Abstract
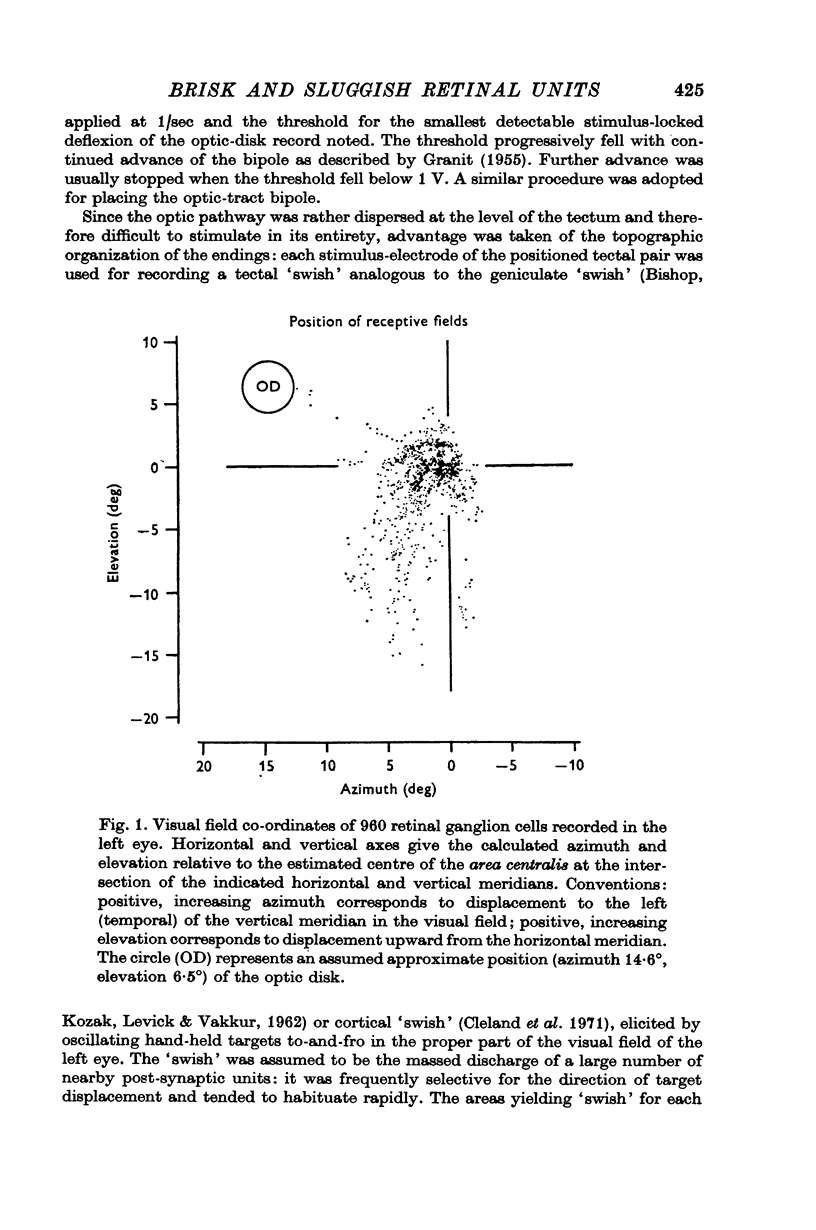
1. Nine hundred and sixty cat retinal ganglion cells were evaluated with respect to receptive-field organization and latency to antidromic activation of their axons from optic-tract and mid-brain positions.
