Abstract
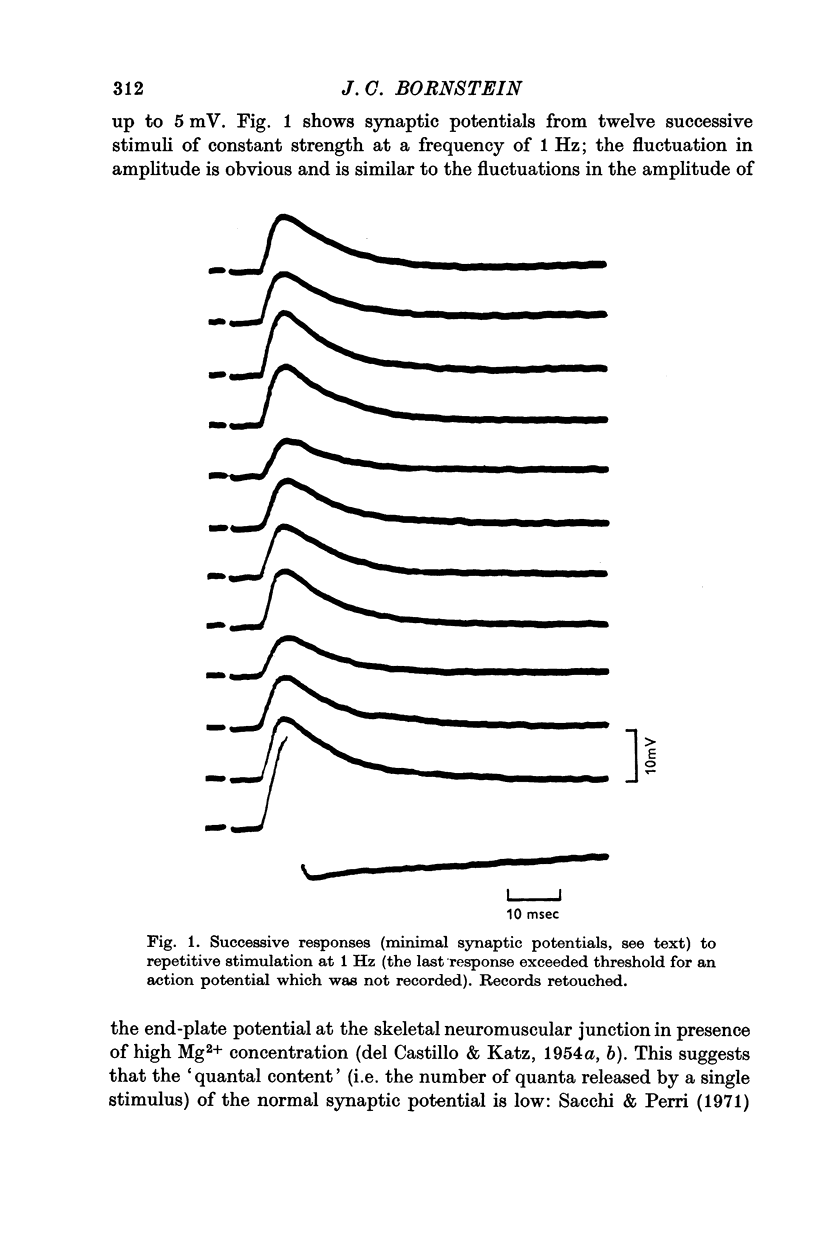
1. Synaptic potentials were recorded with intracellular electrodes from cells in the inferior mesenteric ganglion of the guinea-pig.
