Abstract
Leishmania donovani promastigote membrane antigens (LAg) encapsulated in positively charged liposomes have been found to induce very significant levels of protection against experimental visceral leishmaniasis. The protectively immunized animals exhibited profound delayed-type hypersensitivity and antibody responses. The extent of protection induced by the same antigens, however, varied depending on the charge of the vesicles, with maximum induction by positively charged liposomes, followed by neutral liposomes and last negatively charged liposomes. Characterization of LAg and LAg entrapped in liposomes of different charges by Western blot analysis revealed the immunodominance of gp63 in all three vaccine preparations. The strong reactivity of antigens in a restricted antigen profile that included, in addition to gp63, 72-, 52-, 48-, 45-, 39-, and 20-kDa components in neutral and positively charged liposomes contrasted with the reactivity of a greater number of LAg components in negatively charged liposomes. Resistance to visceral leishmaniasis appears to depend on the immunity induced by gp63 and a few select antigens in association with the right liposomes. A striking similarity between the immunogenic profile of partially purified soluble antigens and that of LAg in neutral and positively charged liposomes suggests the potentiality of these antigens in future vaccine studies of L. donovani.
Leishmania species are dimorphic obligate intracellular protozoa that cause a spectrum of diseases ranging from cutaneous and mucocutaneous to visceral leishmaniasis affecting millions of people worldwide. The cutaneous form of leishmaniasis is generally a mild disease in humans, giving rise to self-curing localized lesions, and has been extensively studied in human and mouse model systems (9, 22, 41). This disease can be caused by Leishmania major, Leishmania tropica, Leishmania aethiopica, and members of the Leishmania braziliensis and Leishmania mexicana complexes. Visceral leishmaniasis is caused by protozoa belonging to the Leishmania donovani complex, including L. donovani and Leishmania chagasi. The parasites multiply in the macrophages of the liver, spleen, bone marrow, and lymph nodes, and the outcome is a potentially fatal disease, a threat that is exacerbated by the present pandemic of AIDS (38, 50).
Research on the immunopathology of parasitic infections has led to the understanding of several aspects of regulation in the immune system. It is a consensus now that the immune cellular response plays a central role in cutaneous leishmaniasis. A differential activation of a Th1 CD4+ lymphocyte subset in resistant strains of inbred mice and of the Th2 subset in susceptible strains is well documented for infections with L. major (20, 21, 45, 46). In this model, the production of Th1-type cytokines (interleukin-2 [IL-2] and gamma interferon) and that of Th2-type cytokines (IL-4, IL-5, and IL-10) are mutually exclusive. In contrast, there have been reports of the existence of both Th1- and Th2-type responses, together with antibodies, in murine and human visceral leishmaniasis, with a dominance of Th1 with protective immunity (7, 14, 28-30, 40)
The development of vaccines is the essential aim of studies on leishmaniasis. Extensive investigations in this field include human vaccine trials with killed promastigotes and immunization of mice with attenuated, killed, and crude parasite fractions, as well as purified and recombinant antigens and their DNA (11, 17, 31, 34, 53-55, 59). While there is still no effective form of immunoprophylaxis against this disease, the impressive recent advances in this area may soon result in the development of a safe and effective vaccine. The protocols used successfully with L. major and L. mexicana infection, however, have been reported to be unsuccessful against murine visceral leishmaniasis (24, 29, 39). The problems in achieving immunization may be why there are few studies of vaccines against L. donovani (23, 44). Recently, we showed that membrane antigens of L. donovani promastigotes (LAg), when entrapped in liposomes (LAg in liposomes), could induce very significant levels of protection against infection in both hamsters and BALB/c mice (3). The extent of protection induced by these antigens in mice, however, varied depending on the charge of liposomes introduced as adjuvants such that positively charged liposomes induced maximum protection (87%) (3), followed by neutral (73%) (6) and negatively charged liposomes (59.4%) (5). Identification of the key components of the complex antigen mixture (LAg) incorporated in the liposome preparations and determining their involvement in conferring protective immunity are essential for the design of future subunit-based vaccines against kala-azar. For this purpose, immunoblots of LAg and LAg in liposomes probed with infected sera and with sera from immunized BALB/c mice before and after infection were analyzed. Results show that, in addition to gp63, the major promastigote surface glycoprotein, 72-, 52-, 48-, 45-, 39-, and 20-kDa components of LAg are potent antigenic targets for the development of vaccine against L. donovani.
MATERIALS AND METHODS
Parasites and culture conditions.
Promastigotes of L. donovani, strain AG83, were grown at 22°C in medium 199 (pH 7.4) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 100 U of penicillin G sodium, and 100 μg of streptomycin sulfate per ml and subcultured in the same medium at an average density of 2 × 106 cells/ml (6).
Preparation of Leishmania antigens.
LAg were prepared from L. donovani promastigotes as described earlier (3). Briefly, stationary-phase promastigotes, harvested after the third or fourth passage, were washed four times in cold 20 mM phosphate-buffered saline (PBS), pH 7.2, and resuspended at a concentration of 1.0 g of cell pellet in 50 ml of cold 5 mM Tris-HCl buffer, pH 7.6. The suspension was vortexed and centrifuged at 2,310 × g for 10 min. The crude ghost membrane pellet thus obtained was resuspended in the same Tris buffer and sonicated in an ultrasonicator. The suspension was centrifuged at 5,190 × g for 30 min, and the supernatant containing the leishmanial antigens was harvested and stored at −70°C until use. The amount of protein obtained from a 1.0-g cell pellet, as assayed by the method of Lowry et al. (35), was approximately 16 mg.
Soluble leishmanial antigens (SLA) were also extracted from L. donovani promastigote membranes. The washed parasites were suspended in cold 5 mM Tris-HCl buffer (pH 7.6) containing 5 μg of leupeptin/ml, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 1 mM iodoacetamide (lysis buffer) and were vortexed and centrifuged as described for LAg. The membrane pellet was resuspended in 10 ml of lysis buffer and sonicated as described above. The suspension thus obtained was solubilized with 1% (wt/vol) octyl-β-d-glucopyranoside in the lysis buffer, with overnight incubation at 4°C, and was finally ultracentrifuged for 1 h at 100,000 × g. The supernatant containing SLA was then dialyzed against 1 mM Tris-HCl buffer (pH 7.6) and stored at −70°C until use. The amount of protein obtained from a 1.0-g cell pellet was approximately 2 mg.
The 63-kDa membrane glycoprotein, gp63, was purified by monoclonal affinity binding from Nonidet P-40 extracts of Leishmania amazonensis promastigotes and was a kind gift from K. P. Chang.
Electroelution of gp63 from SDS-PAGE gels.
The SLA from L. donovani promastigotes was subjected to sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE), and the protein with a molecular mass of 63 kDa (gp63) was localized in gels, stained with Coomassie blue, and eluted by electrophoresis in running buffer (0.025 M Tris, 0.192 M glycine, 1% SDS) at 10 mA for 5 h. After elution, the protein was dialyzed, lyophilized, resuspended in PBS, and filtered through 0.22-μm-pore-size membranes for further utilization in lymphocyte cultures (8). The protein was quantified by Lowry's method (35).
Entrapment of leishmanial antigens in liposomes.
