Abstract
1. In a reference sample of 960 cat retinal ganglion cells, seventy-three had receptive fields departing from the concentric centre—surround pattern.
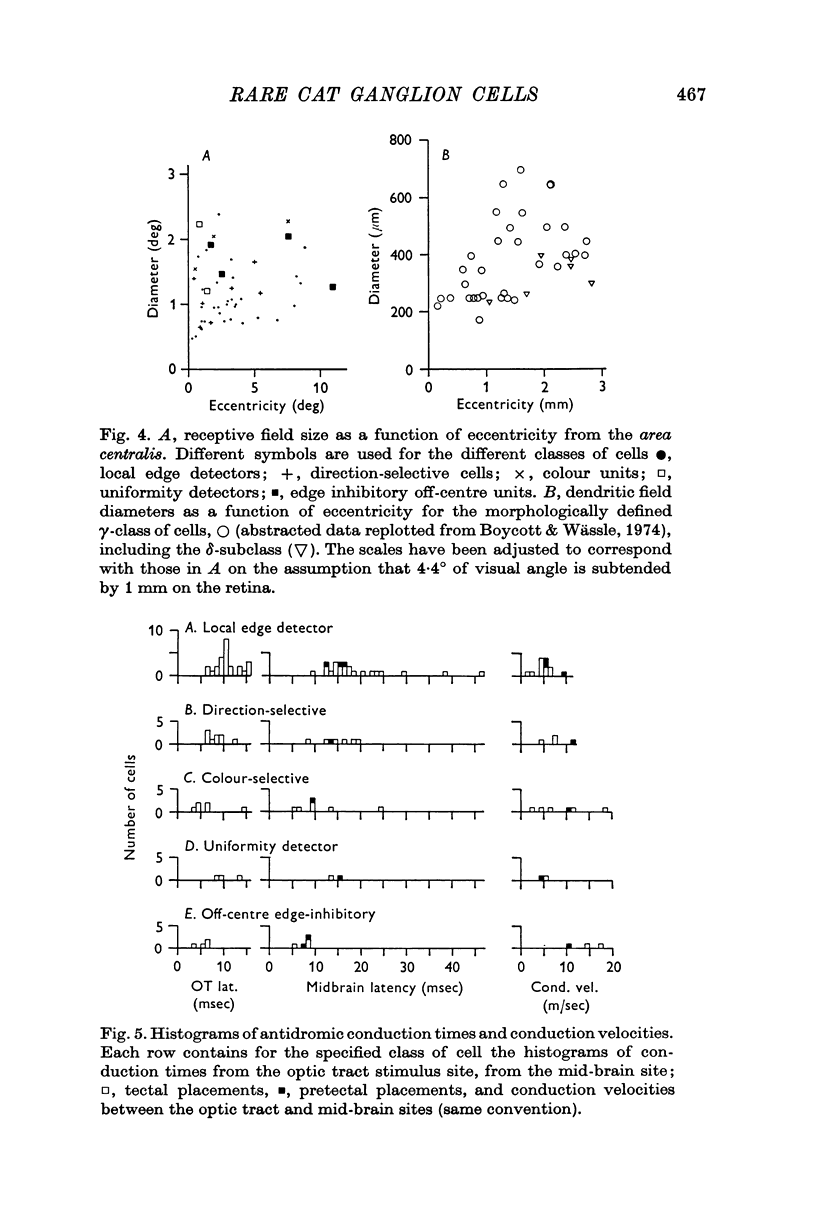
2. Five classes were distinguished among the subset: local edge detectors, direction-selective cells, colour-coded cells, uniformity detectors, edge inhibitory off-centre cells.
3. Local edge detectors (forty-five) possessed a radially symmetrical pattern of responses to both centrifugal and centripetal movements of both black and white small targets, an on—off receptive field with a silent inhibitory surround and a low or zero maintained discharge. Their operation could be interpreted as the detection of a contrasting border confined to a small region of the visual field.
4. With direction-selective units (eleven) it was possible to find an axis through the receptive field along which sharply different responses could be obtained for opposite directions of movement of small black or white targets.