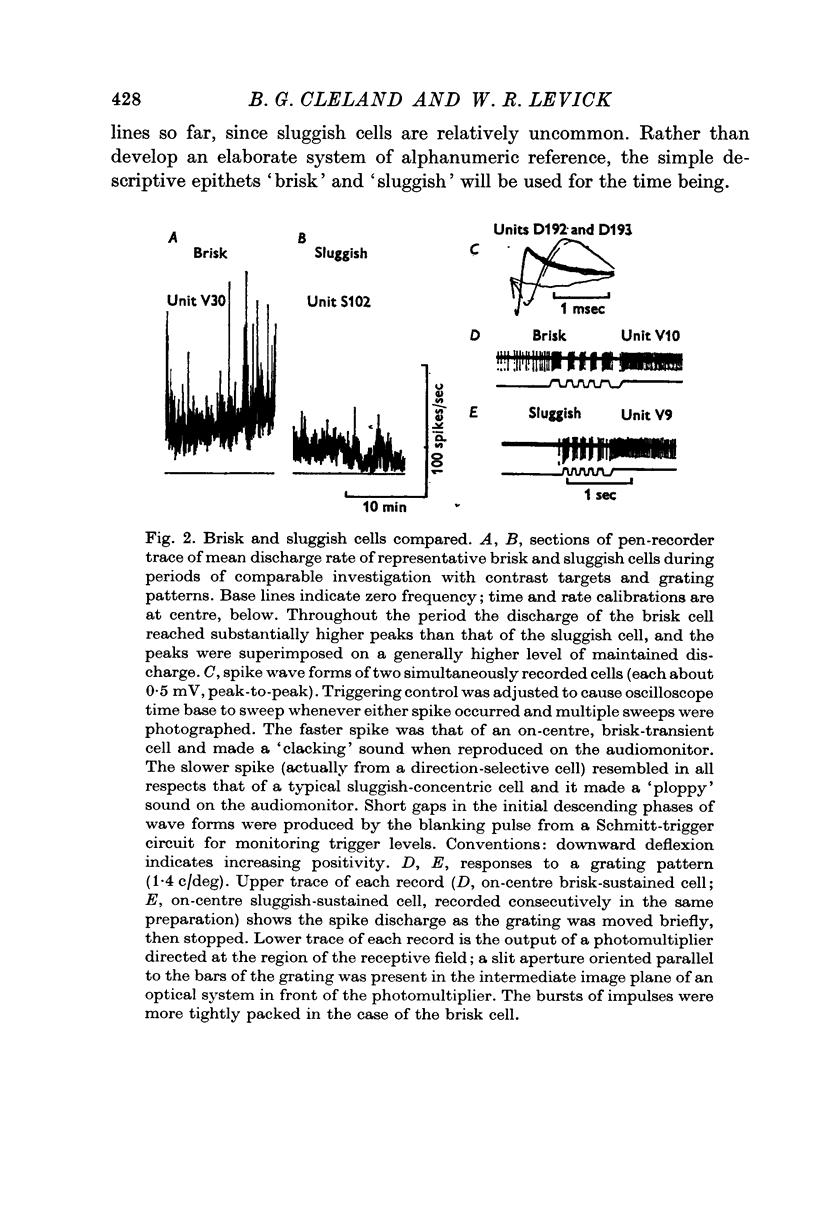
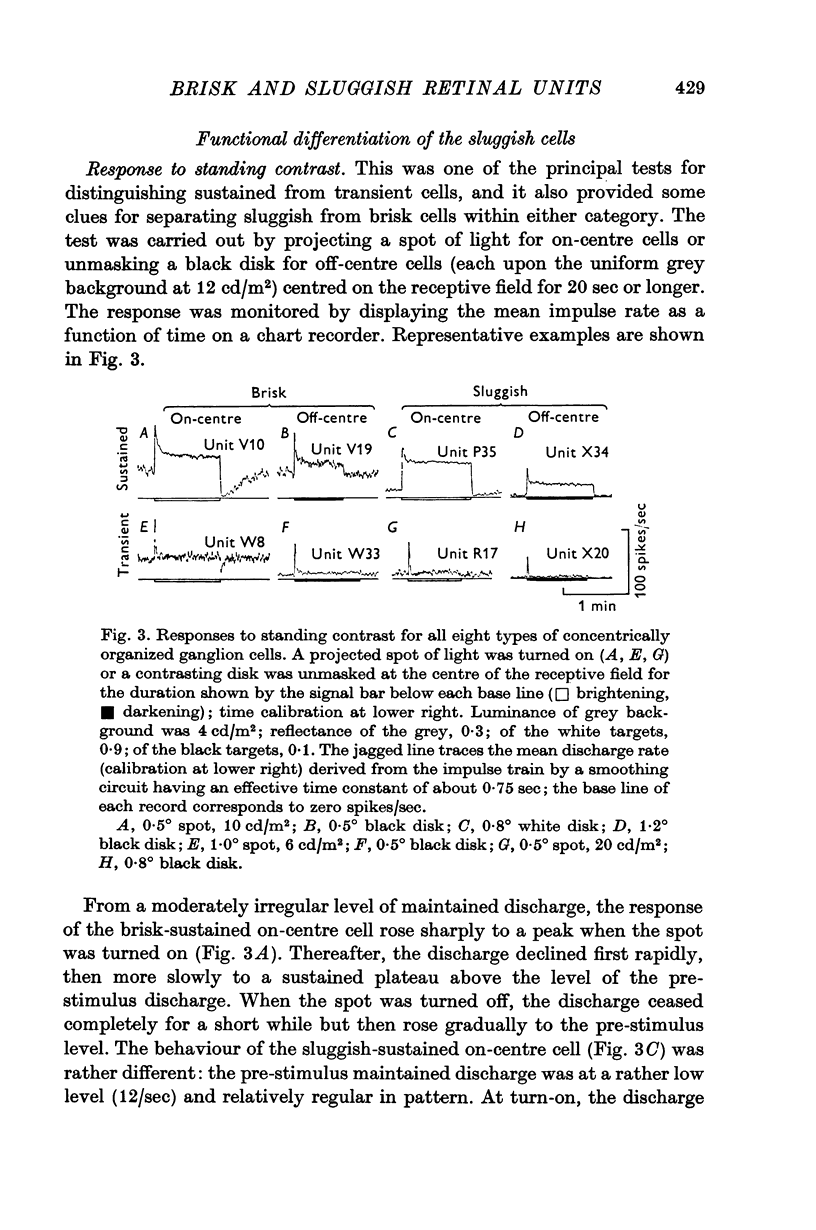
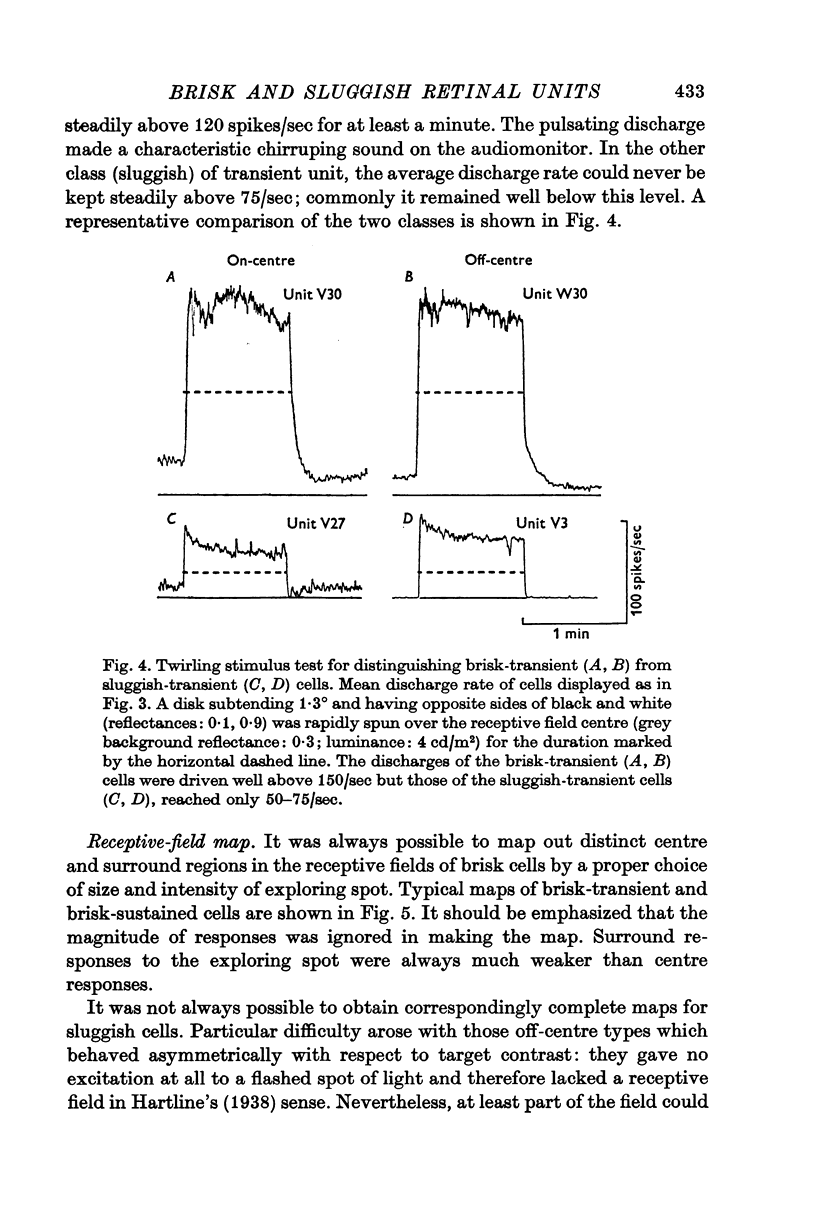
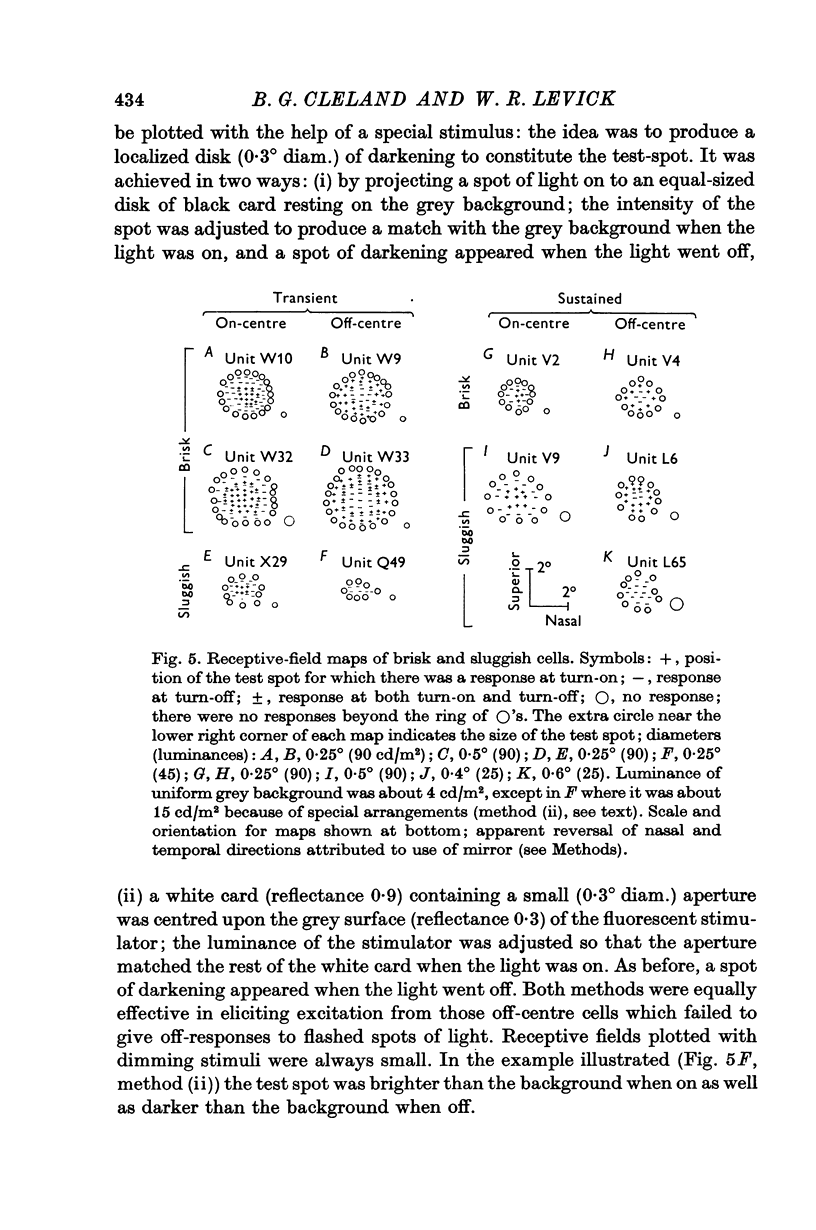
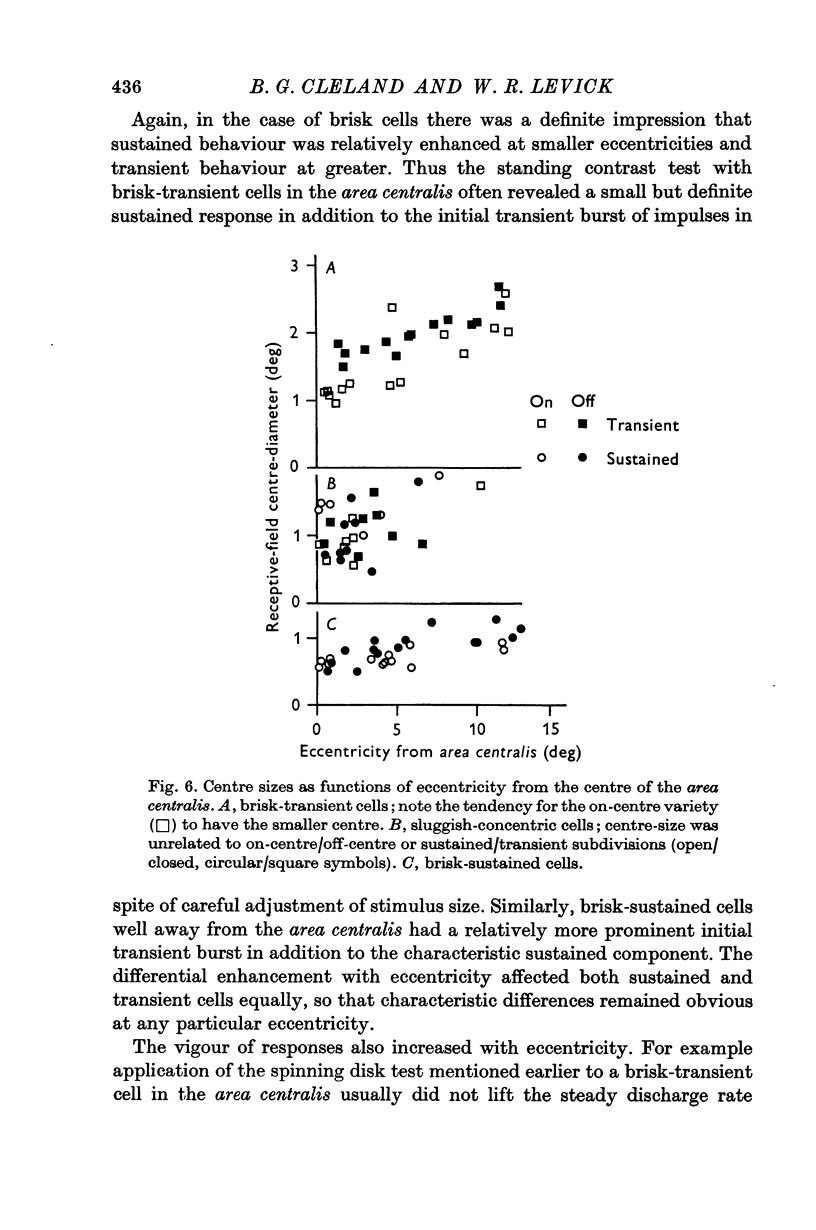
2. The vast majority (92%) had the familiar concentric centre/surround organization. As in earlier work these could be classed as sustained or transient, independently of the centre type. About 13% of the concentric cells were characterized by relatively sluggish responses to conventional visual stimuli which yielded brisk responses from the others. The sluggish cells constituted a previously unspecified class of concentric receptive fields.