Neutral and positively charged liposomes were prepared with egg lecithin and cholesterol (7:2 molar ratio) or with egg lecithin, cholesterol, and stearylamine (7:2:2 molar ratio), respectively, as reported earlier (3, 6). Negatively charged liposomes were prepared with egg lecithin, cholesterol, and phosphatidic acid at a molar ratio of 7:2:2 (5). For encapsulation of the antigens in the vesicles the lipid film was dispersed in PBS containing 2 mg of LAg/ml and sonicated for 30 s in an ultrasonicator. Liposomes with entrapped antigen were separated from the excess free antigen by three successive washing in PBS with ultracentrifugation (105,000 × g, 60 min, 4°C). The amounts of LAg associated per milligram of egg lecithin were 35, 25, and 15 μg for positively charged, neutral, and negatively charged liposomes, respectively. Similarly, electroeluted gp63 was entrapped in the positively charged vesicles. The lipid film was dispersed in PBS containing 1 mg of gp63/ml, and 40 μg of protein was associated with 1 mg of egg lecithin.
Immunization and challenge infection.
BALB/c mice were immunized by three intraperitoneal injections of 20 μg of LAg or 10 μg of gp63, purified by electroelution and free in PBS or entrapped in liposomes, at 2-week intervals. Animals receiving only PBS or empty liposomes served as controls. Ten days after the last immunization, the immunized and control animals were challenged intravenously with 2.5 × 107 freshly transformed L. donovani promastigotes (6). Serum samples collected from each mouse before challenge infection and at 4 months postinfection were stored frozen at −20°C.
DTH.
Delayed-type hypersensitivity (DTH), was determined as an index of cell-mediated immunity. The response was evaluated by measuring the difference between the footpad swelling at 24 h following intradermal inoculation of the test footpad with 50 μl of electroeluted gp63 (800 μg/ml) and the swelling of the control (PBS-injected) footpad (3).
Cell proliferation assay.
The lymphocyte cultures were obtained from spleens of immunized BALB/c mice. The cells were isolated and counted, and, after evaluation of cell viability by trypan blue exclusion, 2 × 105 cells per well were cultured in RPMI 1640 containing 20 mM NaHCO3, 10 mM HEPES, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM l-glutamine, and 10% fetal calf serum (complete medium [CM]), with 50 μM β-mercaptoethanol added.
The cells were cultured in triplicate in a final volume of 200 μl/well with eluted antigen (0.7 μg/well) at optimum concentrations. The cultures were incubated for 96 h at 37°C in a humidified chamber containing 5% CO2. Cells were pulsed with 1 μCi of [3H]thymidine ([3H]TdR; 83 Ci/mmol; Amersham International, Amersham, England) per well 18 h before they were harvested on glass fiber paper. ([3H]TdR uptake was measured in a β-scintillation counter (LS 5000TD; Beckman Instruments, Fullerton, Calif.).
In vitro growth of L. donovani in macrophages.
Macrophages were collected by peritoneal lavage from immunized BALB/c mice 10 days after the last injection and cultured in CM. A total of >90% of the cell preparation was identified as macrophages by microscopic observation, and the macrophages were routinely found to be >98% viable by trypan blue exclusion.
Promastigotes were used to infect cultures of adherent macrophages on glass coverslips (18 mm2; 106 macrophages/coverslip) in 0.5 ml of CM at a ratio of 10 parasites/macrophage. After 3 h of incubation, the unphagocytosed parasites were removed by a washing with PBS. Infected macrophages were further incubated in CM at 37°C for 72 h. Cells were fixed in methanol and stained with Giemsa for determination of intracellular parasite numbers.
ELISA.
Mice immunized with gp63 or gp63-positively charged liposome and control BALB/c mice were bled 10 days after the last immunization by snipping the tail vein, and the sera were collected. Serum IgG levels were determined by enzyme-linked immunosorbent assay (ELISA) (3). Briefly, 96-well microtiter plates were coated overnight at 4°C with gp63 that had been purified by electroelution (15 μg/ml). After being blocked with bovine serum albumin and incubated overnight with serum samples diluted at 1:1,000, the plates were developed by using a 1:5,000 dilution of peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) antibody (Jackson Immunochemical Research Laboratories).
Mice sera were further assayed for gp63-specific IgG subclass antibodies with a mouse antibody isotyping kit (Sigma Immunochemicals, St. Louis, Mo.). gp63-coated wells, incubated with mice sera as described above, were reacted overnight at 4°C with a 1:2,500 dilution of goat anti-mouse isotype-specific antibodies, followed with a 1:5,000 dilution of peroxidase-conjugated rabbit anti-goat IgG (Jackson Immunochemical Research Laboratories). The plates were developed for color reaction and analyzed by measuring the optical density at 450 nm.
Evaluation of infection.
At the times mentioned in Results, the course of infection was monitored by the microscopic examination of Giemsa-stained impression smears of liver and spleen. The parasite load was expressed as Leishman-Donovan units and was calculated by the following formula: number of amastigotes per 1,000 cell nuclei × organ weight (in milligrams) (57).
SDS-PAGE and Western blot analysis.
Components of the L. donovani promastigote membrane were subjected to SDS-PAGE by the method of Laemmli (32). Gels were loaded with proteins (amounts are indicated in the figure legends) of the L. donovani ghost membrane; LAg and SLA free in PBS; LAg entrapped in liposomes of neutral, negative, and positive charge; purified gp63 provided by Chang; and gp63 purified by electroelution. Proteins, separated on 10% polyacrylamide, were silver stained (61).
For Western blot analysis, the resolved proteins were transferred onto nitrocellulose in 25 mM Tris-HCl-192 mM glycine-20% (vol/vol) methanol buffer at 90 V/cm for 90 min (58). Immunoblot assays were performed according to the method described by Rolland-Burger et al. (49) with slight modifications. The nitrocellulose strips were first saturated and then blocked overnight at room temperature in 100 mM Tris-buffered saline (TBS), pH 7.6, containing 0.1% Tween 20 (T-20) and washed once for 15 min with 0.05% T-20 in TBS (washing buffer) with shaking. Incubation of the nitrocellulose strips with sera from mice immunized with LAg in liposomes, before and after infection, was carried out at 1:500 dilution in the washing buffer for 1 h at room temperature, followed by three washes of 20 min each. gp63, SLA, LAg, and LAg-in-liposome blots were probed with rabbit antisera to purified gp63 at 1:200 dilution, kindly provided by K. P. Chang. The blots were then washed and incubated with peroxidase-conjugated anti-mouse or anti-rabbit (Sigma Immunochemicals) IgG, at a 1:500 dilution in the washing buffer for 1 h at room temperature; this was followed by three washes as described above. The last wash was done without T-20. Enzymatic activity was revealed with 15 mg of 3,3′-diaminobenzidine tetrahydrochloride (Sigma Immunochemicals) in 30 ml of TBS containing 15 μl of 30% H2O2. The efficacy of transfer of the leishmanial proteins was regularly checked and confirmed by concurrent gel staining with silver and nitrocellulose membrane staining with 0.1% Ponceau S in 1% acetic acid. The parts of the membrane containing molecular mass standards were marked with ink after the staining with Ponceau S. The specificity of the immune response was assessed by immunoblot analysis employing pooled preimmune sera and only liposome-immunized sera at 1:50 dilution and infected sera at 1:500 dilution in washing buffer.
Statistical analysis.
All data comparisons were tested for significance by using Student's t test; P values of <0.05 were considered significant.
RESULTS
Electrophoretic analysis of the leishmanial antigens and antigens in liposomes.