5. Colour units (six) were mostly of the single opponent type having excitatory input from blue-sensitive cones and inhibitory input from long wave-length cones. Both inputs coexisted at the centre of the field and either could be spatially more extensive than the other. One example changed over to rod input under scotopic conditions, another did not.
6. Uniformity detectors (five) had a brisk maintained discharge which was reduced or abolished temporarily by all forms of visual stimulation.
7. Edge inhibitory off-centre units (three) behaved like uniformity detectors for small targets and fine gratings but like off-centre on-surround units for large targets. Their receptive fields consisted of three concentric regions: a small sized, central edge inhibitory region; a larger zone of off-responsiveness; and an outlying annulus of on-responsiveness.
8. It is argued that the above physiological types belong to the morphologically heterogeneous class of cells called γ cells. The argument is based on similarity in the sizes of receptive fields and dendritic trees and on evidence that the axons are thinner than those of the brisk-sustained and brisk-transient ganglion cells.
9. The physiological classification of cat retinal ganglion cells developed in this paper and the preceding one is summarized in a Table.
10. It now appears that cat and rabbit possess a qualitatively similar complement of receptive field types among their ganglion cells; the differences reside in the quantitative expression of the various classes.
Full text
PDF


































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews D. P., Hammond P. Mesopic increment threshold spectral sensitivity of single optic tract fibres in the cat: cone-rod interaction. J Physiol. 1970 Jul;209(1):65–81. doi: 10.1113/jphysiol.1970.sp009156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Dark adaptation, absolute threshold and Purkinje shift in single units of the cat's retina. J Physiol. 1957 Aug 6;137(3):327–337. doi: 10.1113/jphysiol.1957.sp005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Summation and inhibition in the frog's retina. J Physiol. 1953 Jan;119(1):69–88. doi: 10.1113/jphysiol.1953.sp004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., LEVICK W. R., VAKKUR G. J. The determination of the projection of the visual field on to the lateral geniculate nucleus in the cat. J Physiol. 1962 Oct;163:503–539. doi: 10.1113/jphysiol.1962.sp006991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN K. T., WIESEL T. N. Intraretinal recording with micropipette electrodes in the intact cat eye. J Physiol. 1959 Dec;149:537–562. doi: 10.1113/jphysiol.1959.sp006360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965 Jun;178(3):477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott B. B., Wässle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol. 1974 Jul;240(2):397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Sanderson K. J. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973 Feb;228(3):649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W. Colour-coded ganglion cells in the goldfish retina: extension of their receptive fields by means of new stimuli. J Physiol. 1968 Aug;197(3):567–592. doi: 10.1113/jphysiol.1968.sp008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: evidence for more than one cone process. J Physiol. 1970 Nov;211(1):125–137. doi: 10.1113/jphysiol.1970.sp009270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: one cone process or several? J Physiol. 1969 May;201(3):745–764. doi: 10.1113/jphysiol.1969.sp008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Integrative action in the cat's lateral geniculate body. J Physiol. 1961 Feb;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of optic nerve fibres in the spider monkey. J Physiol. 1960 Dec;154:572–580. doi: 10.1113/jphysiol.1960.sp006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. The outer disinhibitory surround of the retinal ganglion cell receptive field. J Physiol. 1972 Oct;226(2):511–544. doi: 10.1113/jphysiol.1972.sp009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Neurons in the retina; organization, inhibition and excitation problems. Cold Spring Harb Symp Quant Biol. 1952;17:281–292. doi: 10.1101/sqb.1952.017.01.026. [DOI] [PubMed] [Google Scholar]
