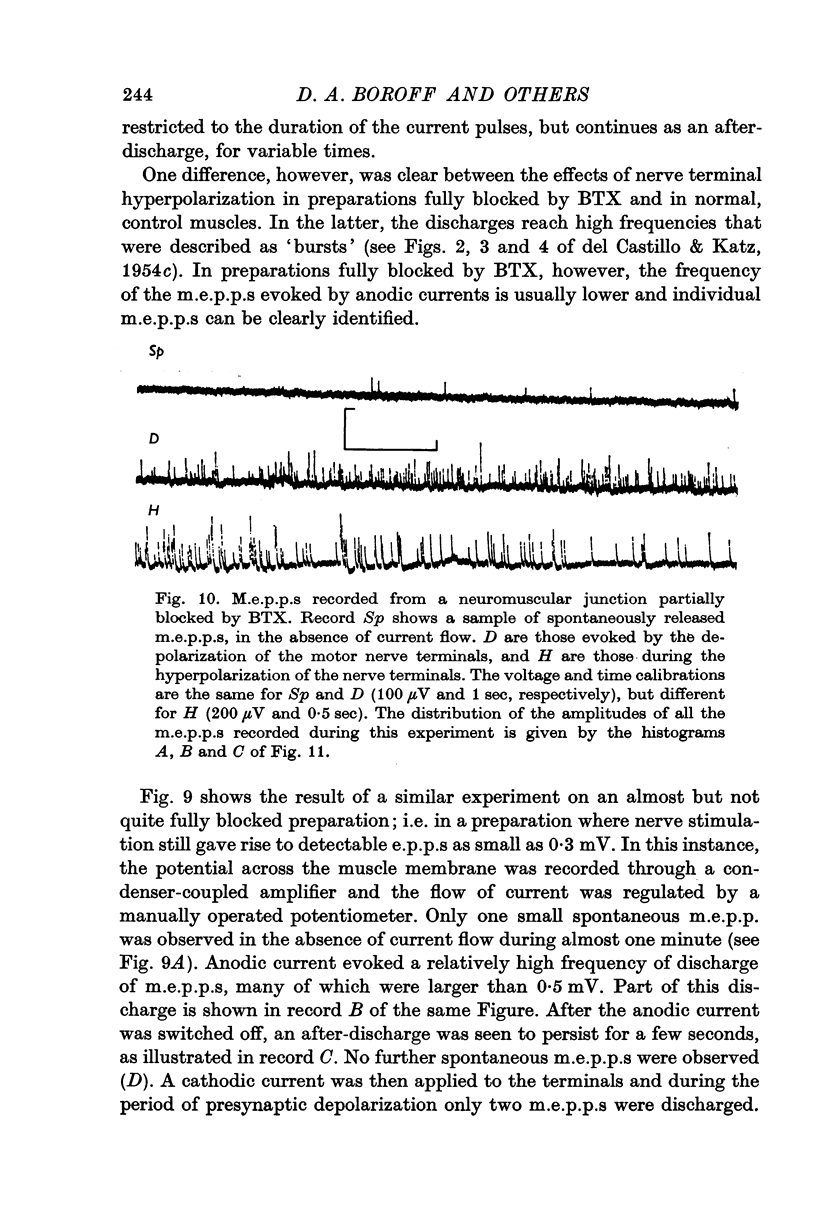
8. Electrical depolarization of nerve terminals in partially blocked preparations evoked a maintained discharge of m.e.p.p.s with an amplitude distribution similar to that of the spontaneous m.e.p.p.s; hyperpolarization of the terminals evokes a distinctly larger class of m.e.p.p.s. In fully blocked preparations, depolarization of the terminals does not evoke transmitter release whereas hyperpolarization continues to yield the larger class of m.e.p.p.s.