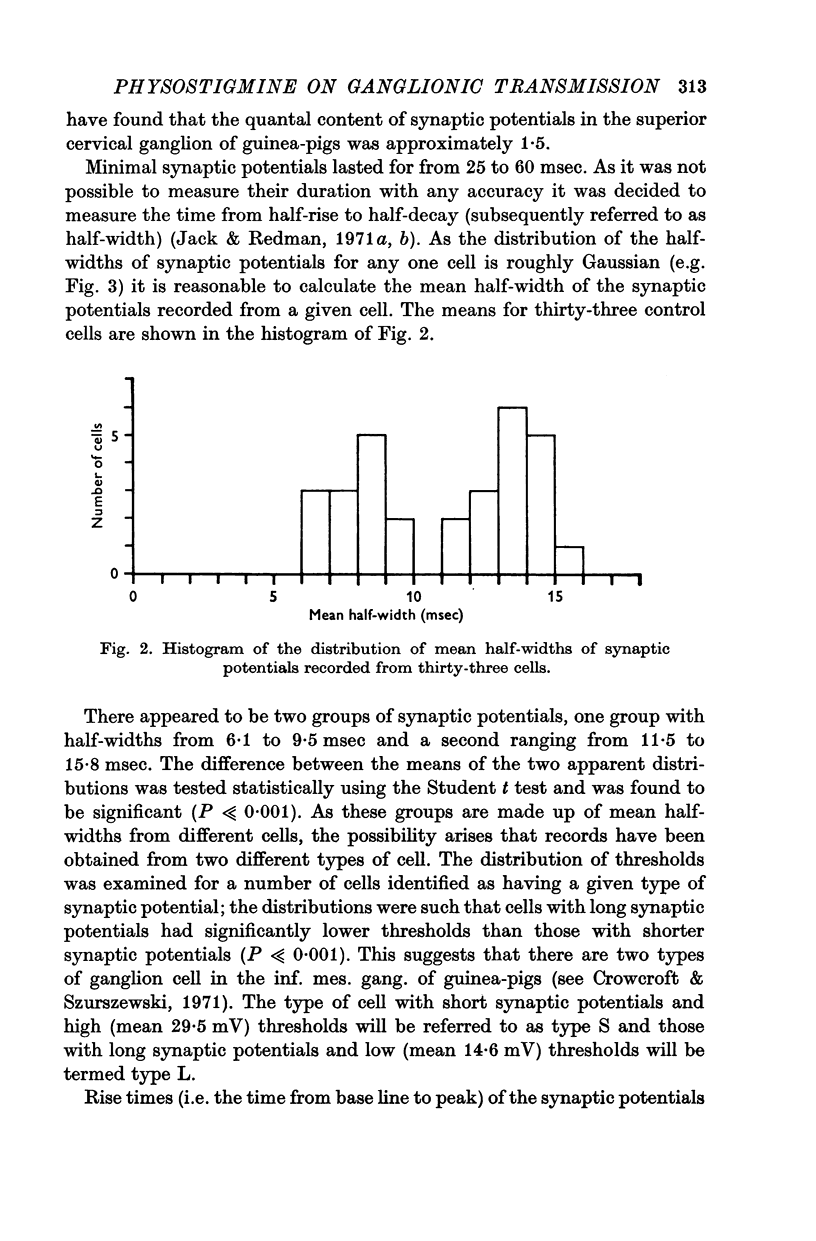
2. Half-widths of the synaptic potentials recorded fell into two groups: type L cells had long synaptic potentials (11·6-15·2 msec) and low thresholds (14·6 mV mean), type S cells had short synaptic potentials (6·1-9·3 msec) and high thresholds (29·9 mV mean).