- LEVICK W. R., OYSTER C. W., DAVIS D. L. EVIDENCE THAT MCILWAIN'S PERIPHERY EFFECT IS NOT A STRAY LIGHT ARTIFACT. J Neurophysiol. 1965 May;28:555–559. doi: 10.1152/jn.1965.28.3.555. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Receptive fields and trigger features of ganglion cells in the visual streak of the rabbits retina. J Physiol. 1967 Feb;188(3):285–307. doi: 10.1113/jphysiol.1967.sp008140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATURANA H. R., LETTVIN J. Y., MCCULLOCH W. S., PITTS W. H. Anatomy and physiology of vision in the frog (Rana pipiens). J Gen Physiol. 1960 Jul;43(6):129–175. doi: 10.1085/jgp.43.6.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCILWAIN J. T. RECEPTIVE FIELDS OF OPTIC TRACT AXONS AND LATERAL GENICULATE CELLS: PERIPHERAL EXTENT AND BARBITURATE SENSITIVITY. J Neurophysiol. 1964 Nov;27:1154–1173. doi: 10.1152/jn.1964.27.6.1154. [DOI] [PubMed] [Google Scholar]
- Maffei L. Inhibitory and facilitatory spatial interactions in retinal receptive fields. Vision Res. 1968 Sep;8(9):1187–1194. doi: 10.1016/0042-6989(68)90026-6. [DOI] [PubMed] [Google Scholar]
- Michael C. R. Receptive fields of single optic nerve fibers in a mammal with an all-cone retina. 3. Opponent color units. J Neurophysiol. 1968 Mar;31(2):268–282. doi: 10.1152/jn.1968.31.2.268. [DOI] [PubMed] [Google Scholar]
- Michael C. R. Receptive fields of single optic nerve fibers in a mammal with an all-cone retina. II: directionally selective units. J Neurophysiol. 1968 Mar;31(2):257–267. doi: 10.1152/jn.1968.31.2.257. [DOI] [PubMed] [Google Scholar]
- Oyster C. W. The analysis of image motion by the rabbit retina. J Physiol. 1968 Dec;199(3):613–635. doi: 10.1113/jphysiol.1968.sp008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman A. L., Daw N. W. Opponent color cells in the cat lateral geniculate nucleus. Science. 1970 Jan 2;167(3914):84–86. doi: 10.1126/science.167.3914.84. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W. Receptive fields in the cat retina: a new type. Science. 1967 Jul 7;157(3784):90–92. doi: 10.1126/science.157.3784.90. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Response of cat retinal ganglion cells to moving visual patterns. J Neurophysiol. 1965 Sep;28(5):819–832. doi: 10.1152/jn.1965.28.5.819. [DOI] [PubMed] [Google Scholar]
- Scobey R. P., Horowitz J. M. The detection of small image displacements by cat retinal ganglion cells. Vision Res. 1972 Dec;12(12):2133–2143. doi: 10.1016/0042-6989(72)90062-4. [DOI] [PubMed] [Google Scholar]
- Spinelli D. N. Receptive field organization of ganglion cells in the cat's retina. Exp Neurol. 1967 Nov;19(3):291–315. doi: 10.1016/0014-4886(67)90027-1. [DOI] [PubMed] [Google Scholar]
- Spinelli D. N. Visual receptive fields in the cat's retina: complications. Science. 1966 Jun 24;152(3730):1768–1768. doi: 10.1126/science.152.3730.1768. [DOI] [PubMed] [Google Scholar]
- Stone J., Fabian M. Specialized receptive fields of the cat's retina. Science. 1966 May 27;152(3726):1277–1279. doi: 10.1126/science.152.3726.1277. [DOI] [PubMed] [Google Scholar]
- Stone J., Hoffmann K. P. Very slow-conducting ganglion cells in the cat's retina: a major, new functional type? Brain Res. 1972 Aug 25;43(2):610–616. doi: 10.1016/0006-8993(72)90416-7. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Winters R. W., Walters J. W. Transient and steady state stimulus-response relations for cat retinal ganglion cells. Vision Res. 1970 Jun;10(6):461–477. doi: 10.1016/0042-6989(70)90003-9. [DOI] [PubMed] [Google Scholar]
- Yoon M. Reversal of Weber's law for an extraordinary unit in the cat's retina. Vision Res. 1970 Aug;10(8):769–774. doi: 10.1016/0042-6989(70)90020-9. [DOI] [PubMed] [Google Scholar]



