Abstract
1. A small electrical potential difference (541 ± 48 μV, aqueous side negative) across rabbit corneal endothelium has been recently found. Its dependence on ambient [Na+], [K+], [H+] and metabolic and specific inhibitors was examined.
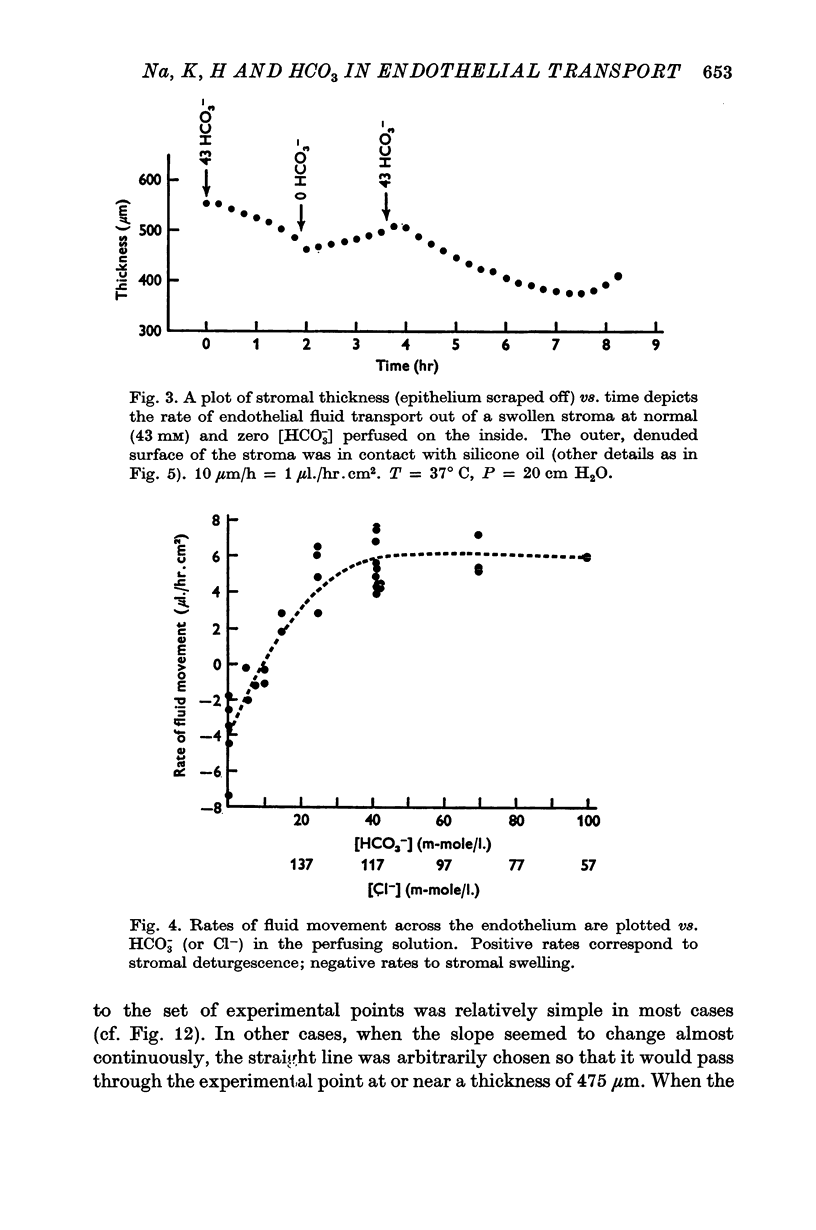
2. Changes in concentration of the ions above either were known or were presently shown to affect the rate of fluid transport across this preparation (normal value: 5·2 ± 0·4 μl./hr.cm2). Ionic concentration changes were also found here to influence potential difference in the same way as fluid transport. In the cases tested, the effects on both fluid transport and potential difference were reversible.