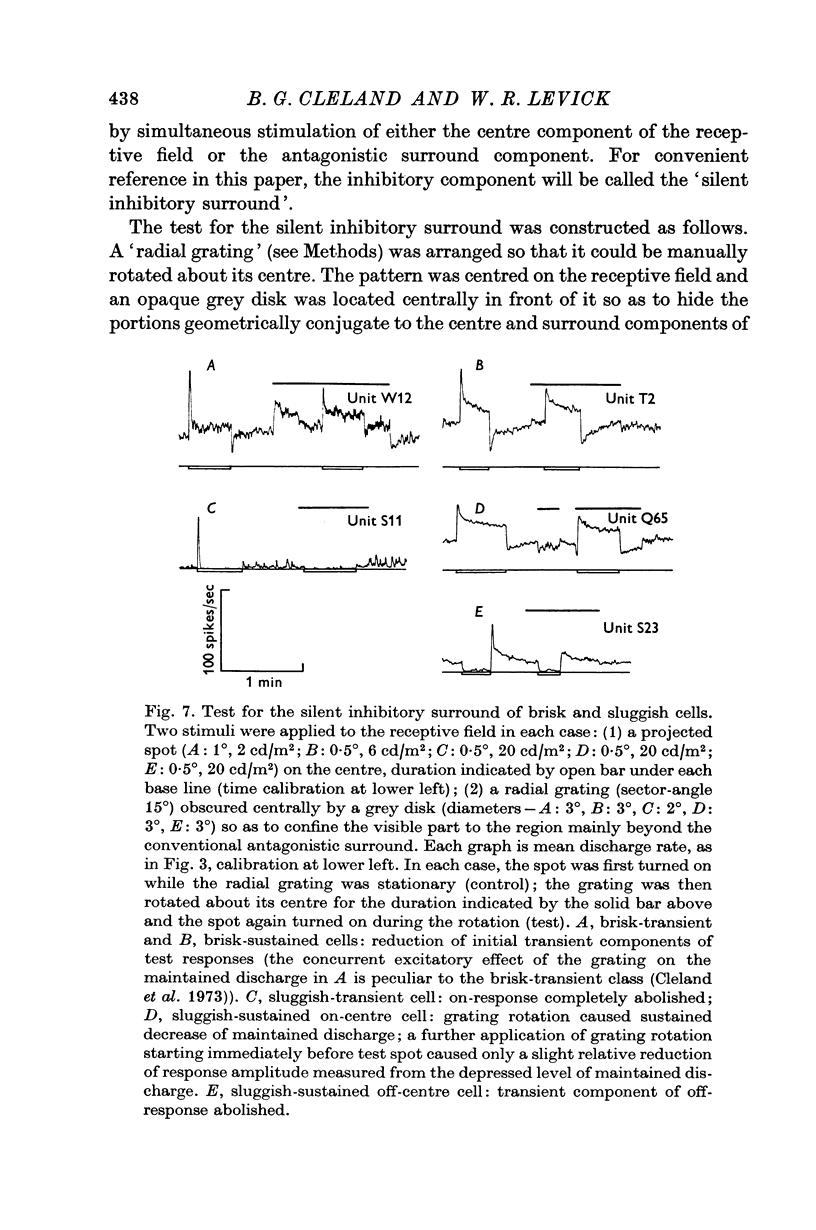
3. The responses of brisk and sluggish cells to a variety of stimuli were described with a view to developing procedures for distinguishing them on functional grounds.
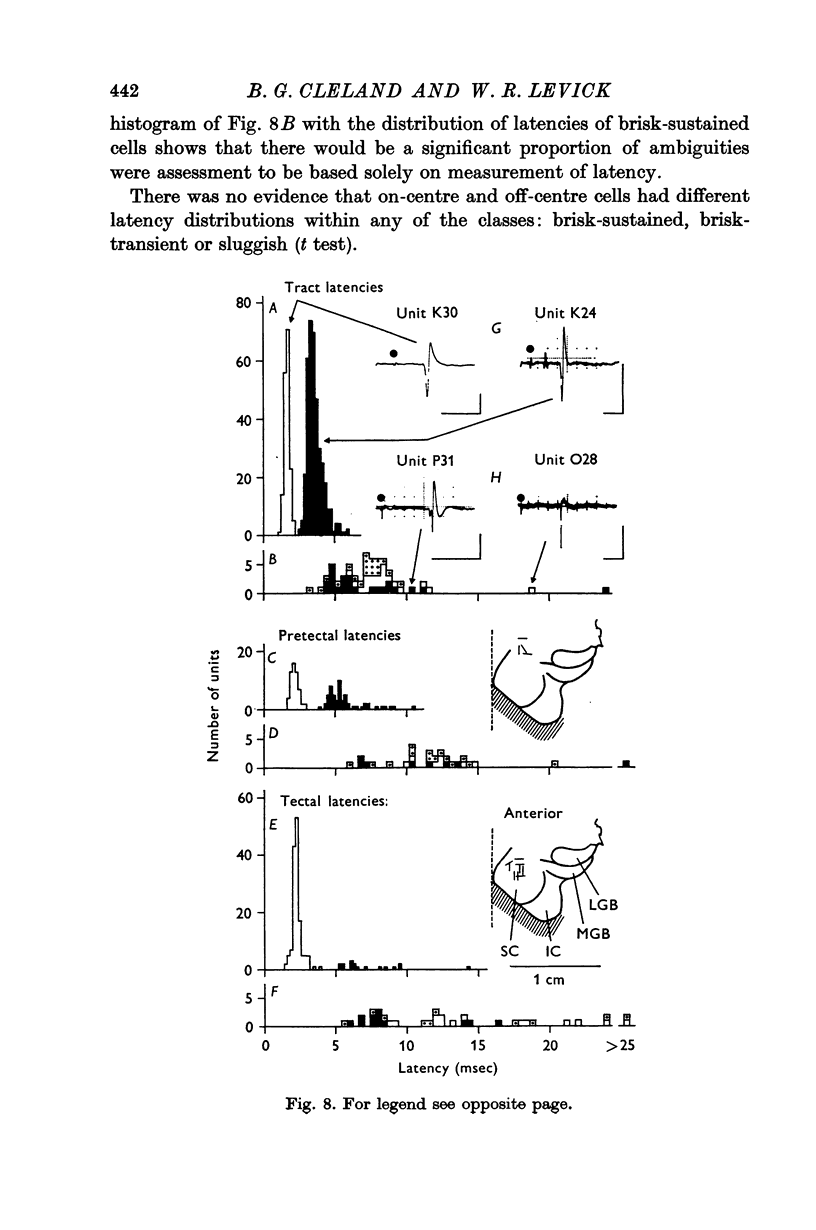
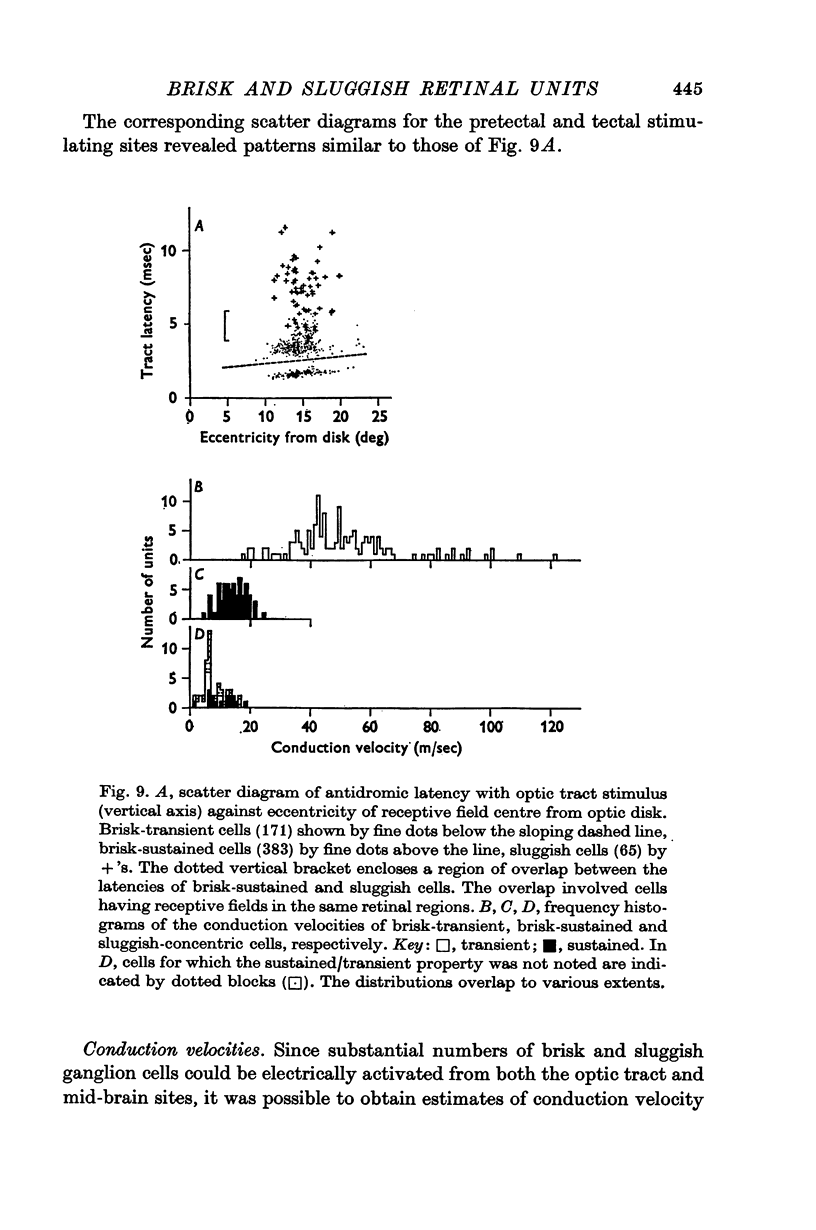
4. Measurements of latency to antidromic activation of retinal axons confirmed earlier work in showing that cells classed as brisk-transient had the shortest conduction times from the optic tract. Cells classed as brisk-sustained had intermediate conduction times and from earlier work would constitute an important input to the lateral geniculate nucleus. A proportion of the brisk-sustained axons reached the pretectal region (especially on-centre types) and a small minority reached the superior colliculus (especially off-centre types).
5. Sluggish cells had generally slower antidromic conduction times; despite some overlap with the brisk-sustained class, the slower conduction provided independent support for the functional differentiation. Sluggish axons reached the pretectal region and superior colliculus.
