Abstract
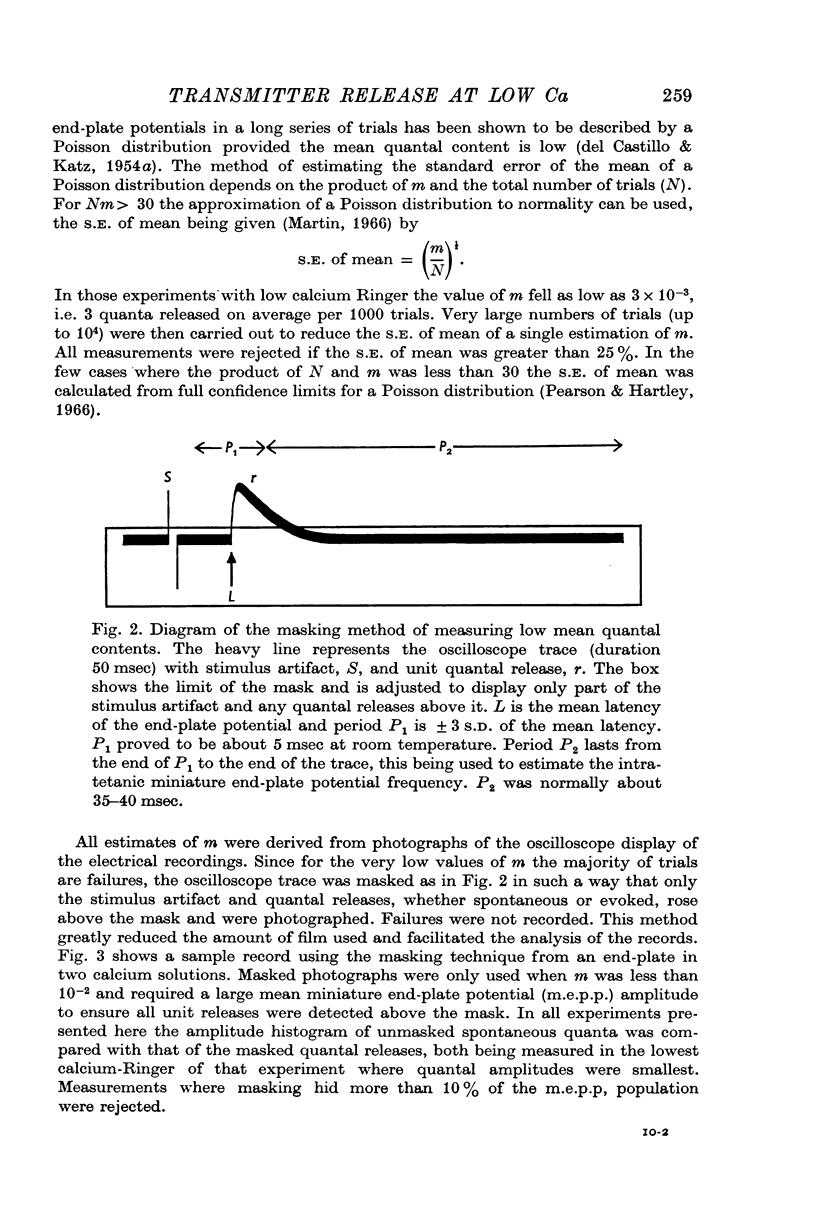
1. The mean quantal content of the frog end-plate potential was examined under conditions that reduced evoked transmitter release to very low values.
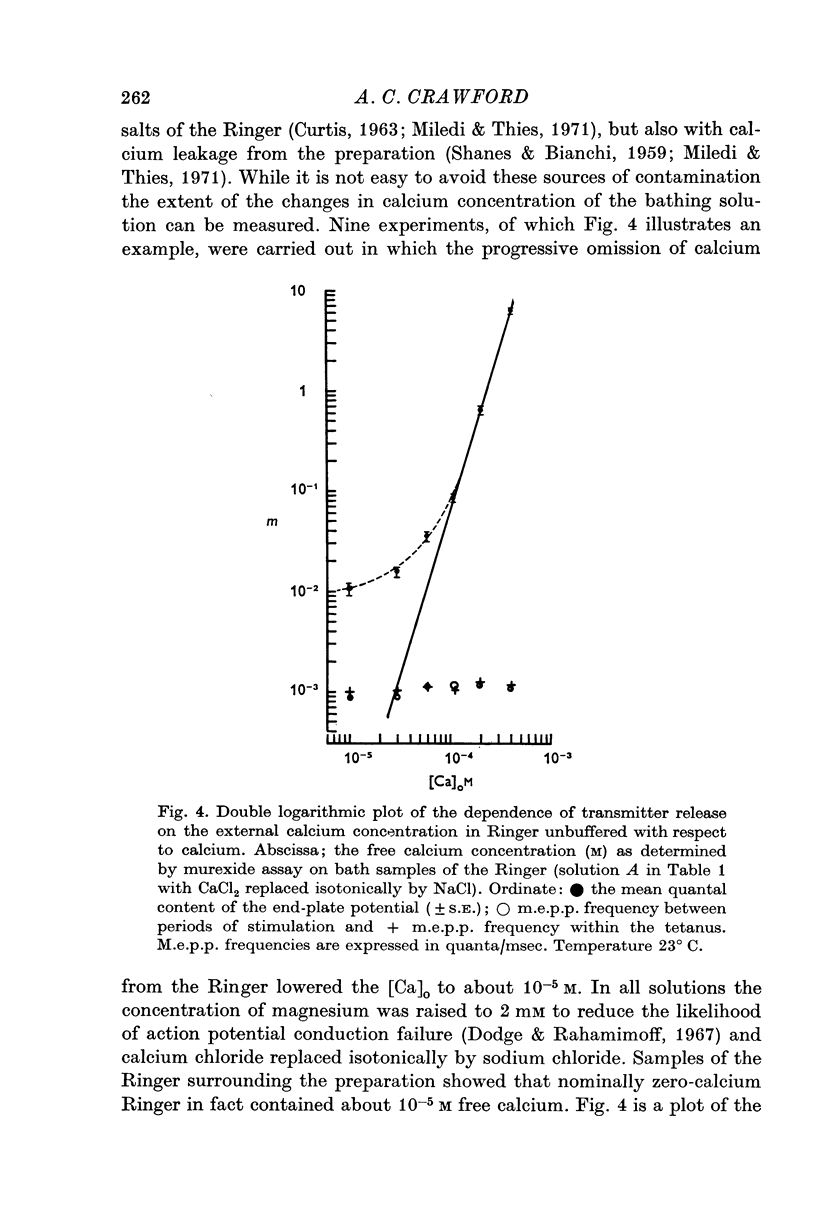
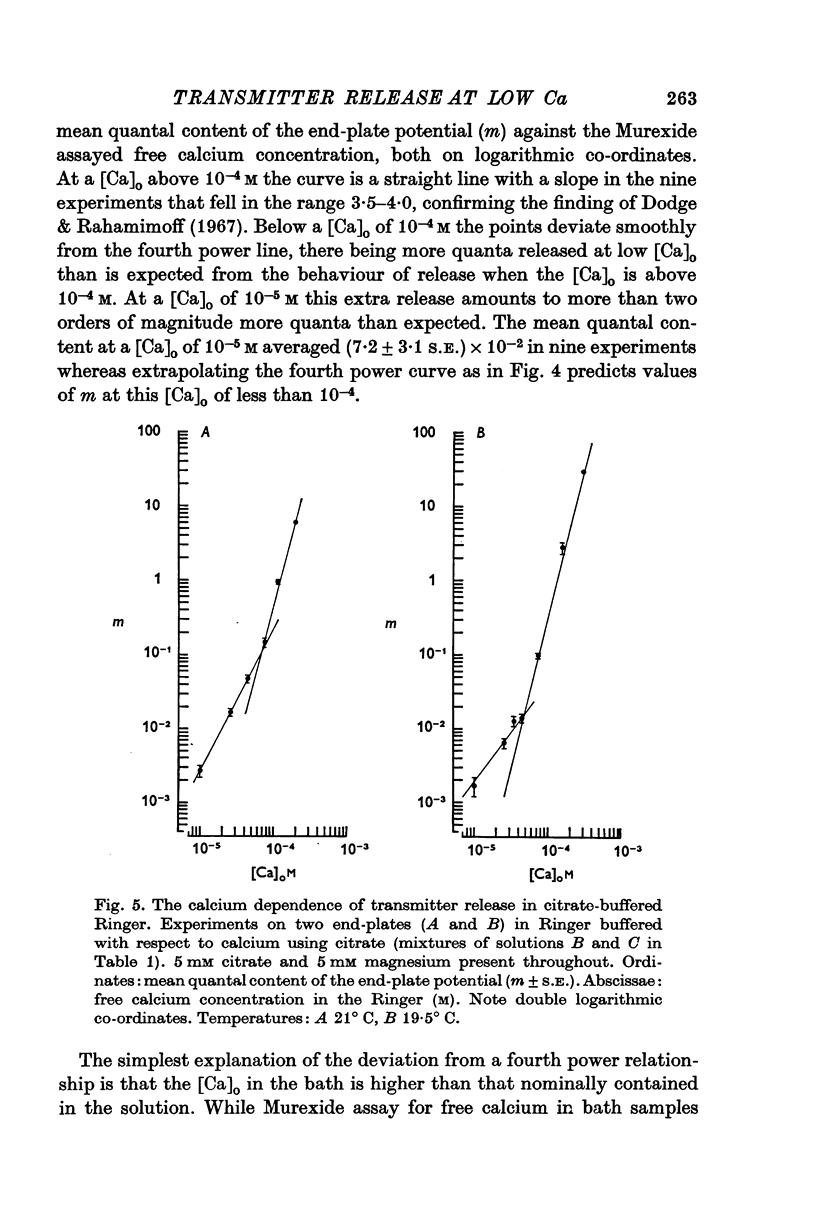
2. When the calcium concentration of the Ringer is reduced below 10-4 M a deviation occurs from the fourth power dependence of the mean quantal content (m) on the calcium concentration such that more quanta are released than is expected from the behaviour of m at higher calcium concentrations.