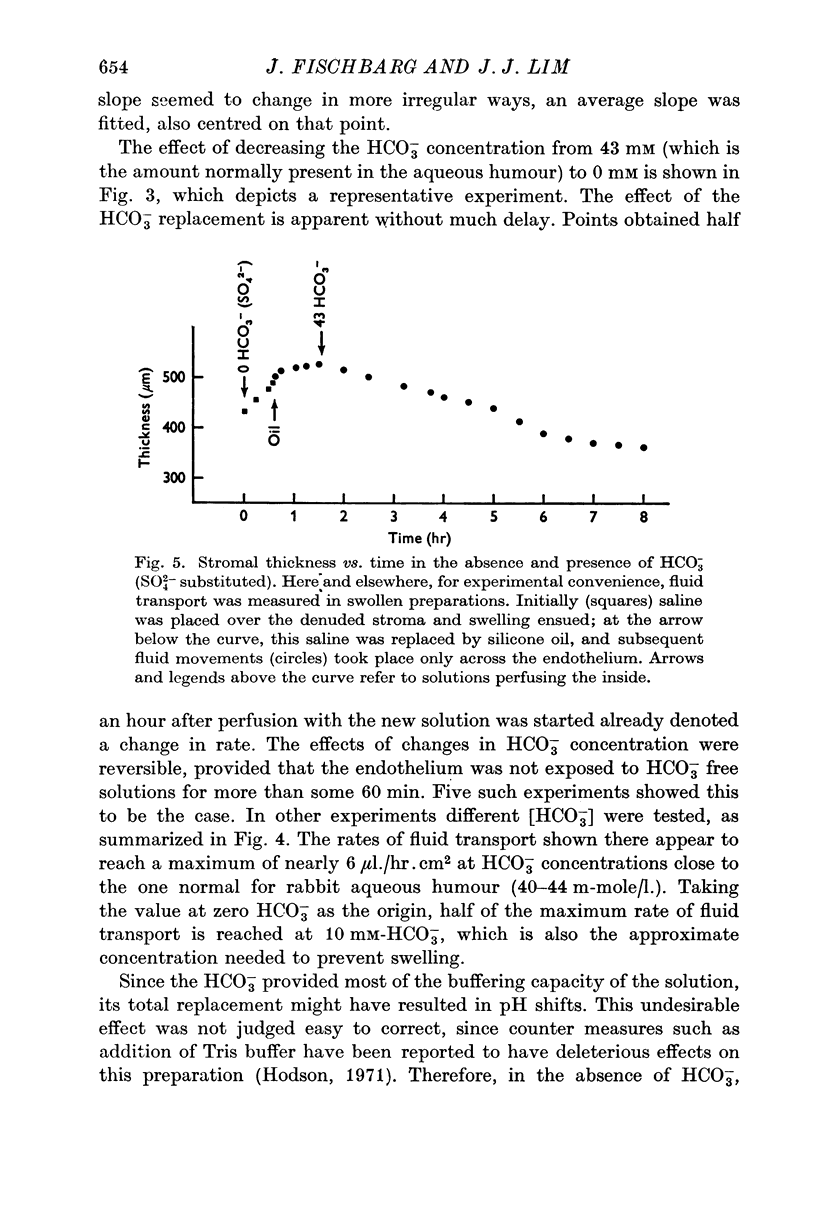
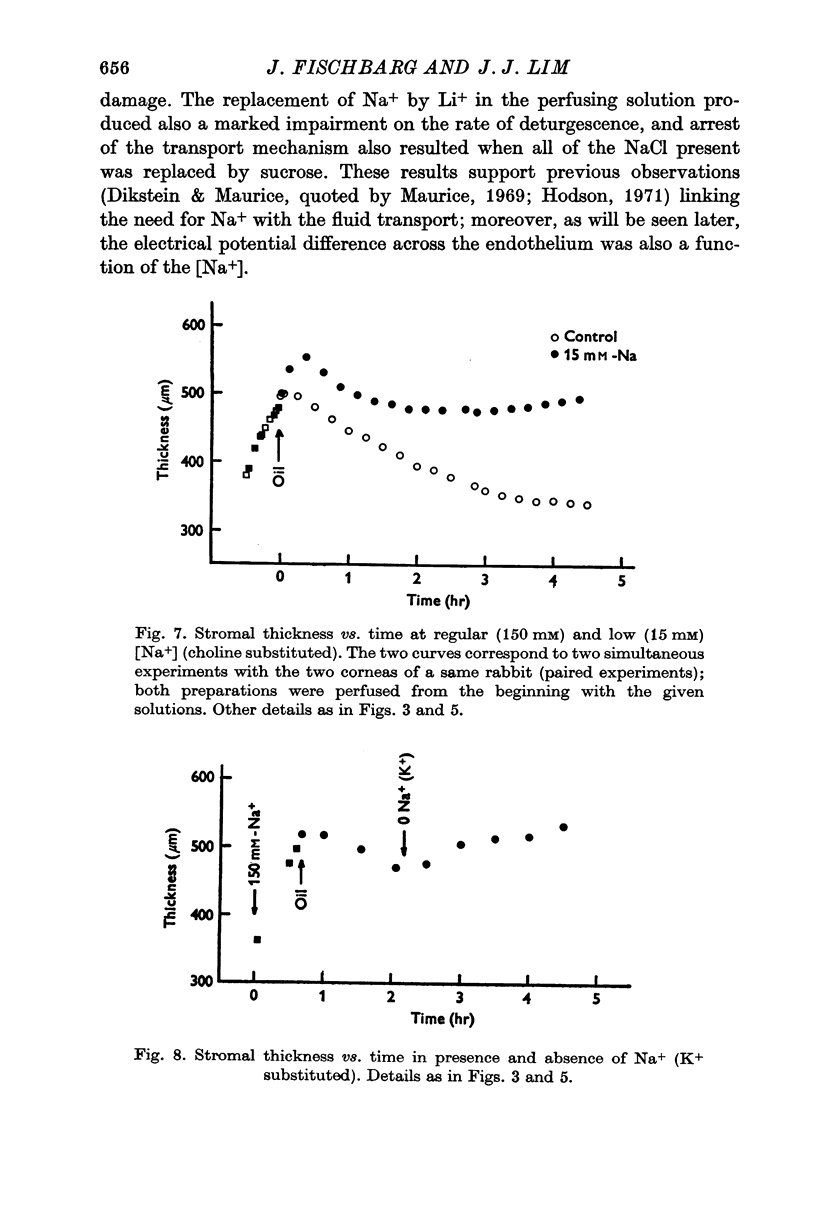
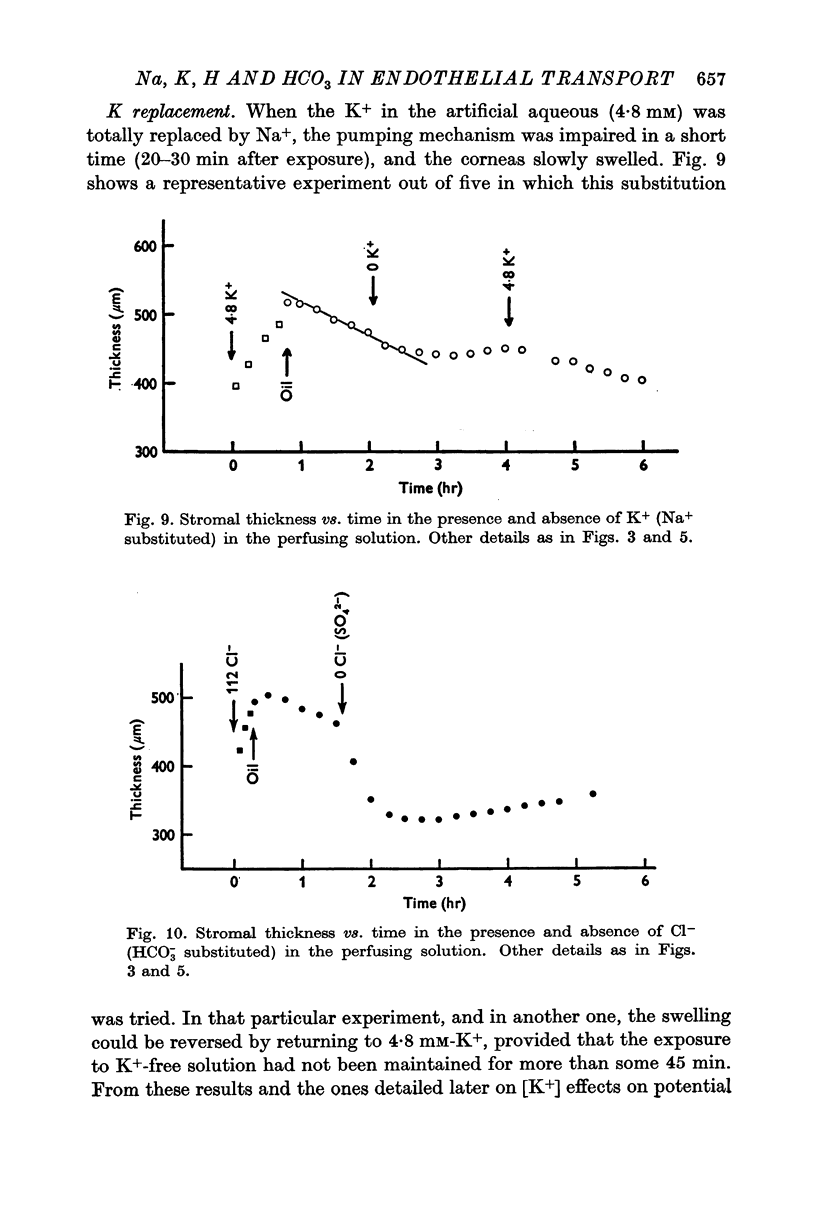
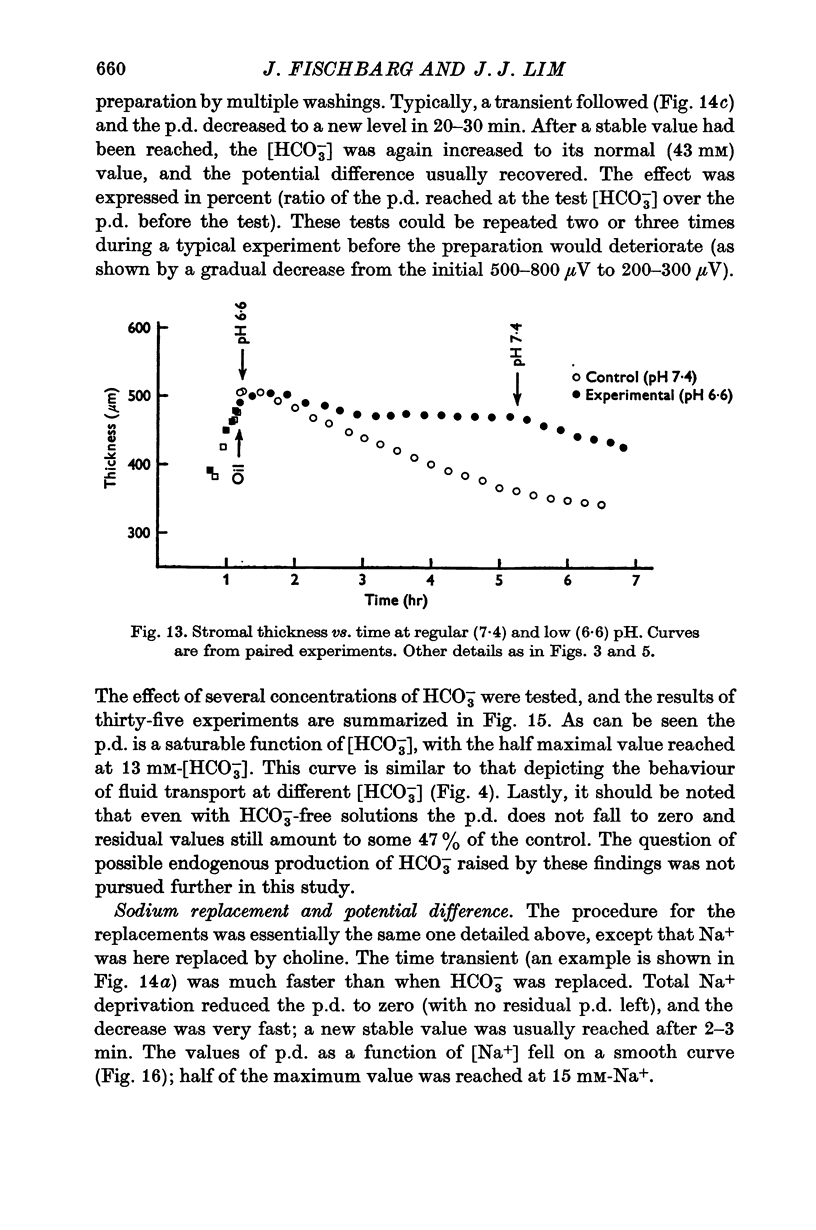