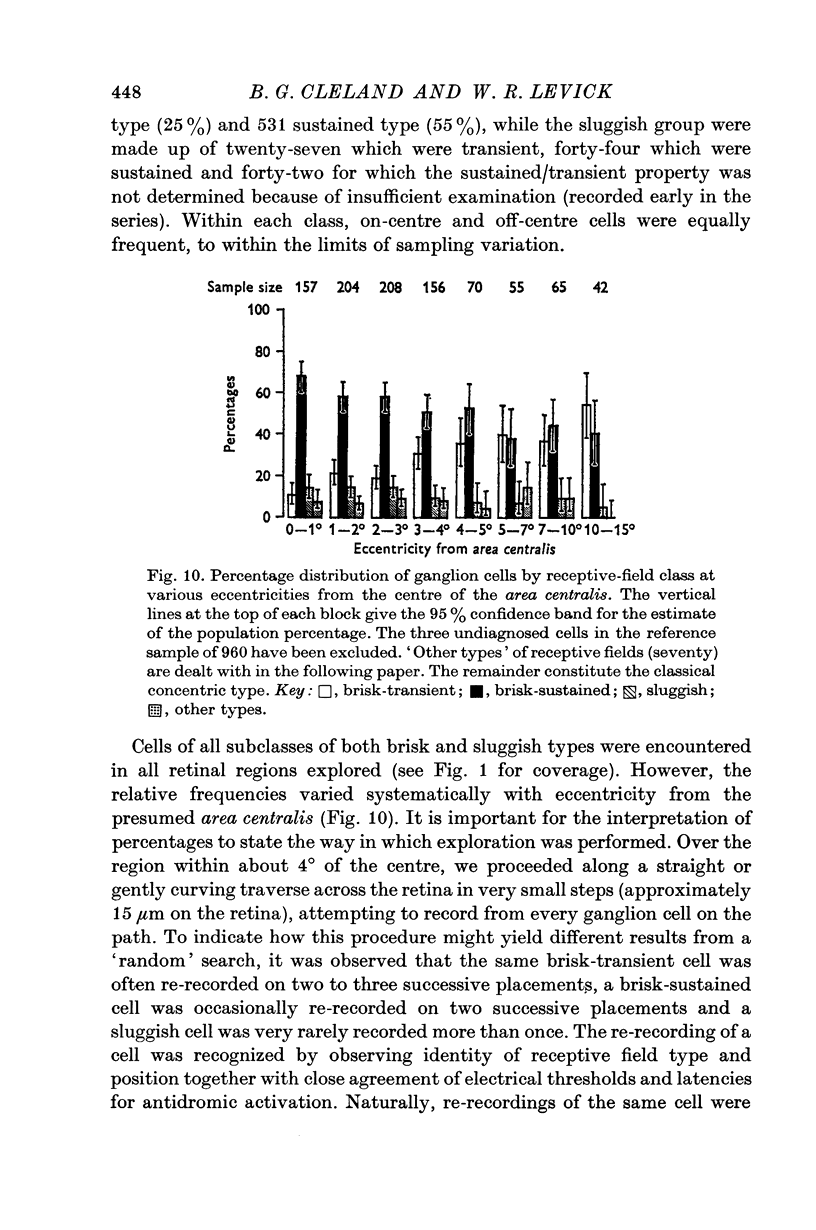
6. The brisk-sustained cells constituted the majority of the recordings in the area centralis.
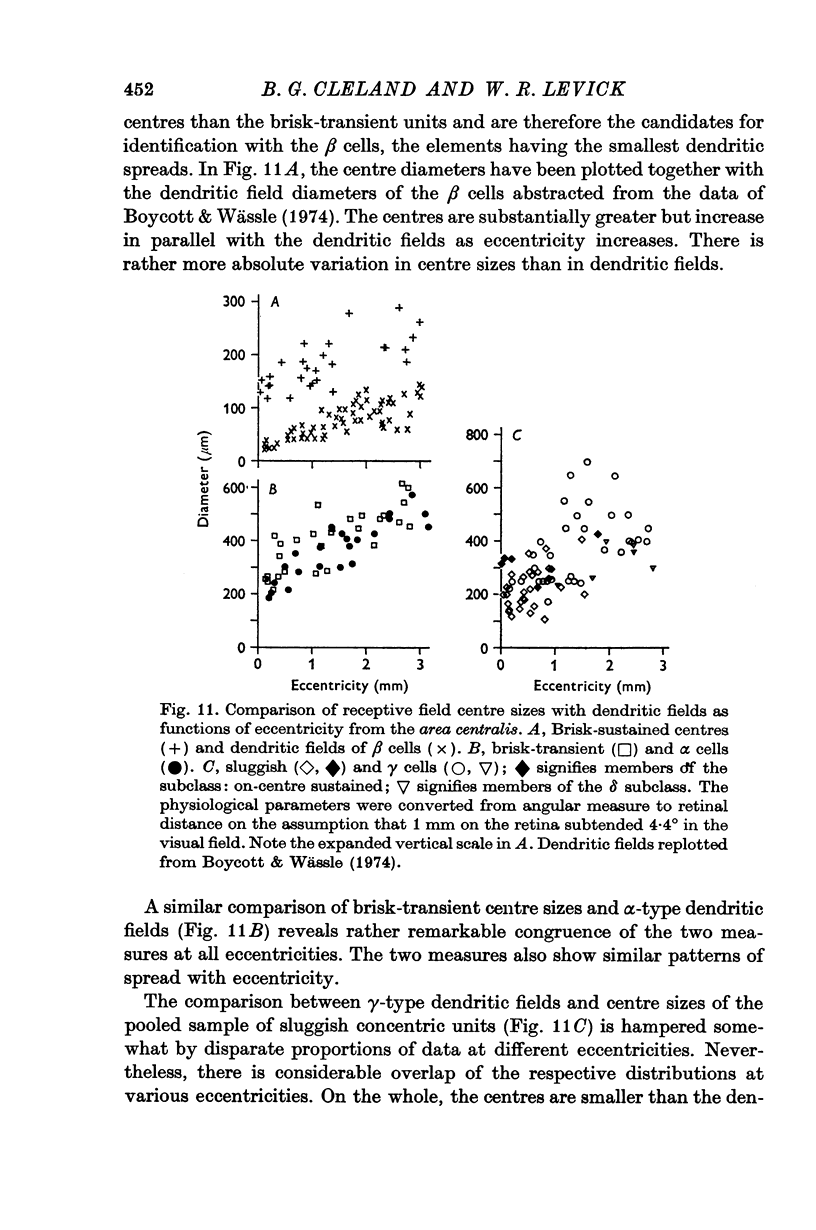
7. A comparison with the morphological data of Boycott & Wässle is made which suggests that the brisk-transient units corresponded with α cells, the brisk-sustained with β cells, and the sluggish units were included amongst the γ cells.