3. At 10-5 M this extra quantal release is more than two orders of magnitude greater than that predicted by the fourth power relationship.
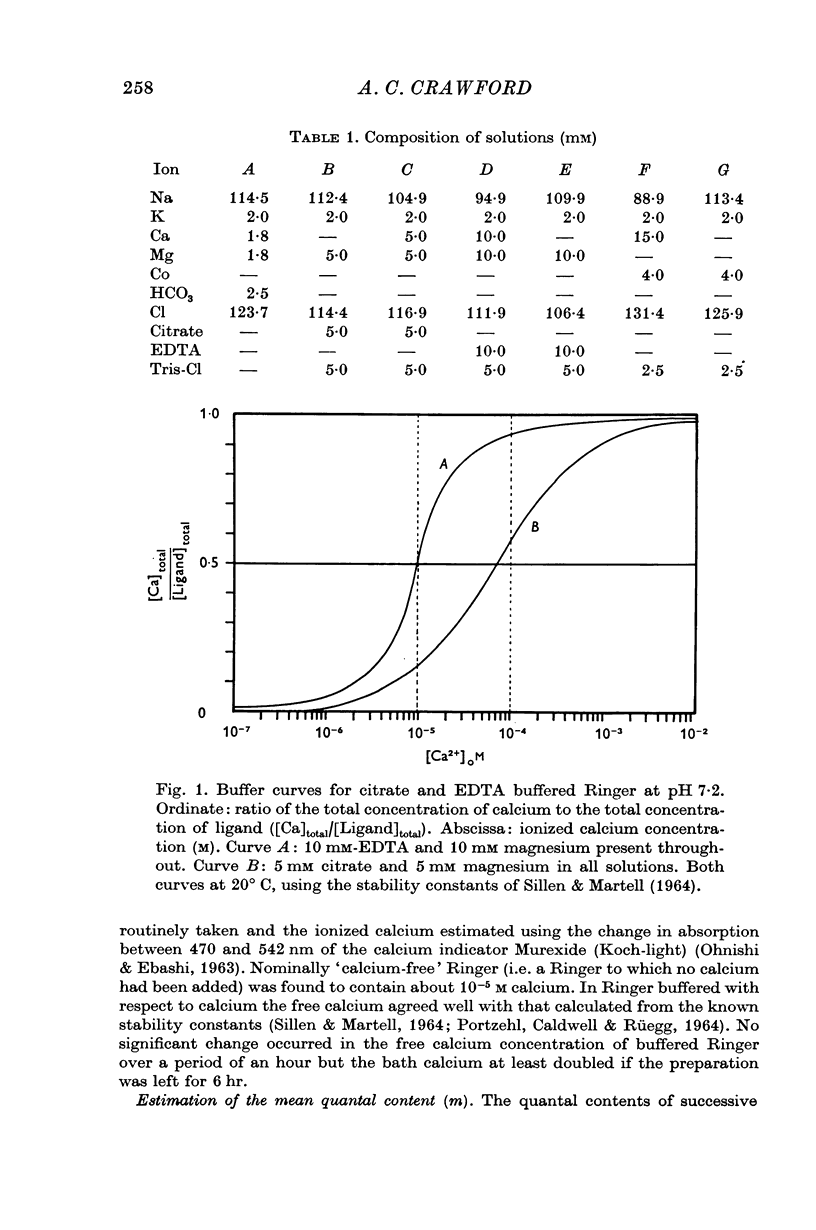