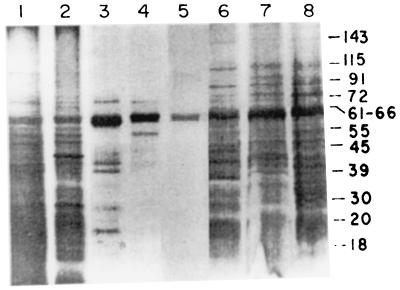
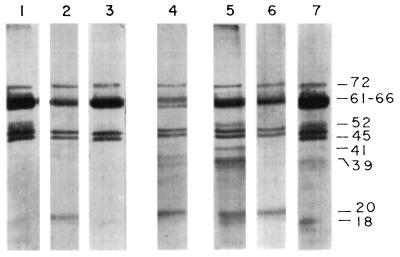
LAg in association with liposomes induce strong protection against virulent homologous challenge (3, 6). These antigens were extracted from the promastigote ghost membrane (Fig. 1, lane 1) and exhibited at least 33 distinct polypeptides (lane 2) ranging in molecular mass from 18 to 153 kDa. Entrapment of LAg in liposomes of different charges (lanes 6 to 8) revealed different polypeptide profiles in vesicles of negative (lane 6), neutral (lane 7), and positive (lane 8) charge. The silver stain data illustrate that preparations of liposomes resulted in preferential entrapment of some proteins. Polypeptides with molecular masses above 64 kDa, weakly displayed in LAg as well as in the ghost membrane, were predominantly associated with liposomes. Again, a 61- to 66-kDa band, present as a 63- to 64-kDa polypeptide in LAg but not as the major component, stained more intensely in neutral and positively charged vesicles than in negatively charged liposomes. This band demonstrated a comigration with the major polypeptide of purified gp63 provided by Chang (lane 4). An attempt at partial purification of LAg through detergent solubilization resulted in the extraction of approximately six polypeptides (72, 63, 43, 41, 30, and 20 kDa) and a faint smear in the region of 45 to 52 kDa, with the major band also comigrating with gp63 (lane 3). gp63 purified by electroelution stained as a single band representing a 63-kDa polypeptide (lane 5). Amounts of empty liposomes equal to the amounts of liposomes with LAg were subjected to SDS-PAGE. No bands were observed (data not shown).
FIG. 1.
Silver-stained SDS-PAGE gel of Leishmania antigens in liposomes. Lanes: 1, ghost membrane (20 μg); 2, LAg (20 μg); 3, SLA (4 μg); 4, gp63 (Chang's; 2 μg); 5, gp63 purified by electroelution (4 μg); 6 to 8, LAg entrapped in negatively charged (lane 6), neutral (lane 7), and positively charged (lane 8) liposomes (6 μg). The positions of prominent bands are shown at the right as apparent molecular masses in kilodaltons.
Immunoblot analysis of LAg and LAg in liposomes with immunized mice sera before and after infection.
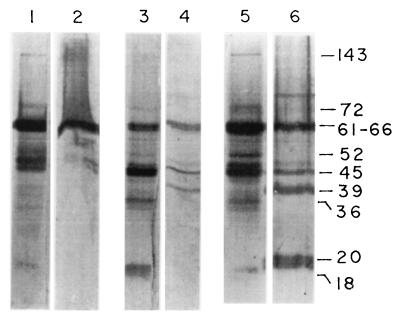
The antigenicities of the components of LAg and LAg in liposomes were studied by performing Western blot analysis on a series of blots of LAg (Fig. 2, lanes 1, 3, and 5) and LAg entrapped in positively charged (lane 2), neutral (lane 4), and negatively charged (lane 6) liposomes. Antigenic profiles were characterized by a dominant 61- to 66-kDa band for LAg in positively charged liposomes as well as for LAg and additional reactivity with 72-, 52-, 48-, and 45-kDa polypeptides on LAg by sera from mice immunized with LAg entrapped in positively charged liposomes (Fig. 2, lanes 1 and 2). Identical blots of LAg and LAg in neutral liposomes probed with sera from mice immunized with LAg in neutral liposomes show recognition of a 62- to 64-kDa band, followed by 45- and 39-kDa bands, on blots of LAg in liposomes (lane 4) and a dominant 45-kDa band, followed by 62- to 64-, 36-, and 18-kDa bands, on LAg blots (lane 3). Sera from mice immunized with LAg in negatively charged liposomes recognized a number of bands on blots of both LAg (lane 5) and LAg in liposomes (lane 6). In addition to the 62- to 64- and 45-kDa bands, which appeared on both blots, the antigenic profiles were characterized by bands at 80, 39, and 20 kDa on the blots of LAg in liposomes and bands at 143, 72, 70, 50, 48, and 36 kDa on LAg blots.
FIG. 2.
Immunoblot profiles of LAg in liposomes following reactions with immunized mouse sera. LAg (20 μg; lanes 1, 3, 5) and equal amounts of LAg in positively charged (6 μg; lane 2), neutral (lane 4), and negatively charged (lane 6) liposomes were subjected to SDS-PAGE and Western blotting. Sera from BALB/c mice immunized with LAg in positively charged (lanes 1 and 2), neutral (lanes 3 and 4), and negatively charged (lanes 5 and 6) liposomes were used at 1:500 dilution. Relative molecular masses of the prominent bands (in kilodaltons) are represented on the right.
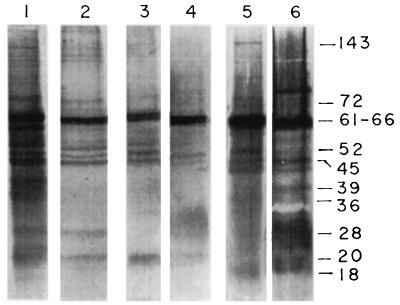
Figure 3 presents the antigenic profiles of similar samples of LAg and LAg in liposomes that reacted with sera from mice immunized with LAg entrapped in liposomes after 4 months of infection. The antibodies with enhanced titers that were elicited after infection of mice immunized with LAg in positively charged liposomes recognized several antigens of LAg (lane 1). The same sera, however, showed reactivity mainly with the 61- to 66-kDa band followed by 72-, 52-, 48-, 45-, 28-, and 20-kDa components of LAg in liposomes (lane 2). Antigen recognition by immunized mouse sera after 4 months of infection with LAg in neutral liposomes was, however, limited for both LAg (lane 3) and LAg in liposomes (lane 4), demonstrating a reactivity predominantly for the 62- to 64-kDa band followed by 52-, 48-, 45-, and 20-kDa moieties. Antibodies elicited in mice immunized with LAg in negatively charged liposomes revealed the strongest reactivity with components of LAg and LAg in liposomes after 4 months of infection. Although the 61- to 66-kDa band was still the dominant component of LAg (lane 5), there were several additional bands as well as smears of LAg in negatively charged liposomes (lane 6) recognized by the antisera.
FIG. 3.
Immunoblot analysis of LAg in liposomes with immunized-mouse sera after 4 months of infection. LAg (lanes 1, 3, and 5) and LAg in liposomes (positively charged, lane 2; neutral, lane 4; negatively charged, lane 6) fractionated by SDS-PAGE were probed with sera from infected mice immunized with LAg entrapped in positively charged (lanes 1 and 2), neutral (lanes 3 and 4), and negatively charged (lanes 5 and 6) liposomes following electrotransfer to nitrocellulose. The amounts of proteins loaded were as described for Fig. 2. Numbers on the right represent the prominent bands (in kilodaltons).