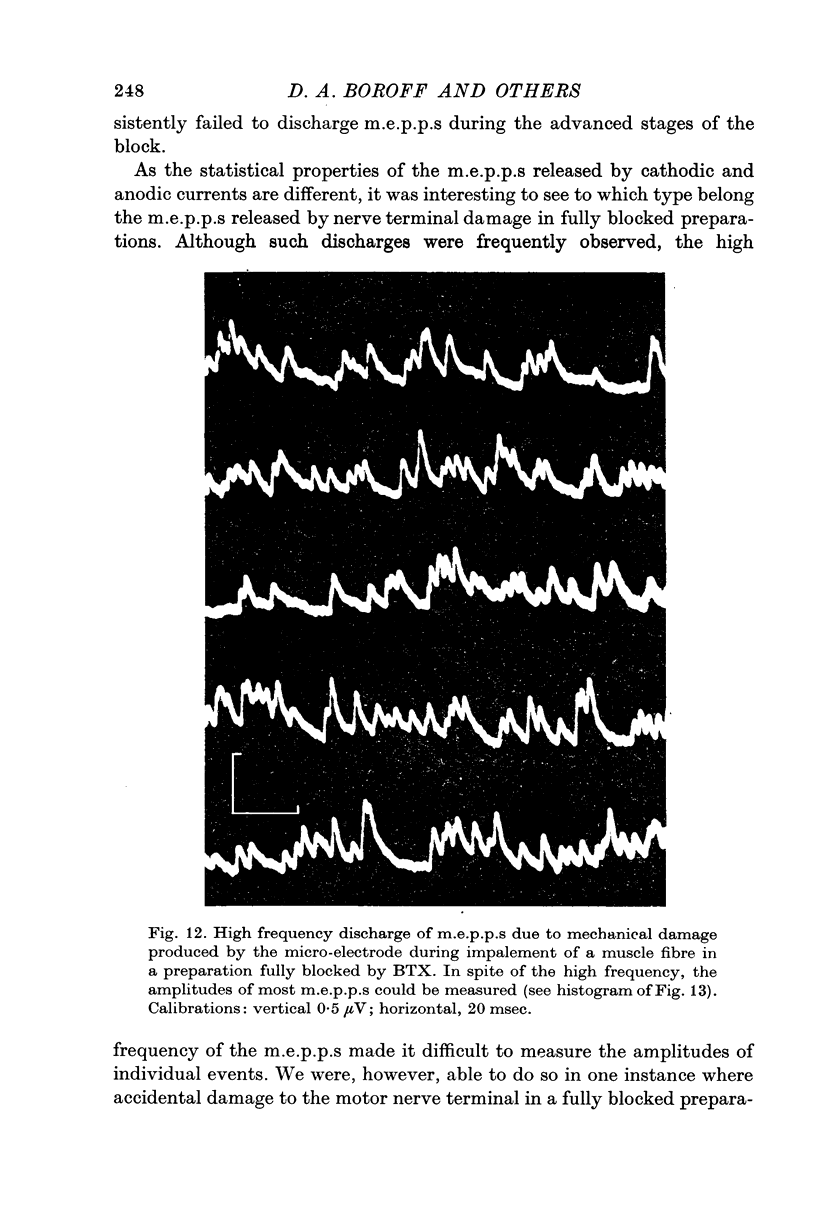
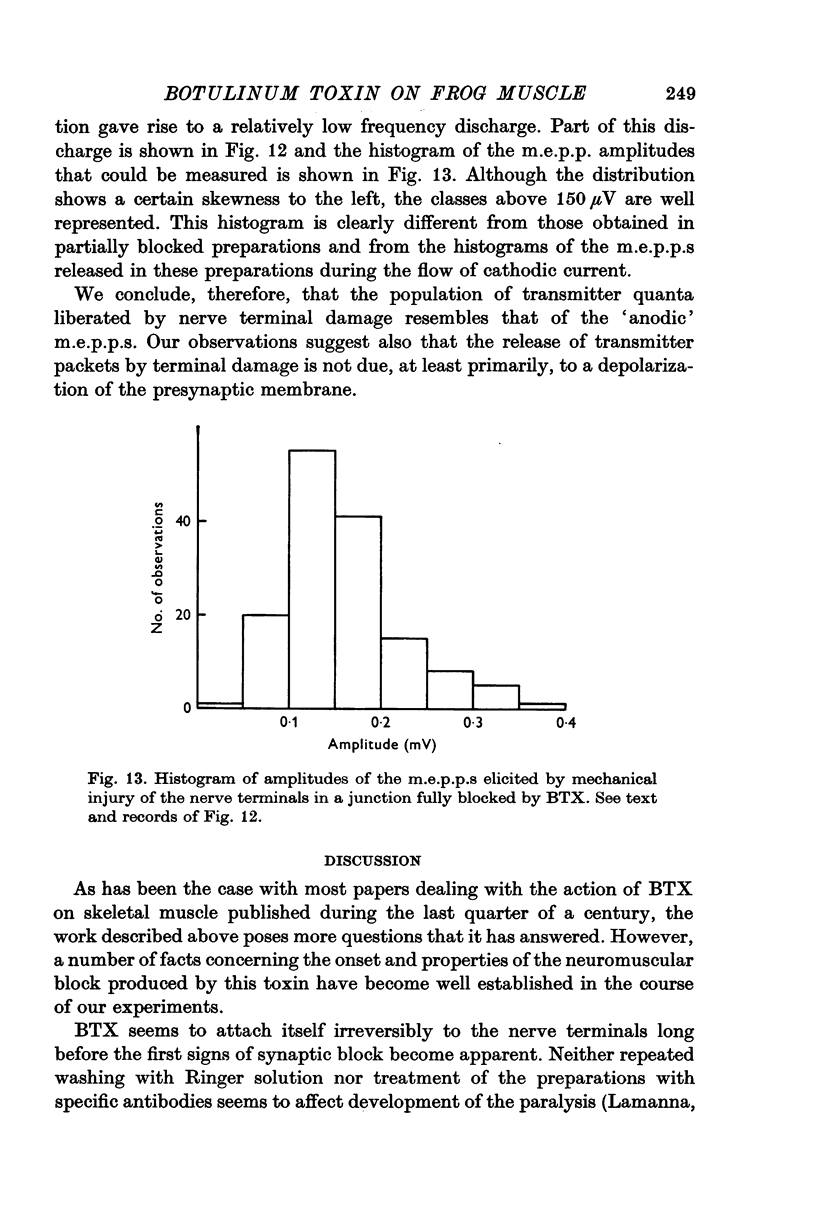
9. It is proposed that the neuromuscular block caused by BTX is due to impairment of a process by which vesicles become charged with transmitter before release.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N. The peripheral action of Cl. botulinum toxin. J Physiol. 1949 Mar 15;108(2):127–141. [PMC free article] [PubMed] [Google Scholar]
- BROOKS V. B. An intracellular study of the action of repetitive nerve volleys and of botulinum toxin on miniature end-plate potentials. J Physiol. 1956 Nov 28;134(2):264–277. doi: 10.1113/jphysiol.1956.sp005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKS V. B. The action of botulinum toxin on motor-nerve filaments. J Physiol. 1954 Mar 29;123(3):501–515. doi: 10.1113/jphysiol.1954.sp005067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGEN A. S. V., DICKENS F., ZATMAN L. J. The action of botulinum toxin on the neuro-muscular junction. J Physiol. 1949 Aug;109(1-2):10–24. doi: 10.1113/jphysiol.1949.sp004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroff D. A., Dasgupta B. R., Fleck U. S. Homogeneity and molecular weight of toxin of Clostridium botulinum type B. J Bacteriol. 1968 May;95(5):1738–1744. doi: 10.1128/jb.95.5.1738-1744.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroff D. A., Fleck U. Statistical analysis of a rapid in vivo method for the titration of the toxin of Clostridium botulinum. J Bacteriol. 1966 Nov;92(5):1580–1581. doi: 10.1128/jb.92.5.1580-1581.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., SANCHEZ V. The electrical activity of the amphibian lymph heart. J Cell Comp Physiol. 1961 Feb;57:29–45. doi: 10.1002/jcp.1030570106. [DOI] [PubMed] [Google Scholar]