Full text
PDF
























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. Single-unit recording from antidromically activated optic radiation neurones. J Physiol. 1962 Aug;162:432–450. doi: 10.1113/jphysiol.1962.sp006943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., LEVICK W. R., VAKKUR G. J. The determination of the projection of the visual field on to the lateral geniculate nucleus in the cat. J Physiol. 1962 Oct;163:503–539. doi: 10.1113/jphysiol.1962.sp006991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., VAKKUR G. J. Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol. 1962 Oct;163:466–502. doi: 10.1113/jphysiol.1962.sp006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G. H., Clare M. H., Landau W. M. Further analysis of fiber groups in the optic tract of the cat. Exp Neurol. 1969 Jul;24(3):386–399. doi: 10.1016/0014-4886(69)90144-7. [DOI] [PubMed] [Google Scholar]
- Boycott B. B., Wässle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol. 1974 Jul;240(2):397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Properties of rarely encountered types of ganglion cells in the cat's retina and an overall classification. J Physiol. 1974 Jul;240(2):457–492. doi: 10.1113/jphysiol.1974.sp010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Sanderson K. J. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973 Feb;228(3):649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldon P., Kruger L. Topography of the retinal projection upon the superior colliculus of the cat. Vision Res. 1970 Feb;10(2):135–143. doi: 10.1016/0042-6989(70)90111-2. [DOI] [PubMed] [Google Scholar]
- Fernald R., Chase R. An improved method for plotting retinal landmarks and focusing the eyes. Vision Res. 1971 Jan;11(1):95–96. doi: 10.1016/0042-6989(71)90207-0. [DOI] [PubMed] [Google Scholar]
- Fukada Y. Receptive field organization of cat optic nerve fibers with special reference to conduction velocity. Vision Res. 1971 Mar;11(3):209–226. doi: 10.1016/0042-6989(71)90186-6. [DOI] [PubMed] [Google Scholar]
- GRANIT R. Centrifugal and antidromic effects on ganglion cells of retina. J Neurophysiol. 1955 Jul;18(4):388–411. doi: 10.1152/jn.1955.18.4.388. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Powell T. P. The projection of the retina in the cat. J Anat. 1968 Jan;102(Pt 2):189–222. [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Neurons in the retina; organization, inhibition and excitation problems. Cold Spring Harb Symp Quant Biol. 1952;17:281–292. doi: 10.1101/sqb.1952.017.01.026. [DOI] [PubMed] [Google Scholar]
- LEVICK W. R., OYSTER C. W., DAVIS D. L. EVIDENCE THAT MCILWAIN'S PERIPHERY EFFECT IS NOT A STRAY LIGHT ARTIFACT. J Neurophysiol. 1965 May;28:555–559. doi: 10.1152/jn.1965.28.3.555. [DOI] [PubMed] [Google Scholar]
- LOEWENFELD I. E., ALTMAN R. Variations of Horsley-Clarke coordinates in cat brains; with description of a stereotaxic instrument especially useful in neuro-ophthalmological work. J Neuropathol Exp Neurol. 1956 Apr;15(2):181–189. doi: 10.1097/00005072-195604000-00003. [DOI] [PubMed] [Google Scholar]
- Laties A. M., Sprague J. M. The projection of optic fibers to the visual centers in the cat. J Comp Neurol. 1966 May;127(1):35–70. doi: 10.1002/cne.901270104. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Levick W. R., Zacks J. L. Responses of cat retinal ganglion cells to brief flashes of light. J Physiol. 1970 Mar;206(3):677–700. doi: 10.1113/jphysiol.1970.sp009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCILWAIN J. T. RECEPTIVE FIELDS OF OPTIC TRACT AXONS AND LATERAL GENICULATE CELLS: PERIPHERAL EXTENT AND BARBITURATE SENSITIVITY. J Neurophysiol. 1964 Nov;27:1154–1173. doi: 10.1152/jn.1964.27.6.1154. [DOI] [PubMed] [Google Scholar]
- Saito H. A., Shimahara T., Fukada Y. Four types of responses to light and dark spot stimuli in the cat optic nerve. Tohoku J Exp Med. 1970 Oct;102(2):127–133. doi: 10.1620/tjem.102.127. [DOI] [PubMed] [Google Scholar]
- Stone J., Freeman R. B., Jr Conduction velocity groups in the cat's optic nerve classified according to their retinal origin. Exp Brain Res. 1971 Nov 30;13(5):489–497. doi: 10.1007/BF00234279. [DOI] [PubMed] [Google Scholar]
- VAKKUR G. J., BISHOP P. O., KOZAK W. VISUAL OPTICS IN THE CAT, INCLUDING POSTERIOR NODAL DISTANCE AND RETINAL LANDMARKS. Vision Res. 1963 Nov;61:289–314. doi: 10.1016/0042-6989(63)90004-x. [DOI] [PubMed] [Google Scholar]
- Venes J. L., Collins W. F., Taub A. Nitrous oxide: an anesthetic for experiments in cats. Am J Physiol. 1971 Jun;220(6):2028–2031. doi: 10.1152/ajplegacy.1971.220.6.2028. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]