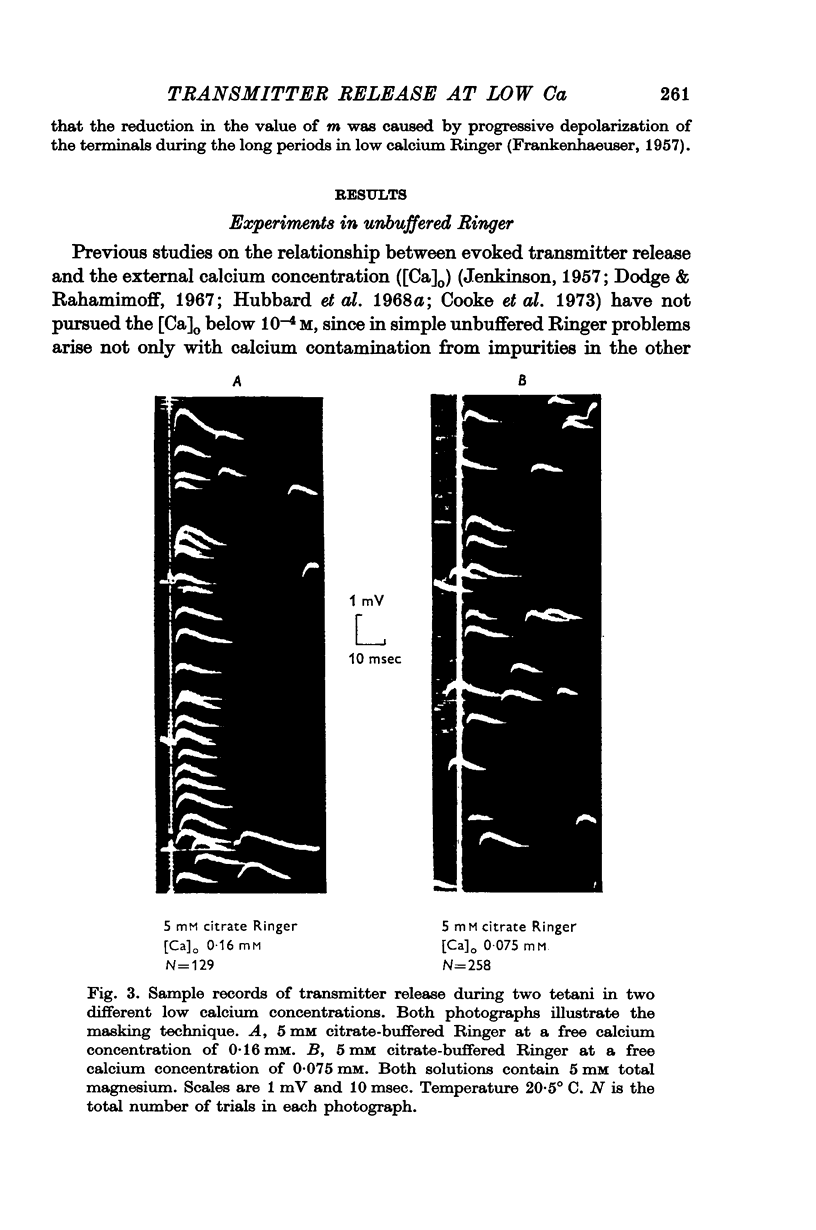
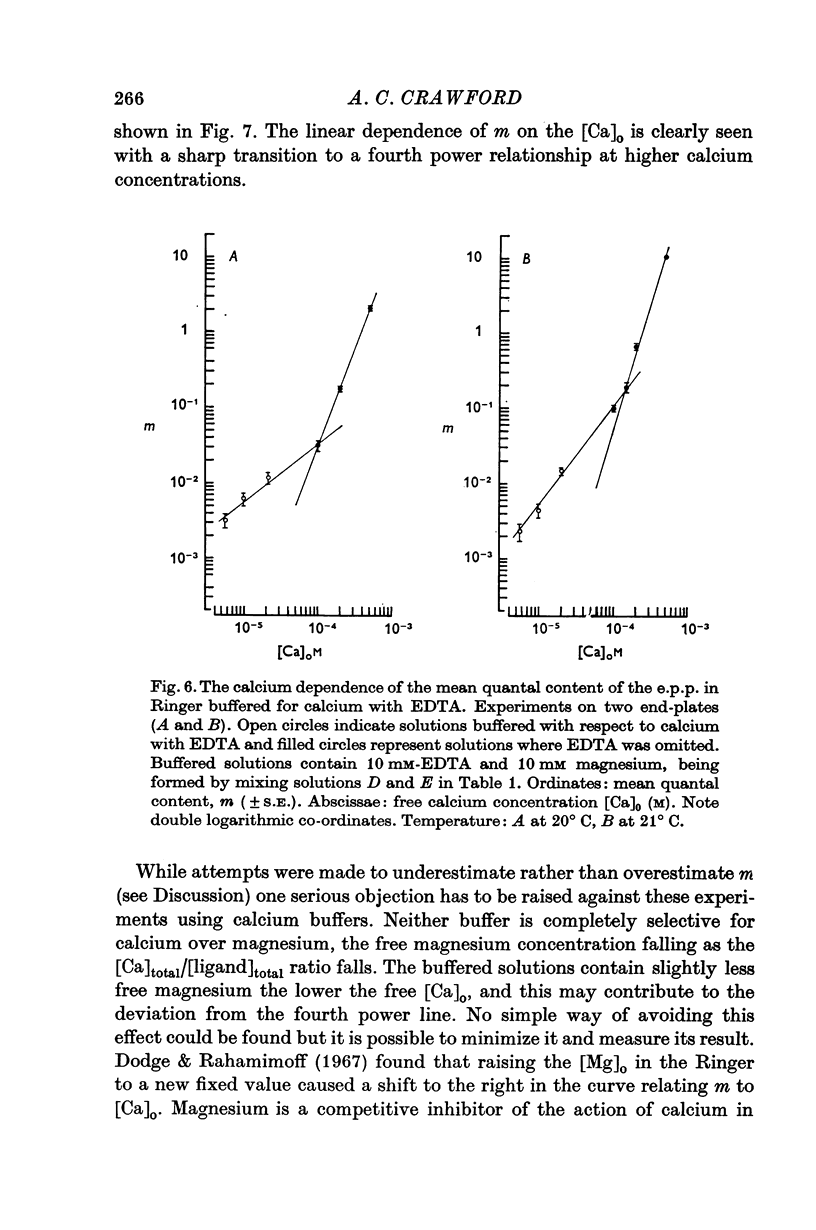
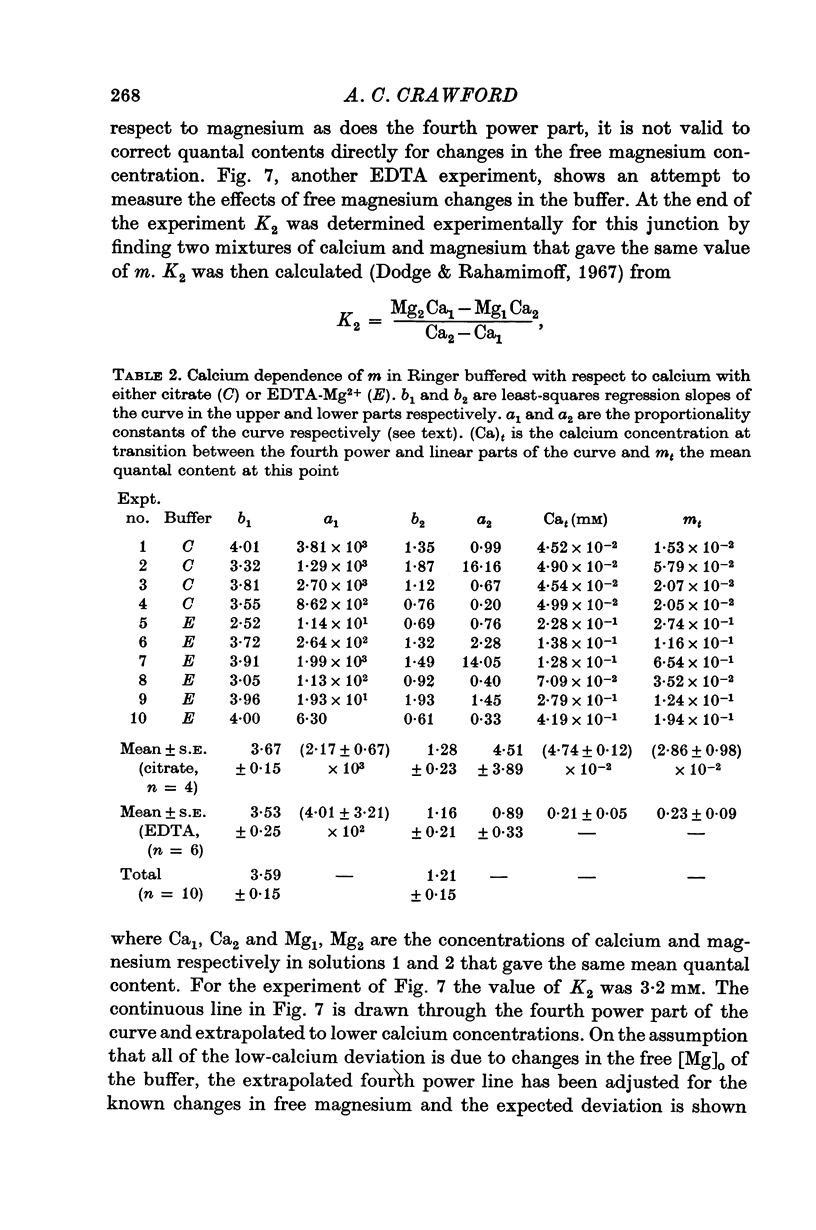
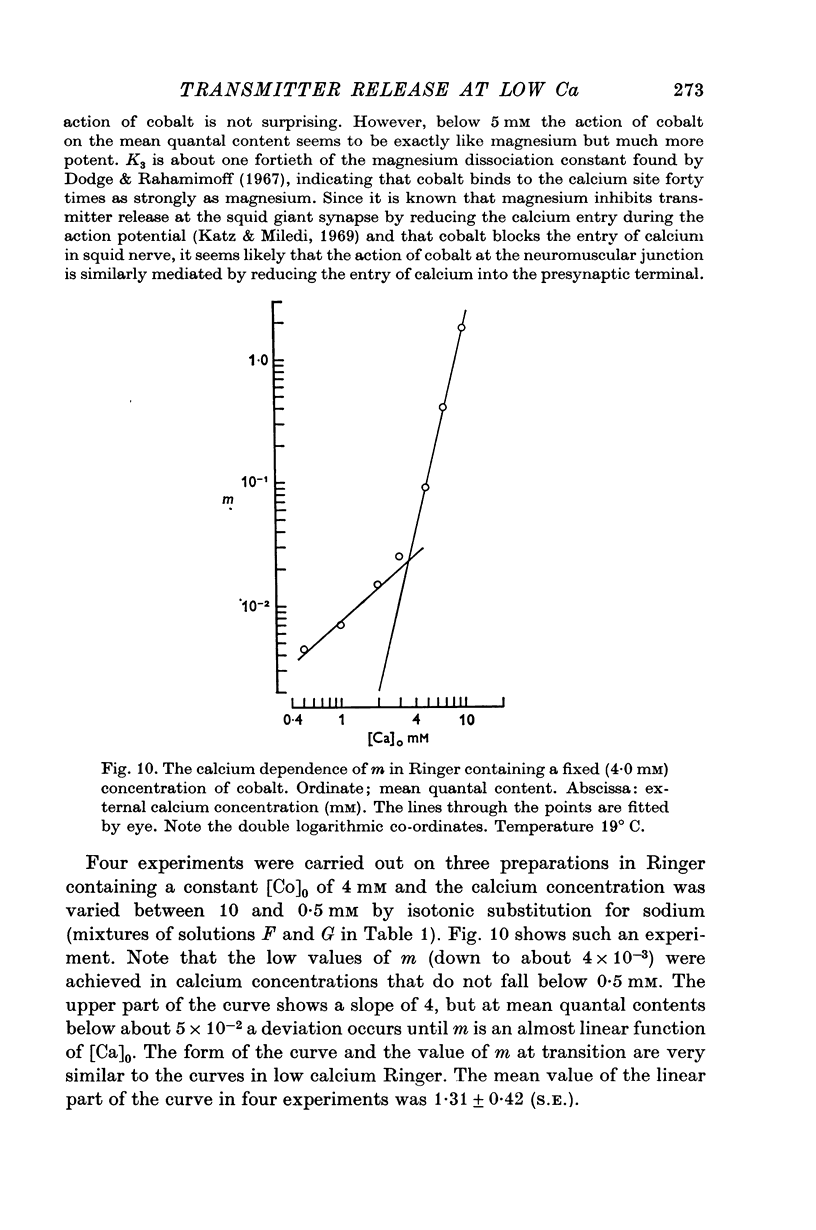