- DUFF J. T., WRIGHT G. G., KLERER J., MOORE D. E., BIBLER R. H. Studies on immunity to toxins of Clostridium botulinum. I. A simplified procedure for isolation of type A toxin. J Bacteriol. 1957 Jan;73(1):42–47. doi: 10.1128/jb.73.1.42-47.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B. R., Boroff D. A., Rothstein E. Chromatographic fractionation of the crystalline toxin of Clostridium botulinum type A. Biochem Biophys Res Commun. 1966 Mar 22;22(6):750–756. doi: 10.1016/0006-291x(66)90212-9. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Tonge D. A. The effects of tetanus toxin on neuromuscular transmission and on the morphology of motor end-plates in slow and fast skeletal muscle of the mouse. J Physiol. 1973 Jan;228(1):157–172. doi: 10.1113/jphysiol.1973.sp010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES R., WHALER B. C. Influence of nerve-ending activity and of drugs on the rate of paralysis of rat diaphragm preparations by Cl. botulinum type A toxin. J Physiol. 1962 Feb;160:221–233. doi: 10.1113/jphysiol.1962.sp006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. J., Miledi R. The effect of type D botulinum toxin on frog neuromuscular junctions. J Physiol. 1971 Sep;217(2):497–515. doi: 10.1113/jphysiol.1971.sp009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUPFER C. Selective block of synaptic transmission in ciliary ganglion by type A botulinus toxin in rabbits. Proc Soc Exp Biol Med. 1958 Nov;99(2):474–476. doi: 10.3181/00379727-99-24388. [DOI] [PubMed] [Google Scholar]
- LAMANNA C. The most poisonous poison. Science. 1959 Sep 25;130(3378):763–772. doi: 10.1126/science.130.3378.763. [DOI] [PubMed] [Google Scholar]