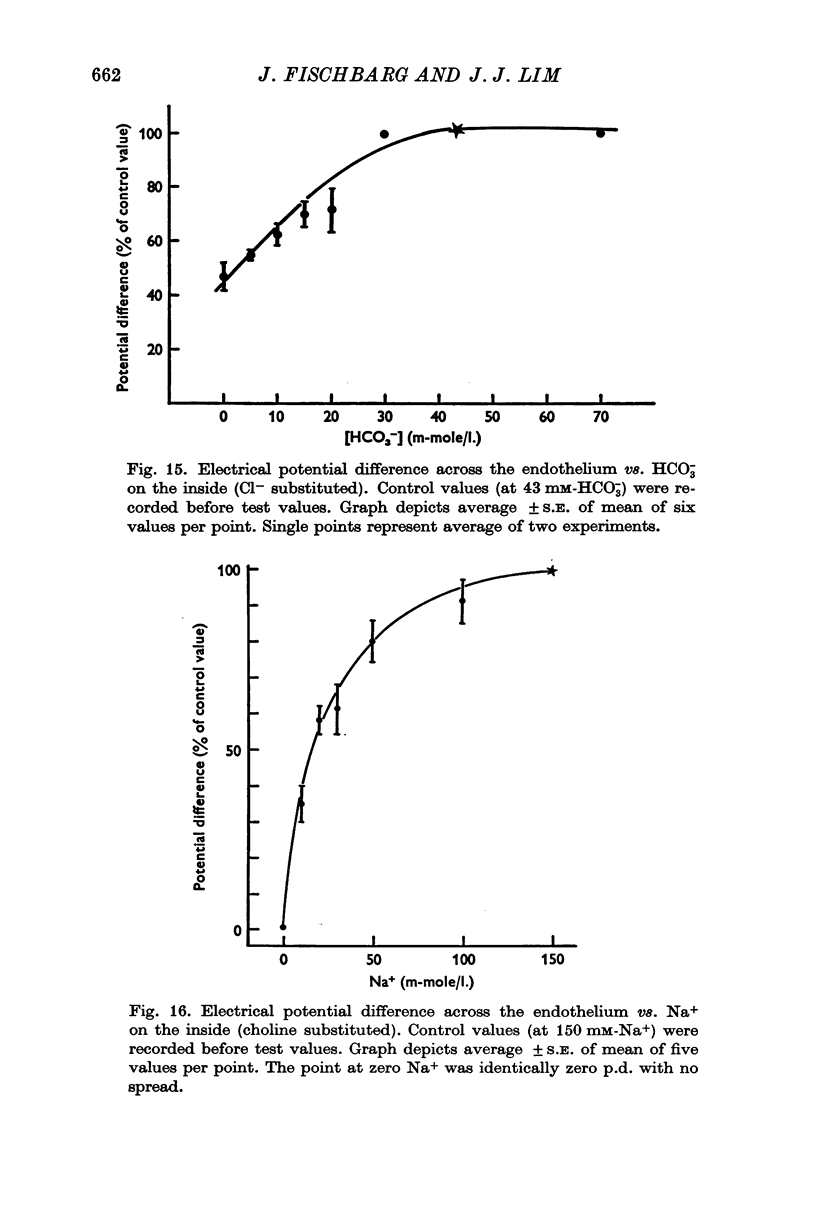
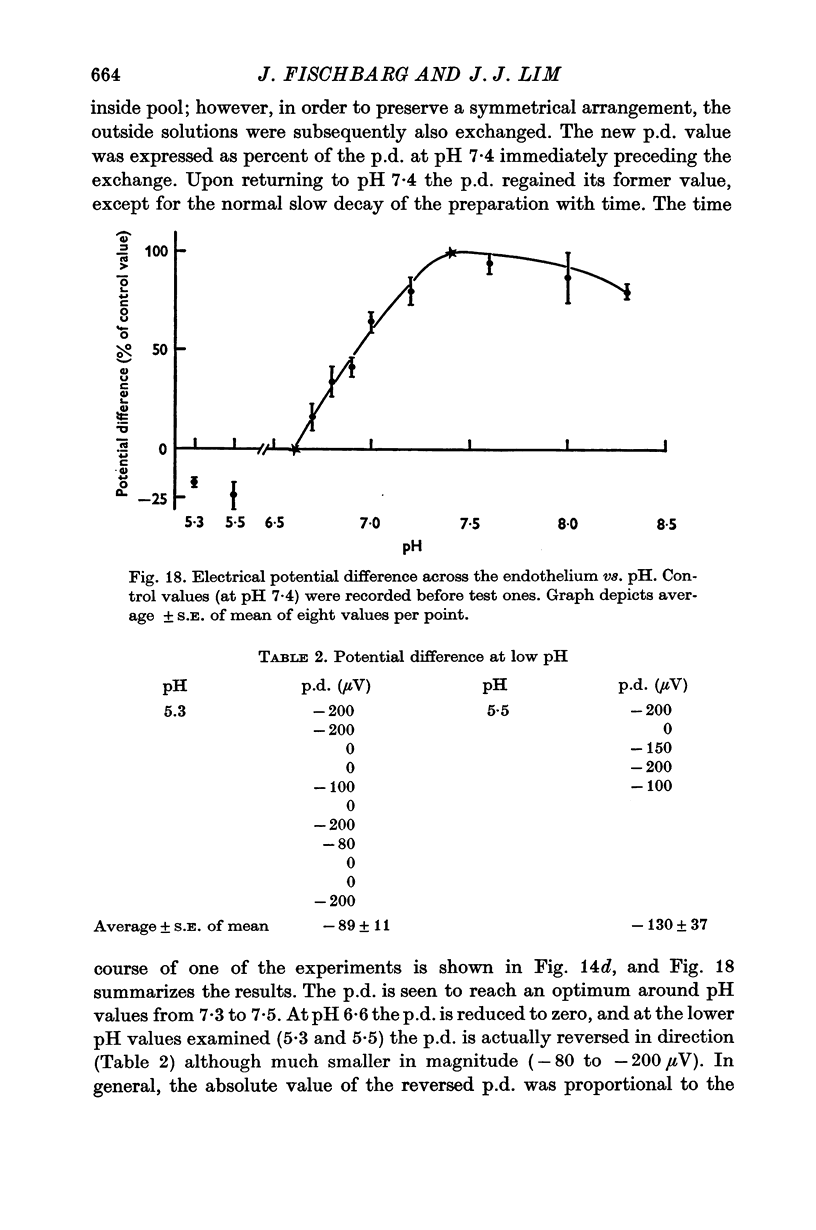
3. Fluid transport and potential difference were both decreased or abolished in absence of Na+, K+ and HCO3-, and when [H+] was decreased. Fluid transport and potential difference were saturable functions of [HCO3-] and half-saturation occurred in both cases at about 13 mM-HCO3-. The potential difference was also a saturable function of [Na+] (half-saturation around 15 mM). There was a pH optimum for potential difference in the range 7·4-7·6. Lower pH values decreases the potential difference and the fluid transport, and a small (-100 μV) reversed potential was observed in the range of 5·3-5·5.