4. The calcium dependence of very low values of m was studied in low calcium Ringer in which the calcium was buffered by either citrate or EDTA. It was found that in the fourth power dependence of m on the external calcium concentration changes rather suddenly to an approximately linear dependence.
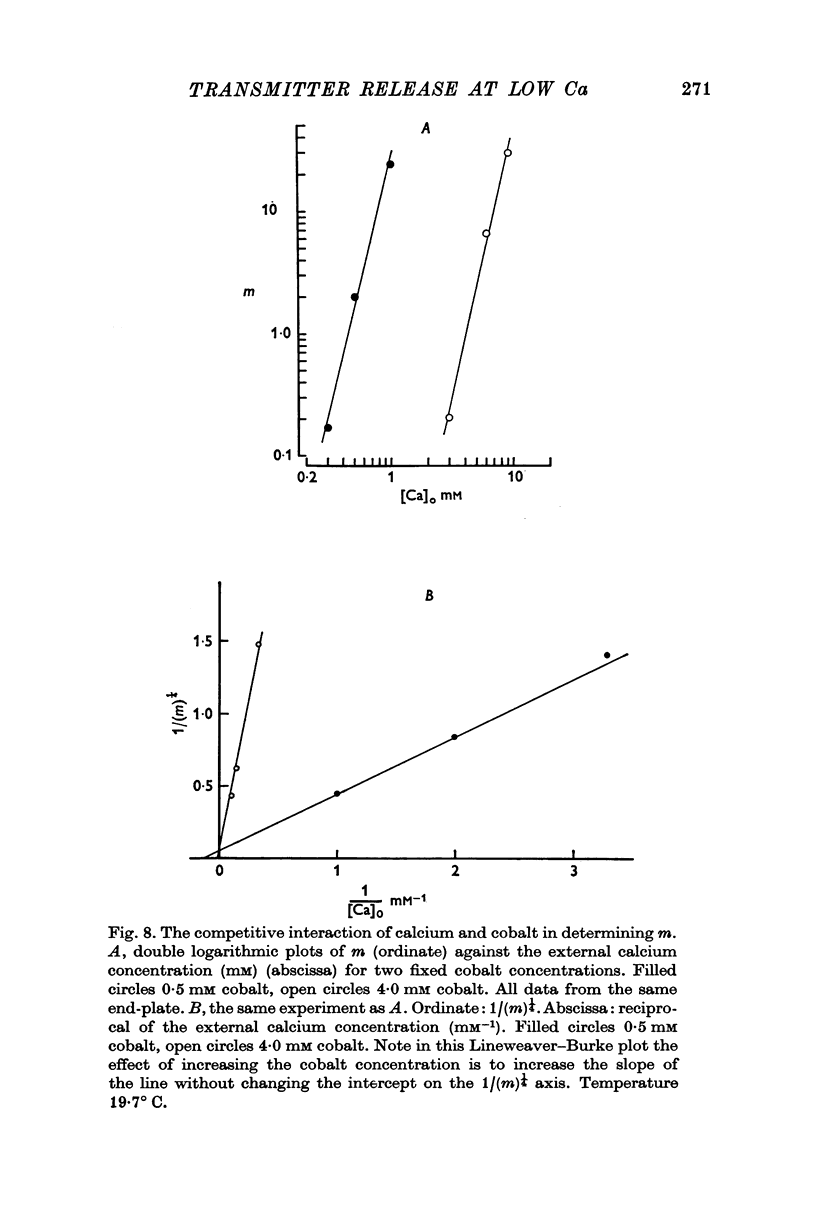
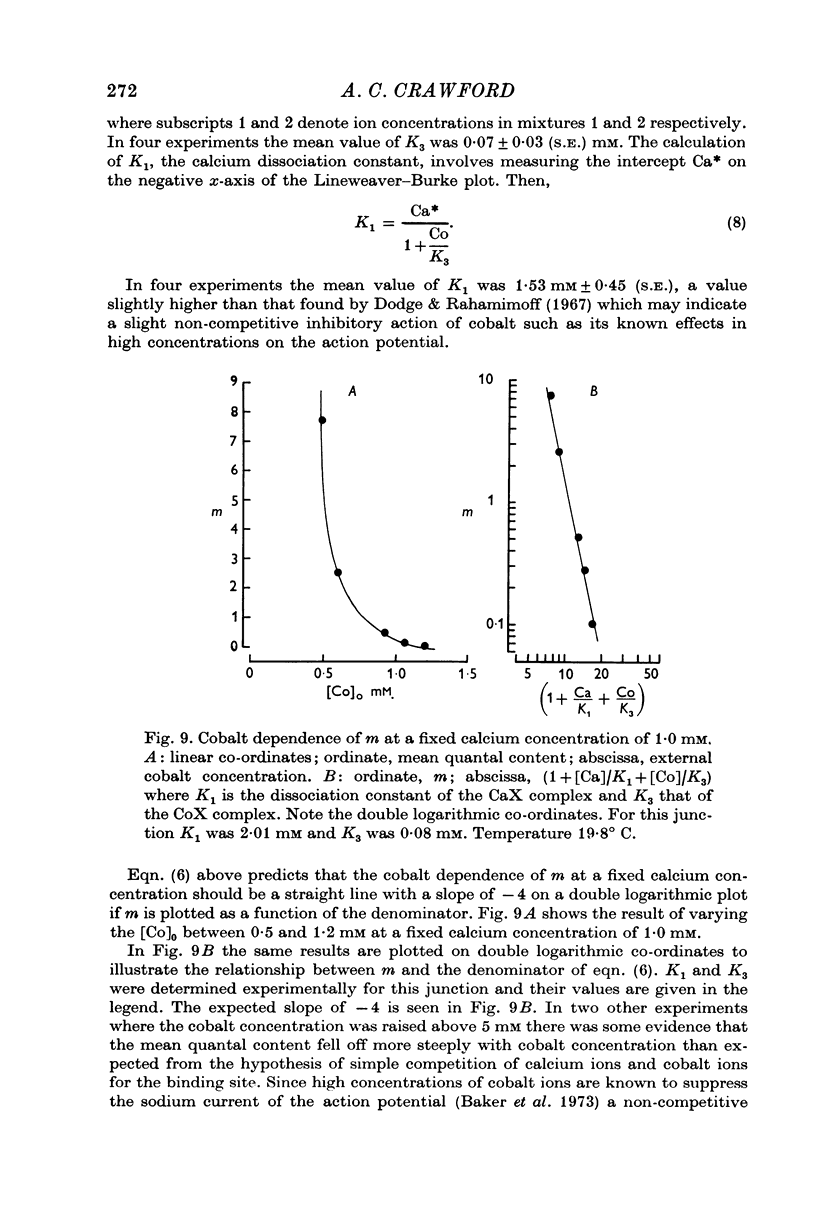
5. The inclusion of small concentrations of cobalt in the Ringer was found to reduce m to very low values even in the presence of millimolar concentrations of calcium.