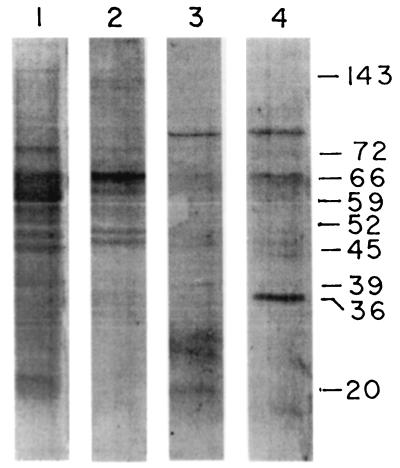
Reactivity of LAg and LAg in liposomes with infected sera is illustrated in Fig. 4. While there was a dominant 61- to 66-kDa band of LAg with immunized-mouse sera before and after infection (Fig. 2 and 3), sera from unimmunized, infected mice exhibited predominantly a 59-kDa band of LAg (Fig. 4, lane 1). Other components recognized by the infected sera include bands of 143, 72, 52, 48, 45, 39, 36, and 20 kDa, as well as smears in the range of 60 to 66, 50 to 58, and 18 to 22 kDa. Infected sera reacted with LAg in liposomes with lower intensity, recognizing only 66-, 60-, 48-, and 45-kDa bands for LAg in positively charged liposomes (lane 2); 80-, 28-, and 20-kDa bands for LAg in neutral liposomes (lane 3); and 80-, 66-, and 36-kDa bands for LAg in negatively charged liposomes (lane 4). No bands were present on identical sets of blots probed with normal mouse sera or sera from mice immunized with empty liposomes (data not shown).
FIG. 4.
Reactivity of LAg and LAg in liposomes with infected-mouse sera. Blots of LAg (lane 1) and LAg in positively charged (lane 2), neutral (lane 3), and negatively charged (lane 4) liposomes were probed with a 1:500 dilution of sera from mice infected for 4 months. Amounts of proteins loaded were identical to those for Fig. 2. Numbers indicate molecular masses in kilodaltons.
Western blot analysis of SLA with sera from mice immunized with LAg in liposomes before and after protection.
The antigenic profile of the components of SLA in sera from mice immunized with LAg entrapped in liposomes (Fig. 5) was characterized by bands at molecular masses of 72, 48, and 45 kDa and a predominant band of 60 to 66 kDa (lanes 1 to 3), with additional bands of 52 kDa for positively charged liposomes (lane 1) and 20 kDa for neutral liposomes (lane 2). The profile of SLA in sera from mice immunized with LAg in neutral liposomes following infection remained the same (lane 6). However, additional bands of 41, 39, and 20 kDa for LAg in positively charged liposomes (lane 5) and of 52, 39, and 18 kDa for LAg in negatively charged liposomes (lane 7) in mouse sera after 4 months of infection were observed. In the sera from unimmunized, infected mice (lane 4) the reaction was of a lower intensity and a doublet of 59 and 63 kDa replaced the immunodominant 61- to 66-kDa band reactive with sera from mice immunized with LAg in liposomes, while the rest of the profile remained the same.
FIG. 5.
Western blot analysis of SLA. SLA (4 μg) was separated by SDS-PAGE, and the proteins were immunoblotted with sera from mice after immunization with LAg entrapped in positively charged (lanes 1 and 5), neutral (lanes 2 and 6), and negatively charged (lanes 3 and 7) liposomes before (lanes 1 to 3) and after (lanes 5 to 7) infection. A similar blot of SLA (lane 4) was probed with sera from unimmunized, infected mice. The positions of prominent bands are shown at the right.
Expression of gp63.
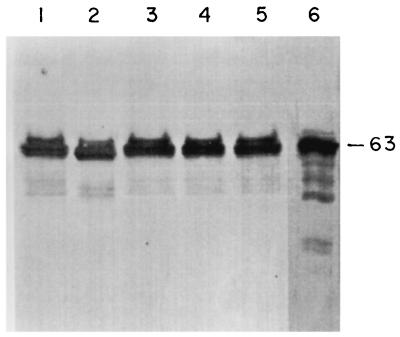
The dominant band of 61 to 66 kDa for LAg in liposomes as well as SLA exhibited a relative mobility very similar to that of the band for purified gp63 obtained from Chang (Fig. 1). The specificity of gp63 in blots of LAg, LAg in liposomes, and SLA was confirmed through reactivity with a polyvalent rabbit antiserum raised against this purified protein (Fig. 6). gp63 recognized by anti-gp63 (lane 6) appeared as a doublet of 61 to 66 kDa in LAg (lane 1), LAg in liposomes (lanes 3 to 5), and SLA (lane 2) with almost equal intensities and specificities. No bands were present on a similar set of blots probed with normal rabbit serum (data not shown).
FIG. 6.
Western blot analysis of gp63 expression in liposomes containing LAg. Blots of LAg (20 μg; lane 1); SLA (4 μg; lane 2); LAg in positively charged (6 μg; lane 3), neutral (6 μg; lane 4), and negatively charged (6 μg; lane 5) liposomes; and gp63 (Chang's; 2 μg; lane 6) were probed with a 1:200 dilution of polyclonal rabbit anti-gp63 sera and developed with diaminobenzidine and H2O2 as described in Materials and Methods.
Vaccination with gp63 entrapped in liposomes.
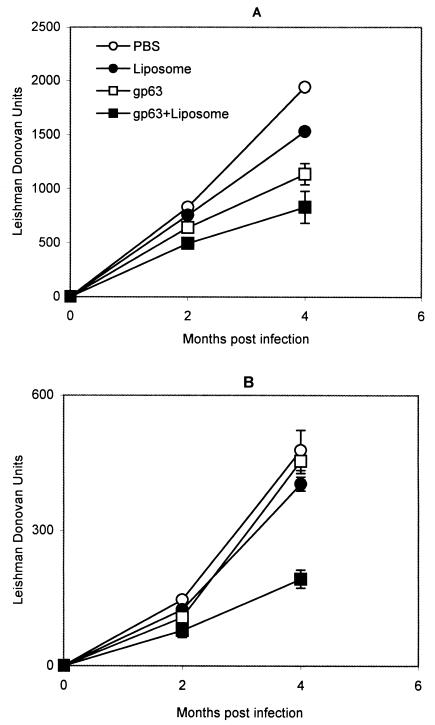
To investigate the protective efficacy of gp63 in a liposomal formulation, BALB/c mice were immunized with gp63 purified by electroelution, both free gp63 and gp63 entrapped in positively charged liposomes, prior to challenge with L. donovani promastigotes. The positively charged vesicles were selected because the maximum level of protection against experimental visceral leishmaniasis was observed with these liposomes. After 2 and 4 months of infection mice were sacrificed and parasite loads in their livers and spleens were quantified (Fig. 7). Animals immunized with gp63 and gp63 entrapped in liposomes demonstrated significantly enhanced resistance to hepatic infection with L. donovani; the protection induced was 22.8 and 40%, respectively, at 2 months, and 41 and 57%, respectively, at 4 months, compared to the resistance of mice receiving only PBS (P < 0.01) (Fig. 7A). The level of protection induced by liposomal gp63 was higher than that with free gp63. However, the difference was not statistically significant. Vaccination with liposomal gp63 could also induce significant protection against parasite growth in the spleen (46% at 2 months and 60% at 4 months; P < 0.02) (Fig. 7B). In contrast, free gp63 failed to provide protection in this organ. These results demonstrated the partial but significant vaccine potentiality of gp63 in a liposomal formulation.