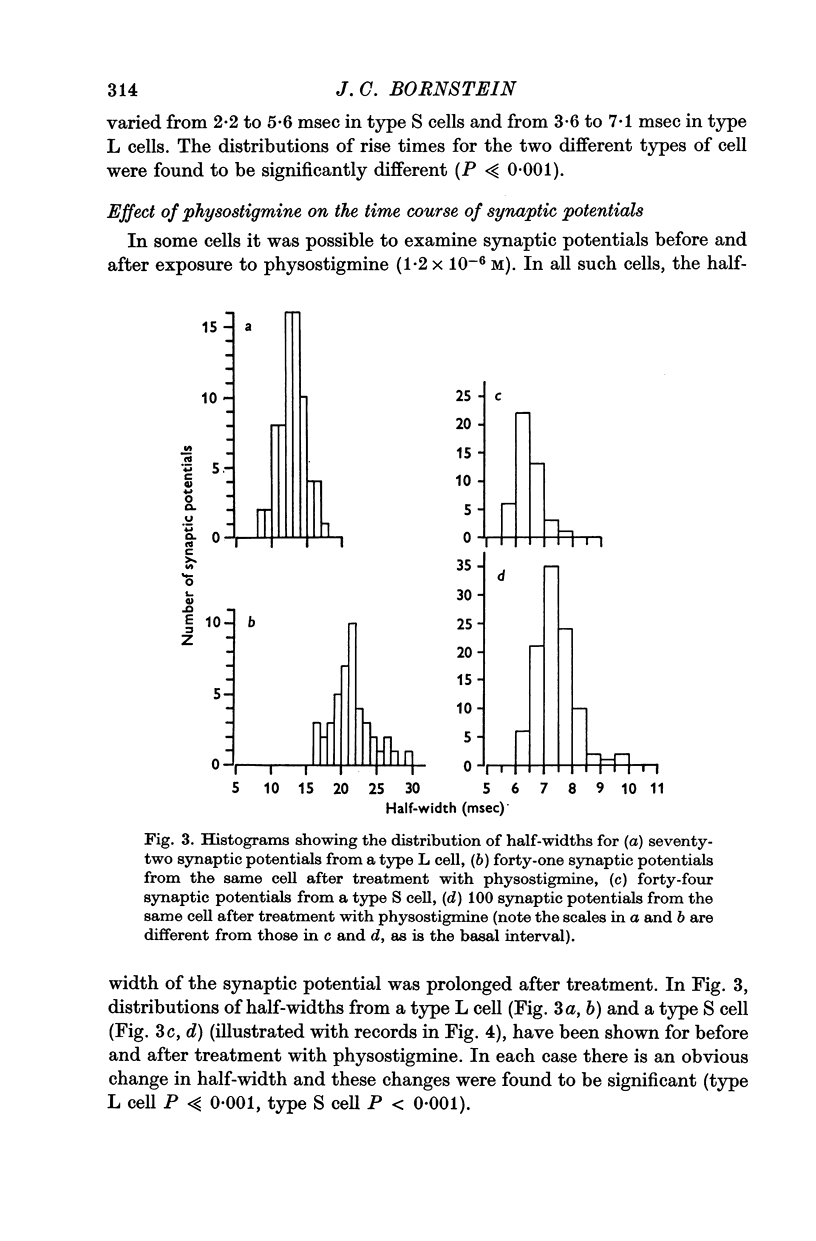
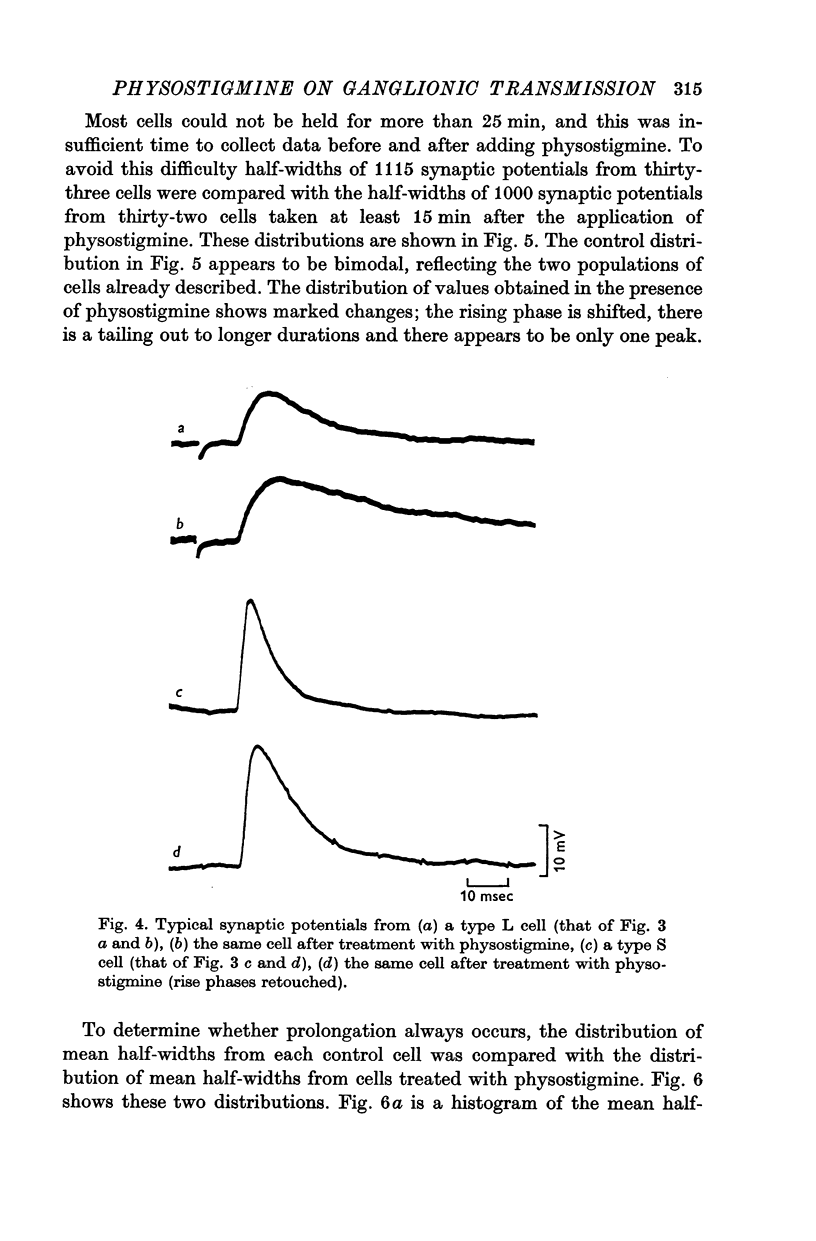
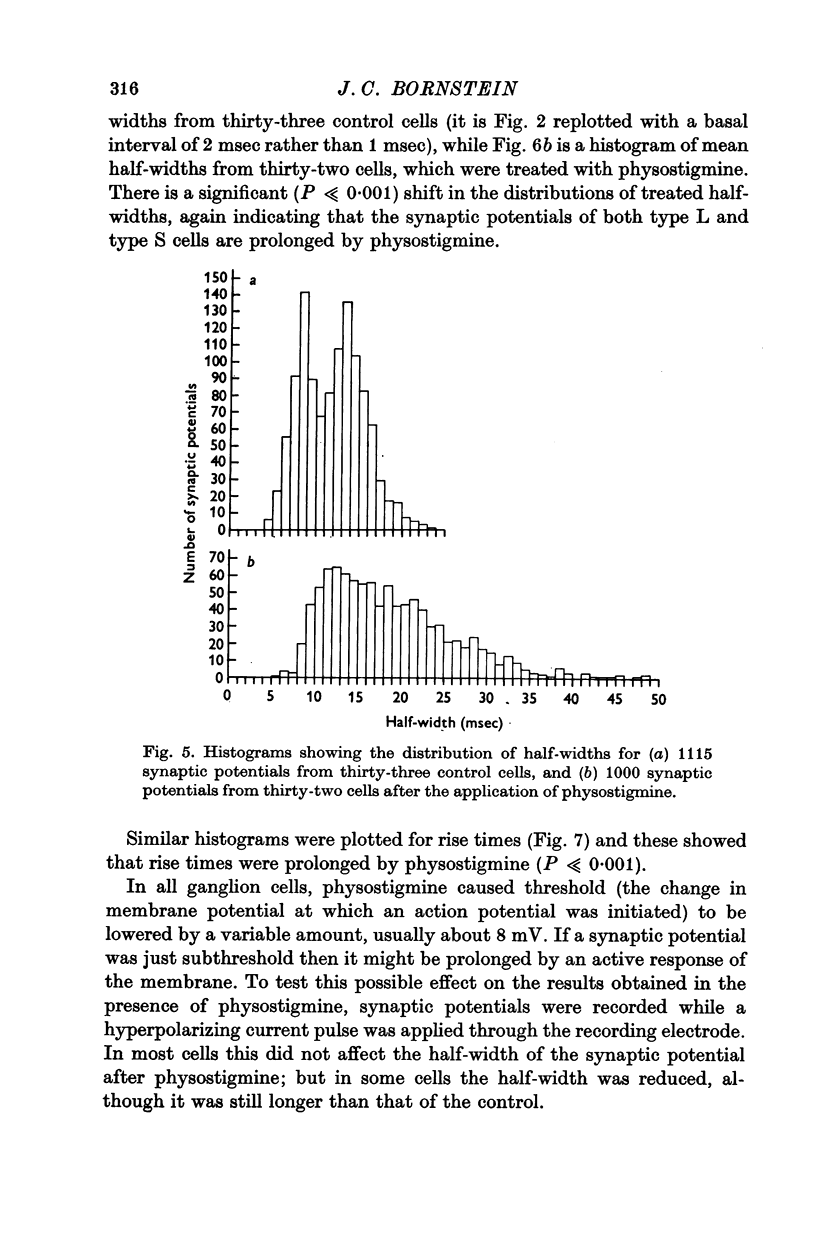
3. Physostigmine (1·2 × 10-6 M) caused a significant increase in the half-width of