- Lamanna C., McElroy O. E., Eklund H. W. The Purification and Crystallization of Clostridium botulinum Type A Toxin. Science. 1946 May 17;103(2681):613–614. doi: 10.1126/science.103.2681.613. [DOI] [PubMed] [Google Scholar]
- STOVER J. H., Jr, FINGERMAN M., FORESTER R. H. Botulinum toxin and the motor end plate. Proc Soc Exp Biol Med. 1953 Oct;84(1):146–147. doi: 10.3181/00379727-84-20570. [DOI] [PubMed] [Google Scholar]
- Shankland D. L., Rose J. A., Donniger C. The cholinergic nature of the cercal nerve-giant fiber synapse in the sixth abdominal ganglion of the American cockroach, Periplaneta americana (L.). J Neurobiol. 1971;2(3):247–262. doi: 10.1002/neu.480020306. [DOI] [PubMed] [Google Scholar]
- Simpson L. L., Tapp J. T. Actions of calcium and magnesium on the rate of onset of botulinum toxin paralysis of the rat diaphragm. Int J Neuropharmacol. 1967 Nov;6(6):485–492. doi: 10.1016/0028-3908(67)90048-2. [DOI] [PubMed] [Google Scholar]
- Simpson L. L. The interaction between divalent cations and botulinum toxin type A in the paralysis of the rat phrenic nerve-hemidiaphragm preparation. Neuropharmacology. 1973 Feb;12(2):165–176. doi: 10.1016/0028-3908(73)90085-3. [DOI] [PubMed] [Google Scholar]
- Spitzer N. Miniature end-plate potentials at mammalian neuromuscular junctions poisoned by botulinum toxin. Nat New Biol. 1972 May 3;237(70):26–27. doi: 10.1038/newbio237026a0. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. Supersensitivity of skeletal muscle produced by botulinum toxin. J Physiol. 1960 Jun;151:598–607. doi: 10.1113/jphysiol.1960.sp006463. [DOI] [PMC free article] [PubMed] [Google Scholar]



