Abstract
Using monoclonal antibodies and human affinity-purified antibodies specific to the Plasmodium falciparum 126-kDa serine-rich protein, SERP, we found that these antibodies have no direct effect upon merozoite invasion at the concentrations tested but can cooperate with blood monocytes to strongly inhibit P. falciparum in vitro growth.
Since the original observation that immunization with a P140 protein purified from cultured P. falciparum parasites could confer protection in Saimiri monkeys (15), there has been a sustained interest in investigating the vaccine potential of this molecule, a 126-kDa serine-rich protein called SERA, SERP, or P126 (1) (referred to here as SERP). Further studies were carried out using two recombinant proteins of 262 (positions 24 to 285) and 483 (positions 24 to 506) amino acids from the N-terminal region of SERP which were used to immunize Aotus monkeys with Freund's adjuvant, i.e., under immunizing conditions which cannot be used in humans but which are optimal to induce strong immune responses (8). Challenge with blood-stage parasites revealed that a high degree of protection was obtained in four of six immunized monkeys (i.e., a 1,000-fold reduction in peak parasitemia), a level of protection among the highest reported for artificial immunization against malaria (10). Attempts to correlate the level of protection with immune responses were not conclusive. High antibody titers, as measured by enzyme-linked immunosorbent assay, were detected in three of the protected monkeys, but similar levels of antibody titers were also found among those less or not protected (8). Since a SERP-specific monoclonal antibody (MAb), 43E5, which reacts with the N-terminal region of the molecule was found to directly inhibit the growth of P. falciparum in vitro (2), the Aotus sera were studied for growth inhibition. However, sera containing high levels of SERP antibodies from immunized Aotus were not able to inhibit P. falciparum multiplication in vitro (J. Inselburg et al., unpublished data).
It was previously demonstrated that protection in humans was mediated by antibodies which have no significant effect upon merozoite invasion but which in contrast inhibited growth indirectly by cooperating with blood monocytes (3). This mechanism, called antibody-dependent cell inhibition (ADCI), is mediated by soluble components released by monocytes which block the division of intraerythrocytic parasites and is triggered by merozoite surface components (4). This has led us to identify a new merozoite surface protein, MSP3, as a main target of ADCI-effective antibodies (11). We recently demonstrated that natural antibodies specific to another parasite antigen, glutamate-rich protein, can also inhibit P. falciparum multiplication through ADCI (17). We therefore thought it of interest to investigate whether the antibodies raised against SERP were implicated in ADCI. To this end we tested a panel of human and mouse antibodies in parallel assays of direct and ADCI-mediated parasite growth inhibition.
The direct merozoite invasion inhibition and ADCI assays were performed as previously described (3, 9). Blood mononuclear cells from healthy donors, separated on Ficoll-Hypaque, were distributed into 96-well flat-bottom plates (TPP, Trasadingen, Switzerland) at a rate of 2 × 105 monocytes per well. After 1 h of incubation at 37°C in a 5% CO2-air mixture, nonadherent cells were removed by washings with RPMI. Monocyte viability was estimated by the nonspecific esterase stain. P. falciparum cultures at the schizont stage were added at a ratio of 200 red blood cells per monocyte. The culture medium (RPMI plus 10% Albumax) was supplemented with each of the antibodies to be tested, including positive control immunoglobulin G (IgG) from African adults and negative control IgG from European donors, in wells with and without monocytes. Starting parasitemia was 0.5% with 2% hematocrit. Only assays in which the final parasitemia reached ≥10% were kept for analysis. The specific growth-inhibitory index (SGI) was calculated as follows: 100 × {1 − [(percent parasitemia with monocyte and test antibody/percent parasitemia with test antibody without monocytes)/(percent parasitemia with negative control antibody with monocytes/percent parasitemia with negative control antibody without monocytes)]}.
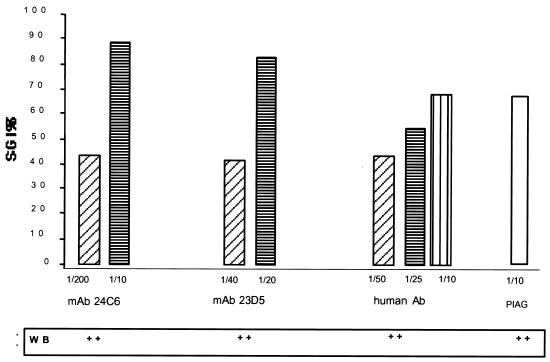
We first studied two MAbs, 24C6 and 23D5, which are specific for two distinct epitopes derived from the N-terminal region of SERP (6, 7) and which recognize the 126-kDa polypeptide on Western blots of P. falciparum asexual blood-stage extracts. Neither antibody showed a significant direct inhibitory effect in the absence of monocytes at the dilutions tested (data not shown). In contrast, ADCI-mediated parasite killing was clearly observed, and parasite growth inhibition was dependent on antibody concentrations (Fig. 1). Results were reproducible with P. falciparum strains NF54, T9-96, and FCIP-150. We then investigated the activity of naturally acquired antibodies specific to SERP. A recombinant protein, SE47′, corresponding to amino acid residues 17 to 382 of the N-terminal domain (16) was used to affinity purify specific antibodies from sera of African (Ivory Coast) immune adults (3) as described in reference 5. These antibodies, which were SERP specific (Fig. 2), had no direct effect on parasite growth but were able to exert a strong inhibitory ADCI-mediated effect in a dose-dependent manner (Fig. 1). Similar results (not shown) were obtained with affinity-purified antibodies from hyperimmune adults from Uganda (Fig. 2).