both types of synaptic potential.
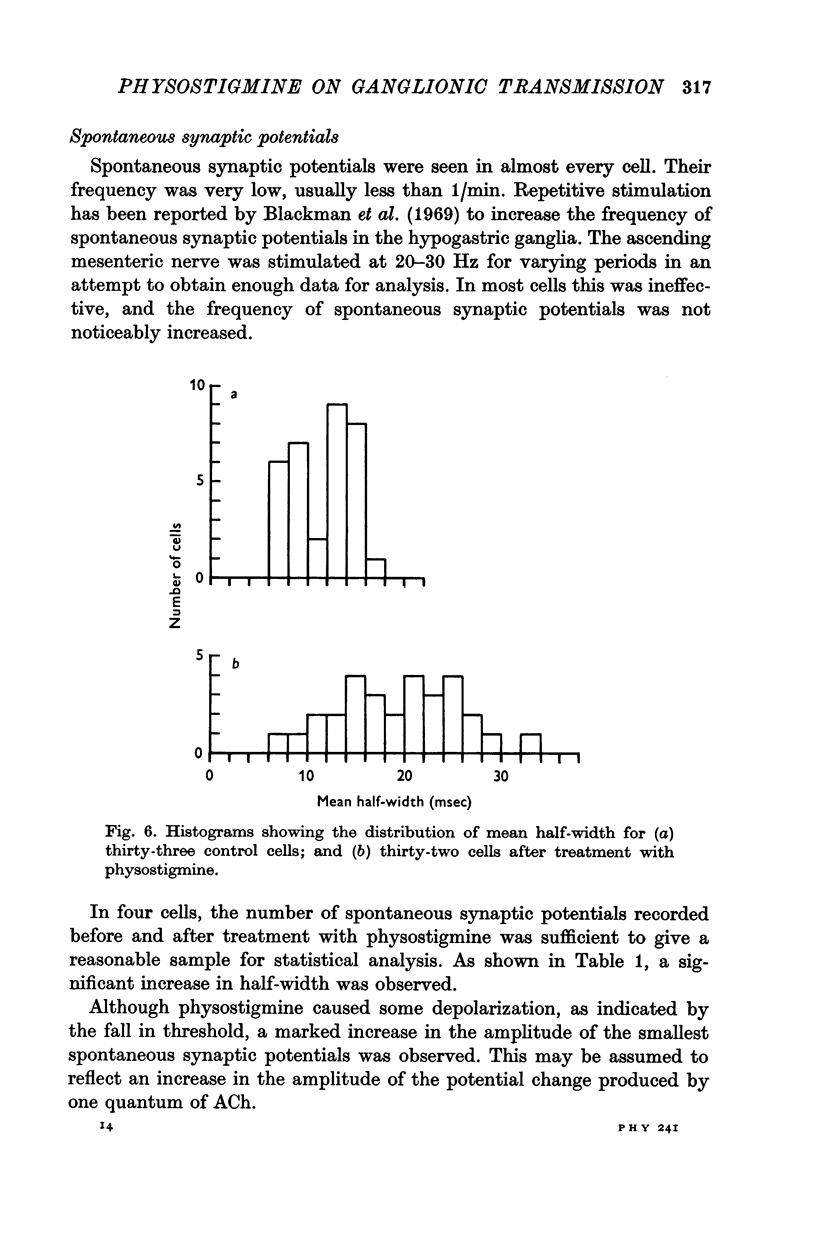
4. Physostigmine caused a significant increase in the half-width of spontaneous synaptic potentials and an increase in their amplitude.