FIG. 7.
Kinetics of protection against L. donovani in liver (A) and spleens (B) of BALB/c mice immunized with PBS, empty liposomes, gp63 in PBS, and gp63 in liposomes. After three intraperitoneal immunizations, mice were challenged intravenously with L. donovani promastigotes and the parasite burdens at 2 and 4 months of infection were calculated as described in Materials and Methods. The mean values ± standard errors (error bars) for three mice per group are given.
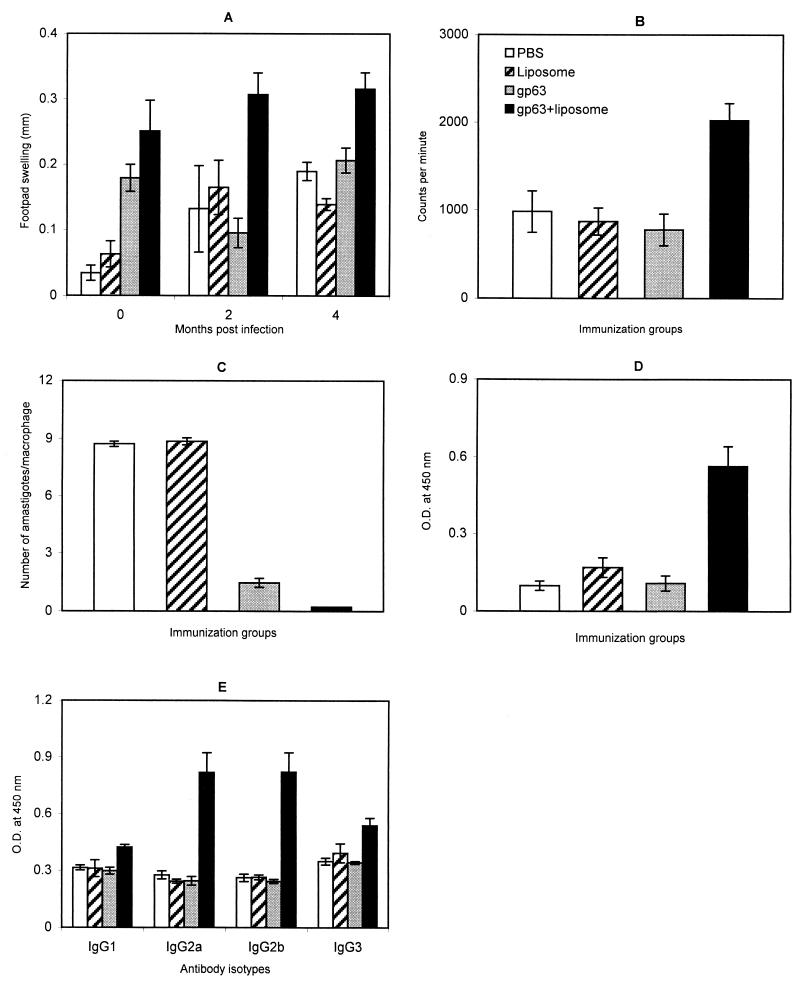
Immunization with liposomal gp63 induced the highest level of DTH before, as well as after, challenge infection, correlating with the acquired resistance to infection observed in this group (Fig. 8A). Free gp63 could also induce DTH responses after immunization. However, after infection the levels of the responses did not differ significantly from those for the controls.
FIG. 8.
Immune responses elicited by gp63 in positively charged liposomes in BALB/c mice. (A) gp63-specific DTH in immunized and challenged mice expressed as the difference (in millimeters) between the thicknesses of the test (gp63-injected) and control (PBS-injected) footpads. (B) Lymphoproliferative responses of gp63-immunized mice. The stimulation index of the liposomal gp63 group (mean counts per minute of cells stimulated with gp63 in triplicate/mean counts per minute of medium controls) was 4.1. (C) Immunization induced activation of mouse peritoneal macrophages to suppress intracellular amastigote proliferation 72 h after infection in vitro with L. donovani. (D and E) Serum IgG (D) and IgG (E) subclass antibody responses in immunized mice were assayed by ELISA on gp63-coated microtiter plates incubated overnight with a 1:1,000 dilution of sera, as described in Materials and Methods. Results are shown as the mean ± standard errors of the means for three individual mice. O.D., optical density.
Vaccine-induced stimulation of the cell-mediated immune response was further investigated through the capacity of spleen cells to proliferate in response to concanavalin A and gp63 after immunization. The in vitro responses to the mitogen evaluated 10 days after immunization were found to be strongly proliferative, with no differences between the control and the antigen-immunized groups (data not shown). In the gp63-specific-restimulation assays, cells from mice immunized with liposomal gp63, but not free gp63, proliferated significantly more than controls (P < 0.05) (Fig. 8B). A slight proliferative response, however, was also displayed by spleen cells from control mice and mice immunized with gp63 alone when restimulated in vitro. Similar lymphoproliferative responses have been observed earlier for mice immunized with gp63 alone or only adjuvant when stimulated in vitro with gp63 or SLA, respectively (48, 2). Immunization with gp63, free as well as in a liposome formulation, could also activate peritoneal macrophages to arrest L. donovani amastigote multiplication in vitro (Fig. 8C). The leishmanicidal activity of macrophages from gp63-liposome immunized mice was, however, significantly higher (P < 0.002) than that of those from free-gp63-immunized mice. In addition, the percentage of infected macrophages for the liposomal antigen-immunized mice was lower than that for free-antigen-immunized mice (12 versus 53%), demonstrating the enhanced adjuvant activity of the liposomal formulation of gp63. Immunization with gp63 in liposomes induced a strong humoral immune response (Fig. 8D), which was not observed in free-gp63-immunized or control animals. Th1 and Th2 cells, implicated in differential host response to infectious diseases, promote cellular as well as humoral immune responses. Since antibody isotype profiles provide a convenient surrogate marker for Th1 and Th2 subsets of CD4+ cells (1, 12), we further evaluated the levels of gp63-specific IgG isotype antibodies in serum from the immunized mice (Fig. 8E). Elicitation of significantly higher levels of IgG2a and IgG2b than of IgG1 (P < 0.01) points to a Th1-dominated immune response (15) stimulated by gp63 entrapped in liposomes.
DISCUSSION
In our attempt to design a vaccine against visceral leishmaniasis we initiated studies with LAg in association with liposomes as the adjuvant. Entrapment of these antigens in the lipid vesicles conferred significant levels of protection against infection in BALB/c mice. However, the extent of protection showed marked variation for the same antigens depending on whether liposomes were positively charged (3), neutral (6), or negatively charged (5). The variations may be a result of differential entrapment of the various components of LAg or an adjuvant-induced difference in the immune responses to LAg. Our data indicate that a few immunodominant parasite antigens can elicit strong protective immune responses when used in the context of appropriate adjuvants.