FIG. 1.
ADCI results obtained using anti-SERP antibodies. Results were obtained with two SERP MAbs and human antibodies affinity purified with the recombinant antigen SE47, at various concentrations (volume/volume in RPMI medium) in the ADCI assay. Results are expressed as the SGI as described in the text; values of >30% are significant. PIAG, positive control from the pool of Ivory Coast adult sera used in passive transfer in humans (3). WB, Western blotting; ++, positive.
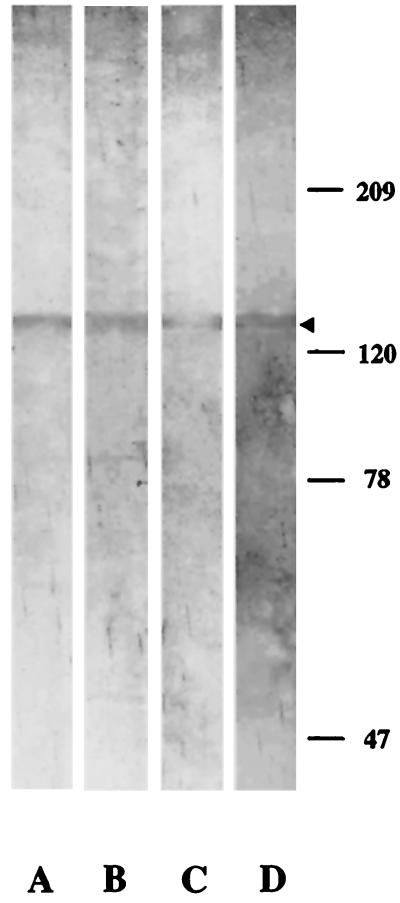
FIG. 2.
Western blot analysis of P. falciparum schizont protein extract using anti-SERP antibodies. Parasite proteins were resolved by sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis under denaturing conditions, electroblotted onto nitrocellulose, and probed with anti-SERP MAbs 24C6 (lane A) and 23D5 (lane B) and affinity-purified human anti-SERP antibodies from hyperimmune sera from residents of Ivory Coast (lane C) and Uganda (lane D). Numbers on the right indicate the positions of molecular weight markers (in thousands). The arrowhead shows the position of SERP.
These results, obtained with mouse and human anti-SERP antibodies, establish SERP as a novel target for the antibody-dependent monocyte-mediated mechanism of inhibition of P. falciparum. This role of SERP in protection is in addition to the direct inhibition of growth obtained at high antibody concentrations (13, 14). Recent immunoepidemiological investigations carried out with sera from children living in an area of holoendemicity in Uganda support ADCI as a natural defense mechanism, since they revealed that higher levels of naturally induced IgG3 cytophilic antibodies against this antigen correlate with lower levels of peripheral parasitemia and an absence of malaria fever (12). The ADCI monocyte-dependent effect can be obtained at antibody concentrations which do not exert any direct inhibition of growth. Finally, our results reenforce the role of ADCI in the acquisition of immunity against malaria and broaden the range of parasite antigens implicated in this defense mechanism. We therefore suggest that ADCI assays should be used to assess the functional role of naturally induced antibodies and to monitor and optimize the protective efficacy of artificial immunization against malaria.
ADDENDUM
We obtained 12 sera corresponding to the pre- and postimmunization samples from the six Aotus monkeys immunized with the SERP antigen, as described in reference 8. We found that only four of these Aotus sera were positive in Western blots of parasite extracts, showing a single polypeptide with a size similar to that of the protein revealed by the two anti-SERP MAbs. The same four sera, but not the remaining eight sera, exerted a strong ADCI effect (70 to 80% inhibitory effect).
Acknowledgments
We express our thanks to Joseph Inselburg for his help and to Georges Snounou for revising the manuscript.
This work received support from grants EC Inco-DC no. 940317 and WHO-tdr no. 980278 and Grants-in-Aid for Scientific Research (13226058 and 13357002) from the Ministry of Education, Science, Sports, Culture and Technology of Japan.