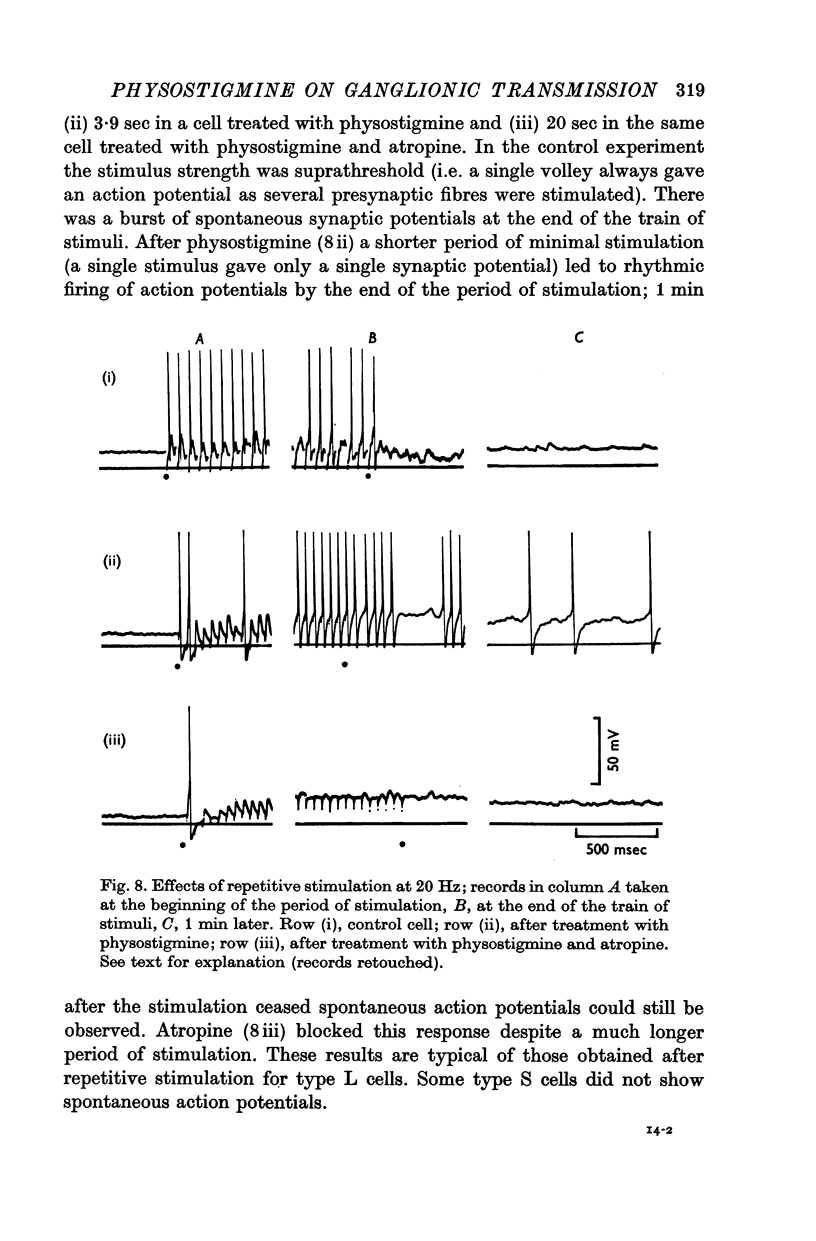
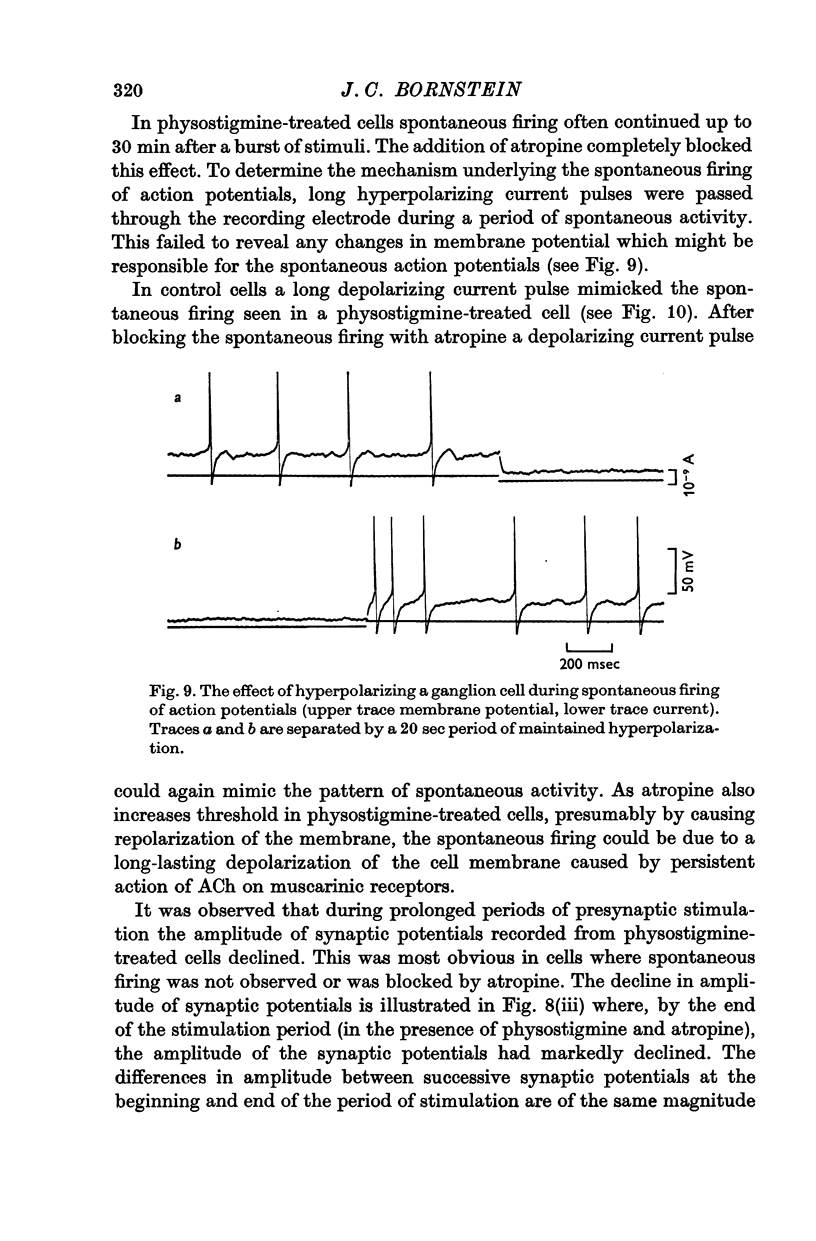
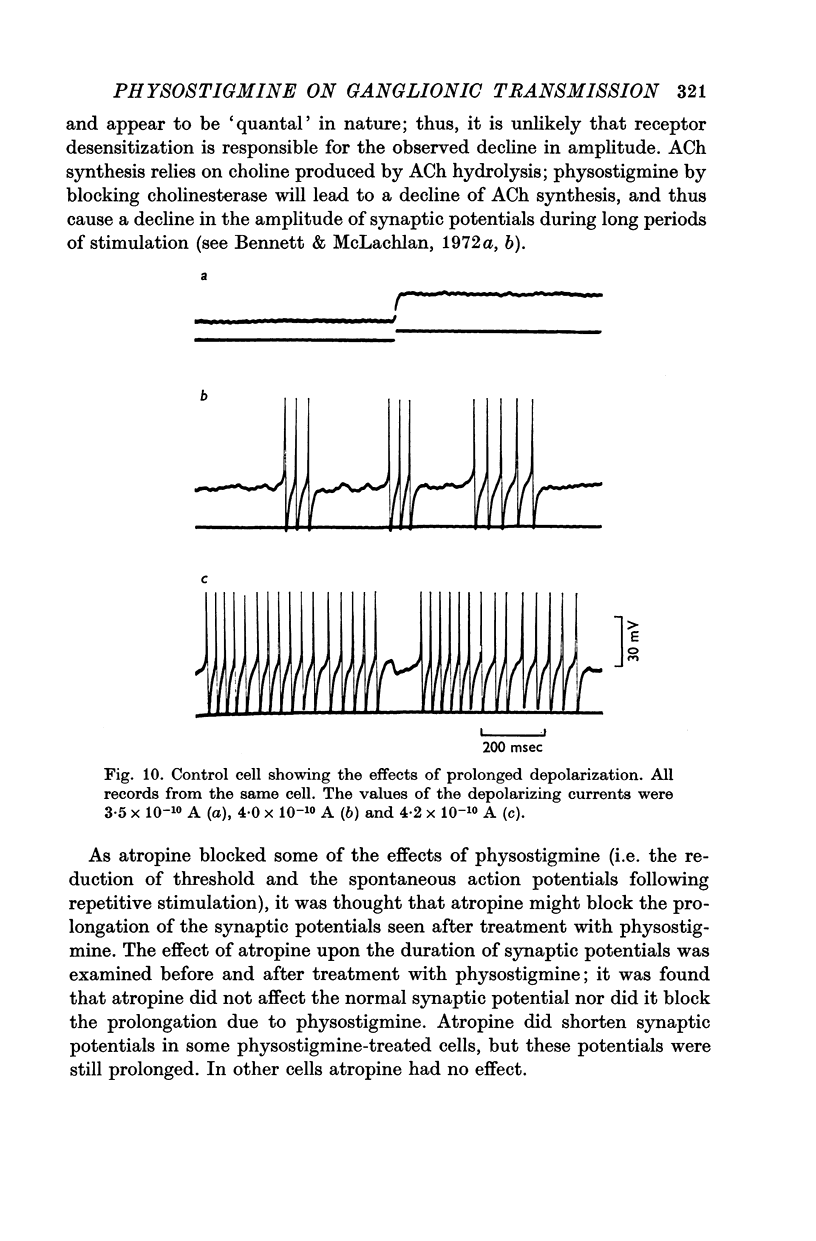
5. Repetitive preganglionic stimulation, in the presence of physostigmine, led to a marked and prolonged depolarization in all cells. In most cells repetitive spontaneous firing of action potentials was then observed. This effect was blocked by atropine (1·4 × 10-7 M).
6. The effect of atropine on the half-width in a physostigmine-treated cell was inconsistent: although synaptic potentials in some cells were slightly shortened their half-widths were always greater than the control.