Characterization of the antigens entrapped in liposomes by SDS-PAGE revealed preferential entrapment of a 61- to 66-kDa promastigote antigen by all the liposome preparations. In addition, a number of other proteins of LAg were entrapped within the vesicles. However, all these components were not immunogenic, and their profiles varied with the charge of the associated vesicle. While a few polypeptides, including the dominant 62- to 64-kDa polypeptide, demonstrated seroreactivity in neutral and positively charged liposomes, a larger number were seroreactive in association with negatively charged vesicles, with antisera obtained through the corresponding vaccine preparation. The reactivity of LAg and LAg in liposomes was enhanced with sera from immunized mice after infection. However, apart from the antigens in negatively charged liposomes, selective seroreactivity of LAg components was again observed for neutral and positively charged liposome-associated antigens. Of the antigens recognized by antisera before and after infection, the 62- to 64-kDa component was the most seroreactive component of LAg in all three vaccine preparations, followed by distinctive bands at 72, 52, 48, 45, and 20 kDa, especially on blots of neutral and positively charged LAg in liposomes. Interestingly, SLA, partially purified from LAg, also demonstrated maximum reactivity at 62 to 64 kDa, followed by strong bands at 72, 52, 48, 45, and 20 kDa, with the sera from mice immunized with LAg in liposomes demonstrating a profile with a striking resemblance to the immunodominant-antigen profiles for neutral and positively charged liposomes. In contrast, sera from unimmunized, infected mice recognized different polypeptides of LAg. Reactivity with the components of LAg entrapped in the different liposomes was low and lacked the dominance of the 62- to 64-kDa antigen observed with the immunized sera.
That the immunodominant antigen among LAg and LAg in liposomes was gp63 was confirmed through reactivity with antiserum against this purified protein. Preferential entrapment of gp63 from the crude mixture of L. major promastigotes in liposomes was also demonstrated by Kahl et al. (27) despite the differences in the use of the phospholipids and the vesicle preparation. These workers identified gp63 as the principal protein antigen conferring protection. The identification of defined parasite proteins and peptides that induce beneficial immune responses may contribute to vaccine development. gp63, the major surface glycoprotein of Leishmania, is highly conserved across species (37), and its role in induction of protection against murine cutaneous leishmaniasis has been extensively investigated (25, 51, 64). The subjects of the studies range from the use of the native protein (13, 16, 51) to the use of recombinant gp63 (rgp63) expressed in Salmonella spp. (64) or plasmid pCMV, which encodes gp63 (62), to the use of T-cell epitopes within gp63 (25, 63). However, even though L. donovani gp63 has been identified (33) and purified in its native and recombinant forms (43, 52), its role in protection against infection with L. donovani has not been established. Partial heterologous protection against L. donovani infection in mice by L. major rgp63 expressed in Salmonella was reported (36). To our knowledge this is the first report of an involvement of L. donovani gp63 in protection against the visceral infection in mice.
Even though gp63 was the immundominant antigen of LAg in all the three liposomes, protection conferred by these preparations varied significantly. The choice of adjuvant is important in inducing the correct immune response. In a study undertaken with pure M2 and three different adjuvants, marked variations in the protection against L. mexicana amazonensis conferred by each preparation were observed (11). It has been shown that cloned T cells with opposite biological effects on murine models of cutaneous leishmaniasis utilize the same or similar T-cell receptors (47), suggesting that a particular antigen may elicit either a protective or an exacerbating immune response against L. major. L. major antigens drive either a Th1- or Th2-type T-cell response, depending on the local cytokine environment during antigen priming (10, 26, 27, 59). Antigens of L. donovani, however, did not induce exacerbation. Investigation of the immune responses to the vaccine preparations showed that LAg in neutral liposomes elicited a weak but exclusively Th1-type response after immunization, as characterized by the antibody isotype profile (4). In contrast, the same antigens in positively and negatively charged liposomes induced both Th1- and Th2-type responses. However, while high levels of IgG2a and IgG2b (markers for the induction of a Th1-like response) were simultaneously stimulated, along with IgG1 (Th2-like response), with positively charged liposomes (3), these isotypes were dominated by the levels of IgG1 stimulated by immunization with LAg in negatively charged vesicles (5). These data indicate that, in contrast to what was found for L. major antigens (55), a concomitant Th2 response with L. donovani antigens does not inhibit the strong Th1 effector function. This conclusion was further substantiated by our investigation of the vaccine potentiality of gp63, purified by electroelution, in association with liposomes. Enhanced protection induced with gp63 in a liposomal formulation corresponded with stronger stimulation of cellular as well as humoral immune responses in comparison with stimulation by gp63 alone. Further analysis of IgG isotypes revealed induction of all the isotypes, suggesting stimulation of both Th1- and Th2-like responses by gp63 in a vaccine formulation. However, stimulation of higher levels of IgG2a and IgG2b antibodies than of IgG1 antibodies indicates the stronger potentiation of a protective Th1 response. The development of a vaccine against L. donovani may, therefore, require that a particular antigen be administered in the context of the right adjuvant for potentiating a dominant Th1 response.
Although gp63 was maximally incorporated in the liposomes, in contrast to the L. major antigens (27), a greater number of other proteins of L. donovani LAg were also entrapped in the vesicles. However, only a few were immunoreactive with sera from protectively immunized animals and therefore likely to be involved in immunoprotection. While the identity of the 61- to 66-kDa band was confirmed, 72-, 52-, 48-, 45-, 39-, 36-, and 20-kDa antigens of LAg remain unidentified. Screening for parasite antigens on the basis of reactivity with sera from infected susceptible animals has led to the identification of several leishmanial antigens having the ability to confer protective immunity. Some of these well-defined antigens detected through antibody reactivity include LPG (18), gp46/M-2 (11), dp72 (24), PSA-2 (19), P4 and P8 (56), Lcr1 (61), and LACK (42, 59). Purified antigens such as L. donovani dp72 and highly conserved polypeptides of Leishmania species such as gp46 and LACK, of 46 and 36 kDa, respectively, may be identified with the immunoreactive components of L. donovani LAg in liposomes and need further testing. In contrast to LAg, SLA, the partially purified antigens from LAg, demonstrated a restricted seroreactivity comparable to that of polypeptides of LAg in positively charged liposomes which exhibited maximum protective activity. Although gp63 is one of the most immunogenic of Leishmania antigens, it is only partially protective against murine visceral leishmaniasis, as observed herein, and against lethal murine cutaneous leishmaniasis, as observed elsewhere (13, 25, 51, 64). It has been suggested that the efficacy of gp63 could be enhanced, possibly with additional Leishmania antigens (13). Since SLA is composed of most of the antigens, including gp63, immunogenic in LAg in liposomes and since it induces better protection than gp63 or LAg in liposomes (Rajesh et al., unpublished data), we propose that its components, polypeptides of 72, 52, 48, 45, 41, 39, and 20 kDa, in addition to gp63, be vaccine candidates for future studies of L. donovani antigens in liposomes.
Acknowledgments
We thank J. Das and S. K. Bhattacharya, past and present directors of IICB, Calcutta, for supporting this work.
We gratefully acknowledge support from the CSIR and the DST, Government of India, and the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases.