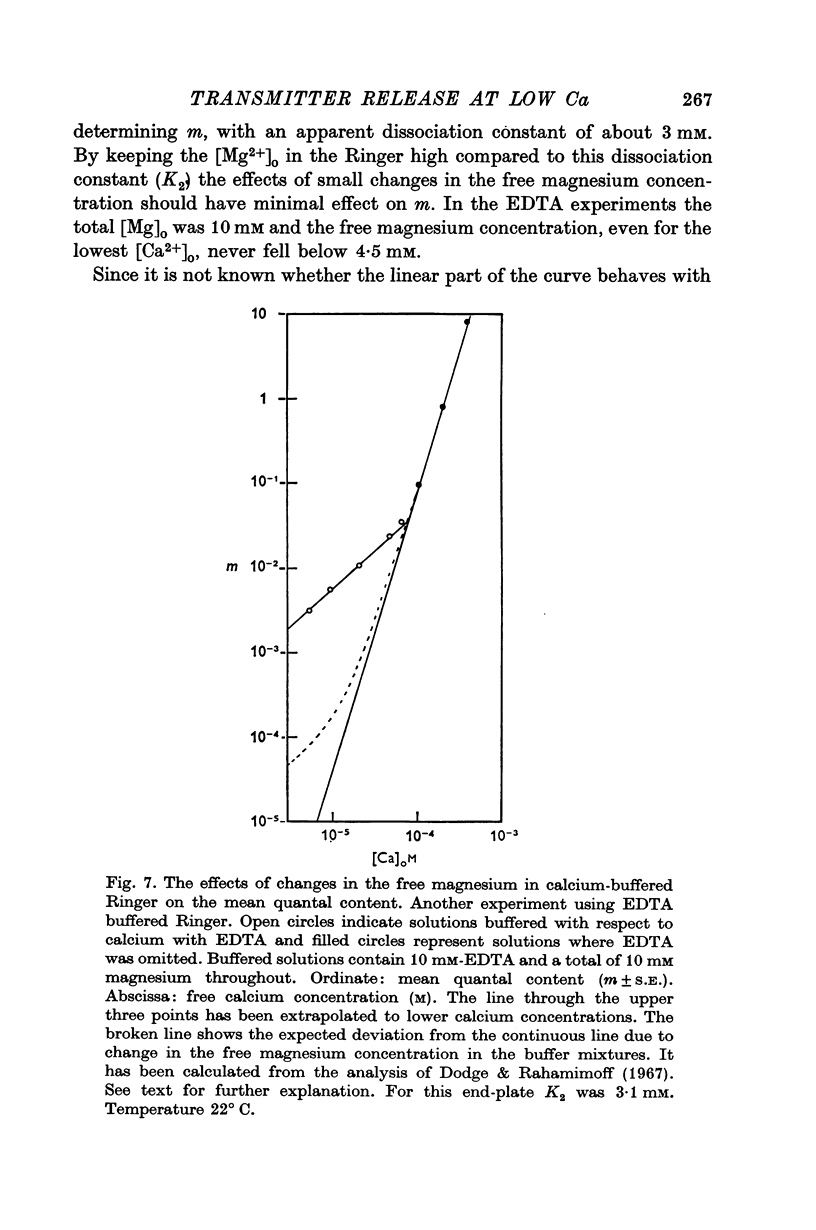
6. The fourth power dependence of m on the external calcium concentration at high values of m was unaffected by the presence of cobalt. At low quantal contents the transition to a linear dependence on external calcium was again seen, but was shifted to calcium concentrations that did not require buffering.
7. The fourth power to linear transition is discussed in terms of its relevance to the relationship between m and the m.e.p.p. frequency.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKS V. B. An intracellular study of the action of repetitive nerve volleys and of botulinum toxin on miniature end-plate potentials. J Physiol. 1956 Nov 28;134(2):264–277. doi: 10.1113/jphysiol.1956.sp005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- CURTIS B. A. Some effects of Ca-free choline-Ringer solution on frog skeletal muscle. J Physiol. 1963 Apr;166:75–86. doi: 10.1113/jphysiol.1963.sp007091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Okamoto K., Quastel D. M. The role of calcium in depolarization-secretion coupling at the motor nerve terminal. J Physiol. 1973 Jan;228(2):459–497. doi: 10.1113/jphysiol.1973.sp010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C. Evoked transmitter release at the frog neuromuscular junction in very low calcium solutions. J Physiol. 1973 May;231(1):47P–48P. [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. A method for altering the intracellular calcium concentration. J Physiol. 1971;217 (Suppl):20P–21P. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. The effect of calcium on the myelinated nerve fibre. J Physiol. 1957 Jul 11;137(2):245–260. doi: 10.1113/jphysiol.1957.sp005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. REPETITIVE STIMULATION AT THE MAMMALIAN NEUROMUSCULAR JUNCTION, AND THE MOBILIZATION OF TRANSMITTER. J Physiol. 1963 Dec;169:641–662. doi: 10.1113/jphysiol.1963.sp007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The timing of calcium action during neuromuscular transmission. J Physiol. 1967 Apr;189(3):535–544. doi: 10.1113/jphysiol.1967.sp008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H., Van der Kloot W. Action of Co and Ni at the frog neuromuscular junction. Nat New Biol. 1973 Sep 12;245(141):52–53. doi: 10.1038/newbio245052a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- OHNISHI T., EBASHI S. SPECTROPHOTOMETRICAL MEASUREMENT OF INSTANTANEOUS CALCIUM BINDING OF THE RELAXING FACTOR OF MUSCLE. J Biochem. 1963 Dec;54:506–511. doi: 10.1093/oxfordjournals.jbchem.a127823. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R., Yaari Y. Delayed release of transmitter at the frog neuromuscular junction. J Physiol. 1973 Jan;228(1):241–257. doi: 10.1113/jphysiol.1973.sp010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANES A. M., BIANCHI C. P. The distribution and kinetics of release of radiocalcium in tendon and skeletal muscle. J Gen Physiol. 1959 May 20;42(5):1123–1137. doi: 10.1085/jgp.42.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich D. Ionic mechanism of post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1971 Jan;212(2):431–446. doi: 10.1113/jphysiol.1971.sp009333. [DOI] [PMC free article] [PubMed] [Google Scholar]