7. It is concluded that cholinesterase plays a role in limiting the time course of the synaptic potential, by limiting the duration of action of acetylcholine.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACKMAN J. G., GINSBORG B. L., RAY C. Spontaneous synaptic activity in sympathetic ganglion cells of the frog. J Physiol. 1963 Jul;167:389–401. doi: 10.1113/jphysiol.1963.sp007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKMAN J. G., GINSBORG B. L., RAY C. Synaptic transmission in the sympathetic ganglion of the frog. J Physiol. 1963 Jul;167:355–373. doi: 10.1113/jphysiol.1963.sp007155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M. An electrophysiological analysis of the storage of acetylcholine in preganglionic nerve terminals. J Physiol. 1972 Mar;221(3):657–668. doi: 10.1113/jphysiol.1972.sp009774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M. An electrophysiological analysis of the synthesis of acetylcholine in preganglionic nerve terminals. J Physiol. 1972 Mar;221(3):669–682. doi: 10.1113/jphysiol.1972.sp009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Crowcroft P. J., Devine C. E., Holman M. E., Yonemura K. Transmission from pregnanglionic fibres in the hypogastric nerve to peripheral ganglia of male guinea-pigs. J Physiol. 1969 May;201(3):723–743. doi: 10.1113/jphysiol.1969.sp008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowcroft P. J., Szurszewski J. H. A study of the inferior mesenteric and pelvic ganglia of guinea-pigs with intracellular electrodes. J Physiol. 1971 Dec;219(2):421–441. doi: 10.1113/jphysiol.1971.sp009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- Eccles J. C. Facilitation and inhibition in the superior cervical ganglion. J Physiol. 1935 Oct 26;85(2):207–238.3. doi: 10.1113/jphysiol.1935.sp003314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C. The discharge of impulses from ganglion cells. J Physiol. 1937 Oct 18;91(1):1–22. doi: 10.1113/jphysiol.1937.sp003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C. The nature of synaptic transmission in a sympathetic ganglion. J Physiol. 1944 Jun 15;103(1):27–54. doi: 10.1113/jphysiol.1944.sp004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Gaddum J. H. The chemical transmitter at synapses in a sympathetic ganglion. J Physiol. 1934 Jun 9;81(3):305–319. doi: 10.1113/jphysiol.1934.sp003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Vartiainen A. Further observations on the physiology and pharmacology of a sympathetic ganglion. J Physiol. 1934 Dec 14;83(1):103–128. doi: 10.1113/jphysiol.1934.sp003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis R. A., Flacke W., Garfield J. M., Alper M. H. Actions of anticholinesterase agents upon ganglionic transmission in the dog. J Pharmacol Exp Ther. 1968 Oct;163(2):277–286. [PubMed] [Google Scholar]
- Jack J. J., Redman S. J. An electrical description of the motoneurone, and its application to the analysis of synaptic potentials. J Physiol. 1971 Jun;215(2):321–352. doi: 10.1113/jphysiol.1971.sp009473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J. The propagation of transient potentials in some linear cable structures. J Physiol. 1971 Jun;215(2):283–320. doi: 10.1113/jphysiol.1971.sp009472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOELLE W. A., KOELLE G. B. The localization of external or functional acetylcholinesterase at the synapses of autonomic ganglia. J Pharmacol Exp Ther. 1959 May;126(1):1–8. [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Libet B. Actions of noradrenaline and acetylcholine on sympathetic ganglion cells. J Physiol. 1970 Jun;208(2):353–372. doi: 10.1113/jphysiol.1970.sp009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz H. W., Lees G. M., Wallis D. I. Resting and action potentials recorded by the sucrose-gap method in the superior cervical ganglion of the rabbit. J Physiol. 1968 Mar;195(1):39–53. doi: 10.1113/jphysiol.1968.sp008445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McISAAC R. J., KOELLE G. B. Comparison of the effects of inhibition of external, internal and total acetylcholinesterase upon ganglionic transmission. J Pharmacol Exp Ther. 1959 May;126(1):9–20. [PubMed] [Google Scholar]
- McLENNAN H. Acetylcholine metabolism of normal and axotomized ganglia. J Physiol. 1954 Apr 28;124(1):113–116. doi: 10.1113/jphysiol.1954.sp005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S. Cholinergic and adrenergic receptors at sympathetic preganglionic nerve terminals. Fed Proc. 1970 Nov-Dec;29(6):1957–1965. [PubMed] [Google Scholar]
- OGSTON A. G. Removal of acetylcholine from a limited volume by diffusion. J Physiol. 1955 Apr 28;128(1):222–223. doi: 10.1113/jphysiol.1955.sp005300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi O., Perri V. Quantal release of acetylcholine from the nerve endings of the guinea-pig superior cervical ganglion. Pflugers Arch. 1971;329(3):207–219. doi: 10.1007/BF00586615. [DOI] [PubMed] [Google Scholar]
- TAKESHIGE C., VOLLE R. L. Bimodal response of sympathetic ganglia to acetylcholine following eserine or repetitive preganglionic stimulation. J Pharmacol Exp Ther. 1962 Oct;138:66–73. [PubMed] [Google Scholar]
- VOLLE R. L., KOELLE G. B. The physiological role of acetyl-cholinesterase (AChE) in sympathetic ganglia. J Pharmacol Exp Ther. 1961 Aug;133:223–240. [PubMed] [Google Scholar]
- VOLLE R. L. The responses to ganglionic stimulating and blocking drugs of cell groups within a sympathetic ganglion. J Pharmacol Exp Ther. 1962 Jan;135:54–61. [PubMed] [Google Scholar]