F.A. and R.R. contributed equally to this work.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Abdelhak, S., H. Louzir, J. Timm, L. Blel, Z. Benlasfar, H. Lagranderie, M. Gheorghiu, K. Dellagi, and B. Gicquel. 1995. Recombinant BCG expressing the leishmania surface antigen Gp63 induces protective immunity against Leishmania major infection in BALB/c mice. Microbiology 141:1585-1592. [DOI] [PubMed] [Google Scholar]
- 3.Afrin, F., and N. Ali. 1997. Adjuvanicity and protective immunity elicited by Leishmania donovani antigens encapsulated in positively charged liposomes. Infect. Immun. 65:2371-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afrin, F., and N. Ali. 1998. Isotype profiles of Leishmania donovani-infected BALB/c mice: preferential stimulation of IgG2a/b by liposome-associated promastigote antigens. J. Parasitol. 84:743-748. [PubMed] [Google Scholar]
- 5.Afrin, F., K. Anam, and N. Ali. 2000. Induction of partial protection against Leishmania donovani by promastigote antigens in negatively charged liposomes. J. Parasitol. 86:730-735. [DOI] [PubMed] [Google Scholar]
- 6.Ali, N., and F. Afrin. 1997. Protection of mice against visceral leishmaniasis by immunization with promastigote antigen incorporated in liposomes. J. Parasitol. 83:70-75. [PubMed] [Google Scholar]
- 7.Anam, K., F. Afrin, D. Banerjee, N. Pramanik, S. K. Guha, R. P. Goswami, P. N. Gupta, S. K. Saha, and N. Ali. 1999. Immunoglobulin subclass distribution and diagnostic value of Leishmania donovani antigen-specific immunoglobulin G3 in Indian kala-azar patients. Clin. Diagn. Lab. Immunol. 6:231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyrodt, C. G. P., A. R. Pinto, E. Freymuller, and C. L. Barbieri. 1997. Characterization of an antigen from Leishmania amazonensis amastigotes able to elicit protective responses in a murine model. Infect. Immun. 65:2052-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogdan, C., A. Gesser, W. Solbach, and M. Rollinghoff. 1996. Invasion, control and persistence of Leishmania parasites. Curr. Opin. Immunol. 8:517-525. [DOI] [PubMed] [Google Scholar]
- 10.Bretscher, P. A., G. Wei, J. N. Menon, and H. Bielefeldt-Ohmann. 1992. Establishment of cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science 257:539-542. [DOI] [PubMed] [Google Scholar]
- 11.Champsi, J., and D. McMahon-Pratt. 1988. Membrane glycoprotein M-2 protects against Leishmania amazonensis infection. Infect. Immun. 56:3272-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coffman, R. L., D. A. Lebman, and P. Rothman. 1993. Mechanism and regulation of immunoglobulin isotype switching. Adv. Immunol. 54:229-270. [DOI] [PubMed] [Google Scholar]
- 13.Connell, N. D., E. Medina-Acosta, W. R. McMaster, B. R. Bloom, and D. G. Russell. 1993. Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette-Guerin expressing the Leishmania surface proteinase gp63. Proc. Natl. Acad. Sci. USA 90:11473-11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fargeas, C., M. Hommel, R. Maingon, C. Dourado, M. Monsigny, and R. Mayer. 1996. Synthetic peptide-based enzyme-linked immunosorbent assay for serodiagnosis of visceral leishmaniasis. J. Clin. Microbiol. 34:241-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Germann, T., M. Bongartz, H. Dlugonska, H. Hess, E. Schmitt, L. Kolbe, E. Kolsch, F. J. Podlaski, M. K. Gately, and E. Rude. 1995. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur. J. Immunol. 25:823-829. [DOI] [PubMed] [Google Scholar]
- 16.Guimaraes, T. M. P. D., V. P. C. P. de Toledo, C. A. da Costa, R. T. da Costa, O. Genaro, P. Williams, and W. Maybrink. 1996. Assessment of immunity induced in mice by glycoproteins derived from different strains and species of Leishmania. Mem. Inst. Oswaldo Cruz 91:63-70. [DOI] [PubMed] [Google Scholar]
- 17.Gurunathan, S., C. Prussin, D. L. Sacks, and R. A. Seder. 1998. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 4:1409-1415. [DOI] [PubMed] [Google Scholar]
- 18.Handman, E., and G. F. Mitchell. 1985. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc. Natl. Acad. Sci. USA 82:5910-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handman, E., F. M. Symons, T. M. Baldwin, J. M. Curtis, and J.-P. Y. Scheerlinck. 1995. Protective vaccination with promastigote surface antigen 2 from Leishmania major is mediated by a Th1 type of immune response. Infect. Immun. 63:4261-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinzel, F. P., M. D. Sadick, B. J. Holaday, R. L. Coffman, and R. M. Locksley. 1989. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 169:59-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinzel, F. P., M. D. Sadick, S. S. Mutah, and R. M. Locksley. 1991. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc. Natl. Acad. Sci. USA 88:7011-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 23.Holbrook, J. W., and J. A. Cook. 1983. Immunization of mice against Leishmania donovani by subcutaneous injections of dead promastigotes. Am. J. Trop. Med. Hyg. 32:51-53. [DOI] [PubMed] [Google Scholar]
- 24.Jaffe, C. L., N. Rachamim, and R. Sarfstein. 1990. Characterization of two proteins from Leishmania donovani and their use for vaccine against visceral leishmaniasis. J. Immunol. 144:699-706. [PubMed] [Google Scholar]
- 25.Jardim, A., J. Alexander, H. S. Teh, D. Qu, and R. W. Olafson. 1990. Immunoprotective Leishmania major synthetic T cell epitopes. J. Exp. Med. 172:645-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahl, L. P., C. A. Scott, R. Lelchuk, G. Gregoriadis, and F. Y. Liew. 1989. Vaccination against murine cutaneous leishmaniasis by using Leishmania major antigen/liposomes. J. Immunol. 142:4441-4449. [PubMed] [Google Scholar]
- 27.Kahl, L. P., R. Lelchuk, C. A. Scott, and J. Beesley. 1990. Characterization of Leishmania major antigen-liposomes that protect BALB/c mice against cutaneous leishmaniasis. Infect. Immun. 58:3233-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karp, C. L., S. H. El-Safi, T. A. Wynn, M. M. H. Satti, A. M. Kordofani, F. A. Hashim, M. Hag-Ali, F. A. Neva, T. B. Nutman, and D. L. Sacks. 1993. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin 10 and interferon gamma. J. Clin. Investig. 91:1644-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaye, P. M., A. J. Curry, and J. F. Blackwell. 1991. Differential production of Th1- and Th2-derived cytokines does not determine the genetically controlled or vaccine-induced rate of cure in murine visceral leishmaniasis. J. Immunol. 146:2763-2770. [PubMed] [Google Scholar]
- 30.Kenney, R. T., D. L. Sacks, A. A. Gam, H. W. Murray, and S. Sundar. 1998. Splenic cytokine response in Indian kala-azar before and after treatment. J. Infect. Dis. 177:815-819. [DOI] [PubMed] [Google Scholar]
- 31.Kimsey, P. B., C. M. Theodos, T. K. Mitchen, S. J. Turco, and R. G. Titus. 1993. An avirulent lipophosphoglycan-deficient Leishmania major clone induces CD4+ T cells which protect susceptible BALB/c mice against infection with virulent L. major. Infect. Immun. 61:5205-5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 33.Lepay, D. A., N. Noguiera, and Z. Cohn. 1983. Surface antigens of Leishmania donovani promastigotes. J. Exp. Med. 157:1562-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liew, F. Y., J. G. Howard, and C. Hale. 1984. Prophylactic immunization against experimental leishmaniasis. III. Protection against fatal Leishmania tropica infection induced by irradiated promastigotes involves Lyt-1+2− T cells that do not mediate cutaneous DTH. J. Immunol. 132:456-461. [PubMed] [Google Scholar]
- 35.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 36.McSorley, S. J., D. Xu, and F. Y. Liew. 1997. Vaccine efficacy of Salmonella strains expressing glycoprotein 63 with different promoters. Infect. Immun. 65:171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medina-Acosta, E., R. E. Karess, and D. G. Russell. 1993. Structurally distinct genes for the surface protease of Leishmania mexicana are developmentally regulated. Mol. Biochem. Parasitol. 57:31-46. [DOI] [PubMed] [Google Scholar]
- 38.Medrano, F. J., C. Canavate, M. Leal, C. Rey, E. Lissen, and J. Alvar. 1998. The role of serology in the diagnosis and prognosis of visceral leishmaniasis in patients coinfected with immunodeficiency virus type-1. Am. J. Trop. Med. Hyg. 59:155-162. [DOI] [PubMed] [Google Scholar]
- 39.Melby, P. C., J. Yang, W. Zhao, L. E. Pervez, and J. Cheng. 2001. Leishmania donovani p36 (LACK) DNA vaccine is highly immunogenic but not protective against experimental visceral leishmaniasis. Infect. Immun. 69:4719-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miralles, G. D., M. Y. Stoeckle, D. F. McDermott, F. D. Finkelman, and H. W. Murray. 1994. Th1 and Th2 cell-associated cytokines in experimental leishmaniasis. Infect. Immun. 62:1058-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modabber, F. 1993. Leishmaniasis, p. 77-87. In UNDP/World Bank/W. H. O. Special Programme for Research and Training in Tropical Diseases, Tropical Disease Research: 11th Programme Report. World Health Organization, Geneva, Switzerland.