Editor: J. M. Mansfield
REFERENCES
- 1.Banic, D. M., J. de Oliveira-Ferreira, L. R. Pratt-Riccio, V. Conseil, D. Goncalves, R. R. Fialho, H. Gras-Masse, C. Daniel-Ribeiro, and D. Camus. 1998. Immune response and lack of immune response to Plasmodium falciparum P126 antigen and its amino-terminal repeat in malaria-infected humans. Am. J. Trop. Med. Hyg. 58:768-774. [DOI] [PubMed] [Google Scholar]
- 2.Banyal, H. S., and J. Inselburg. 1985. Isolation and characterization of parasite-inhibitory P. falciparum monoclonal antibodies. Am. J. Trop. Med. Hyg. 34:1055-1064. [DOI] [PubMed] [Google Scholar]
- 3.Bouharoun-Tayoun, H., P. Attanath, A. Sabchareon, T. Chongsuphajaisiddhi, and P. Druilhe. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 172:1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouharoun-Tayoun, H., C. Oeuvray, F. Lunel, and P. Druilhe. 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182:409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahimi, K., J.-L. Pérignon, M. Bossus, H. Gras, A. Tartar, and P. Druilhe.1993. Fast immunopurification of small amounts of specific antibodies on peptides bound to ELISA plates. J. Immunol. Methods 162:69-75. [DOI] [PubMed] [Google Scholar]
- 6.Delplace, P., J. F. Dubremetz, B. Fortier, and A. Vernes. 1985. A 50 kilodalton exoantigen specific to the merozoite release-reinvasion stage of Plasmodium falciparum. Mol. Biochem. Parasitol. 17:239-251. [DOI] [PubMed] [Google Scholar]
- 7.Fortier, B., P. Delplace, J. F. Dubremetz, F. Ajana, and A. Vernes. 1987. Enzyme immunoassay for detection of antigen in acute Plasmodium falciparum malaria. Eur. J. Clin. Microbiol. 6:596-598. [DOI] [PubMed] [Google Scholar]
- 8.Inselburg, J., D. J. Bzik, W.-B. Li, K. M. Gren, J. Kansopon, B. K. Hahm, I. C. Bathurst, P. J. Barr, and R. N. Rossan. 1991. Protective immunity induced in Aotus monkeys by recombinant SERA proteins of P. falciparum. Infect. Immun. 59:1247-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khusmith, S., and P. Druilhe. 1983. Cooperation between antibodies and monocytes that inhibit in vitro proliferation of Plasmodium falciparum. Infect. Immun. 41:219-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long, C. A. 1993. Immunity to blood stages of malaria. Curr. Opin. Immunol. 5:548-556. [DOI] [PubMed] [Google Scholar]
- 11.Oeuvray, C., T. H. Bouharoun, M. H. Gras, E. Bottius, T. Kaidoh, M. Aikawa, M. C. Filgueira, A. Tartar, and P. Druilhe. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84:1594-1602. [PubMed] [Google Scholar]
- 12.Okech, B. A., A. Nalunkuma, D. Okello, X.-L. Pang, K. Suzue, J. Li, T. Horii, and T. G. Egwang. 2001. Natural human IgG subclass responses to Plasmodium falciparum serine repeat antigen (SERA) in Uganda. Am. J. Trop. Med. Hyg. 65:912-917. [DOI] [PubMed] [Google Scholar]
- 13.Pang, X.-L., and T. Horii. 1999. Complement-mediated killing of Plasmodium falciparum erythrocytic schizont with antibodies to the recombinant serine repeat antigen (SERA). Vaccine 16:1299-1305. [DOI] [PubMed] [Google Scholar]
- 14.Pang, X.-L., T. Mitamura, and T. Horii. 1999. Antibodies reactive with the N-terminal domain of Plasmodium falciparum serine repeat antigen inhibit cell proliferation by agglutinating merozoites and schizonts. Infect. Immun. 67:1821-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perrin, L. H., B. Merkli, M. Loche, C. Chizzolini, J. Smart, and R. Richle. 1984. Antimalarial immunity in saimiri monkeys: immunization with surface components of asexual blood stages. J. Exp. Med. 160:441-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugiyama, T., K. Suzue, M. Okamoto, J. Inselburg, K. Tai, and T. Horii. 1996. Production of recombinant SERA proteins of Plasmodium falciparum in E. coli by using synthetic genes. Vaccine 14:1069-1076. [DOI] [PubMed] [Google Scholar]
- 17.Theisen, M., S. Soe, C. Oeuvray, A. W. Thomas, J. Vuust, S. Daneelsen, S. Jepsen, and P. Druilhe. 1998. The glutamate rich protein (GLURP) of P. falciparum is a target for antibody dependent monocyte mediated inhibition of parasite growth in vitro. Infect. Immun. 66:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]