- 42.Mougneau, E., F. Altare, A. E. Wakil, S. Zheng, T. Coppala, Z.-E. Wang, R. Waldmann, R. M. Locksley, and N. Glaichenhaus. 1995. Expression cloning of a protective Leishmania antigen. Science 268:563-566. [DOI] [PubMed] [Google Scholar]
- 43.Okong'o-Odera, E. A., J. A. L. Kurtzhals, A. S. Hey, and A. Kharazmi. 1993. Measurement of serum antibodies against native Leishmania gp63 distinguishes between ongoing and previous Leishmania donovani infection. APMIS 101:642-646. [DOI] [PubMed] [Google Scholar]
- 44.Rachamim, N., and C. L. Jaffe. 1993. Pure protein from Leishmania donovani protects mice against both cutaneous and visceral leishmaniasis. J. Immunol. 150:2322-2331. [PubMed] [Google Scholar]
- 45.Reed, S. G., and P. Scott. 1993. T-cell and cytokine response in leishmaniasis. Curr. Opin. Immunol. 5:524-531. [DOI] [PubMed] [Google Scholar]
- 46.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 47.Reiner, S. L., Z.-E. Wang, F. Hatam, P. Scott, and R. M. Locksley. 1993. Th1 and Th2 cell antigen receptors in experimental leishmaniasis. Science 259:1457-1460. [DOI] [PubMed] [Google Scholar]
- 48.Rivier, D., P. Bovay, R. Shah, S. Didisheim, and J. Mauel. 1999. Vaccination against Leishmania major in a CBA mouse model of infection: role of adjuvants and mechanism of protection. Parasite Immunol. 21:461-473. [DOI] [PubMed] [Google Scholar]
- 49.Rolland-Burger, L., X. Rolland, C. W. Grieve, and L. Monjour. 1991. Immunoblot analysis of the humoral immune response to Leishmania donovani infantum polypeptides in human visceral leishmaniasis. J. Clin. Microbiol. 29:1429-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenthal, E., P. Marty, I. Poizot-Martin, J. Reynes, F. Pratlong, A. Lafeuillade, D. Jaubert, O. Boulat, J. Dereure, F. Gambarelli, J. A. Gastant, P. Dujardin, P. Dellamonica, and J. P. Cassuto. 1995. Visceral leishmaniasis and HIV-1 co-infection in southern France. Trans. R. Soc. Trop. Med. Hyg. 89:159-162. [DOI] [PubMed] [Google Scholar]
- 51.Russell, D. G., and J. Alexander. 1988. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J. Immunol. 140:1274-1279. [PubMed] [Google Scholar]
- 52.Schreffler, W. G., J. M. Burns, Jr., R. Badaro, H. W. Ghalib, L. L. Button, W. R. McMaster, and S. G. Reed. 1993. Antibody responses of visceral leishmaniasis patients to gp63, a major surface glycoprotein of Leishmania species. J. Infect. Dis. 167:426-430. [DOI] [PubMed] [Google Scholar]
- 53.Scott, P., E. Pearce, P. Natovitz, and A. Sher. 1987. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with soluble extract of promastigotes. J. Immunol. 139:221-227. [PubMed] [Google Scholar]
- 54.Sharifi, I., A. R. Fekri, M.-R. Aflatonian, A. Khamesipour, A. Nadim, M.-R. A. Mousavi, A. Z. Momeni, Y. Dowlati, T. Godal, F. Zicker, P. G. Smith, and F. Modabber. 1998. Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet 351:1540-1543. [DOI] [PubMed] [Google Scholar]
- 55.Sjolander, A., T. M. Baldwin, J. M. Curtis, and E. Handman. 1998. Induction of Th1 immune response and simultaneous lack of activation of a Th2 response are required for generation of immunity to leishmaniasis. J. Immunol. 160:3949-3957. [PubMed] [Google Scholar]
- 56.Soong, L., M. Duboise, P. Kima, and D. McMahon-Pratt. 1995. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect. Immun. 63:3559-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stauber, L. A., E. M. Franchino, and J. Grun. 1958. An eight day method for screening compounds against Leishmania donovani in golden hamster. J. Protozool. 5:269-273. [Google Scholar]
- 58.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Webb, J. R., W. Kaufmann, A. Campos-Neto, and S. G. Reed. 1996. Molecular cloning of a novel protein antigen of Leishmania major that elicits a potent immune response in experimental murine leishmaniasis. J. Immunol. 157:5034-5041. [PubMed] [Google Scholar]
- 60.Wilson, M. E., B. M. Young, K. P. Andersen, J. V. Weinstock, A. Metwali, K. M. Ali, and J. E. Donelson. 1995. A recombinant Leishmania chagasi antigen that stimulates cellular immune responses in infected mice. Infect. Immun. 63:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wray, W., T. Boulikas, V. P. Wray, and R. Hancock. 1981. Silver staining of proteins in polyacrylamide gels. Anal. Biochem. 118:197-203. [DOI] [PubMed] [Google Scholar]
- 62.Xu, D., and F. Y. Liew. 1995. Protection against leishmaniasis by injection of DNA encoding a major surface glycoprotein, gp63 of L. major. Immunology 84:173-176. [PMC free article] [PubMed] [Google Scholar]
- 63.Yang, D. M., M. V. Rogers, and F. Y. Liew. 1991. Identification and characterization of host protective T cell epitopes of a major surface glycoprotein (gp63) from Leishmania major. Immunology 72:3-9. [PMC free article] [PubMed] [Google Scholar]
- 64.Yang, D. M., N. Fairweather, L. L. Button, W. R. McMaster, L. P. Kahl, and F. Y. Liew. 1990. Oral Salmonella typhimurium (AroA−) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J. Immunol. 145:2281-2285. [PubMed] [Google Scholar]