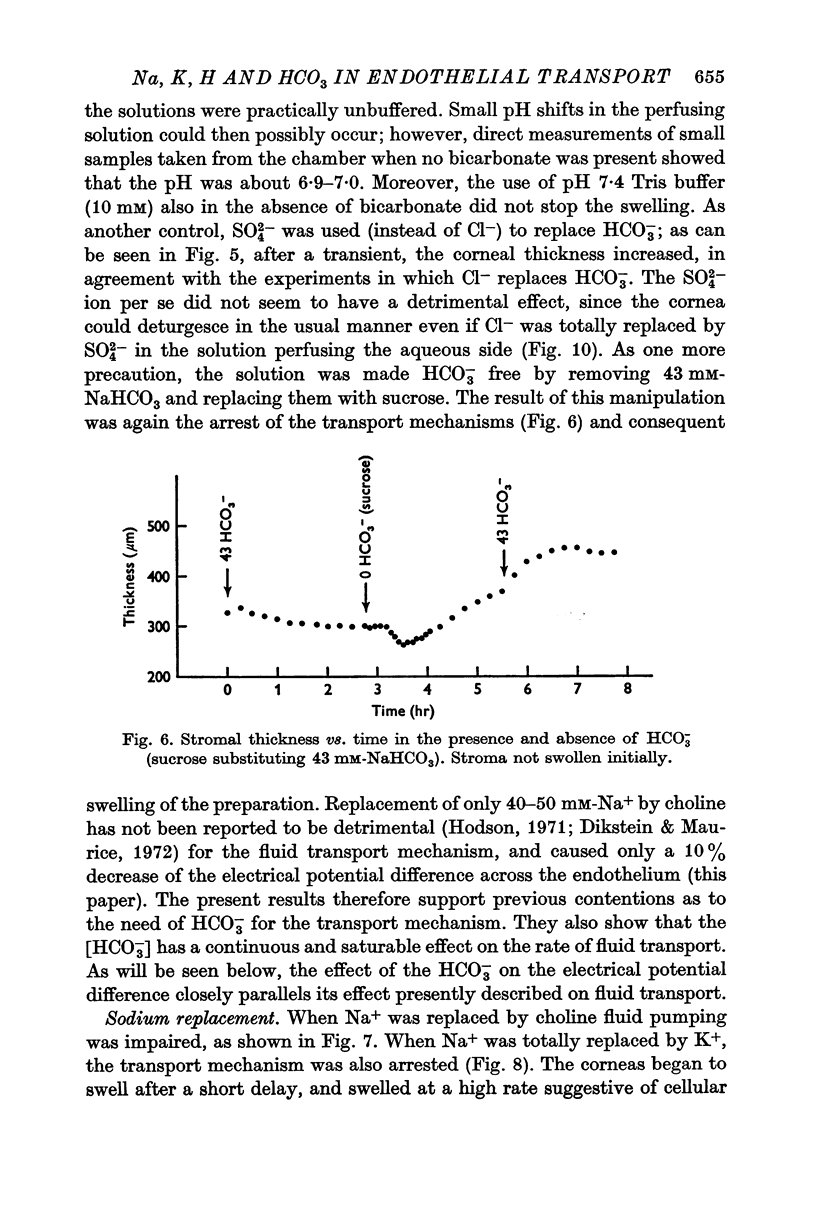
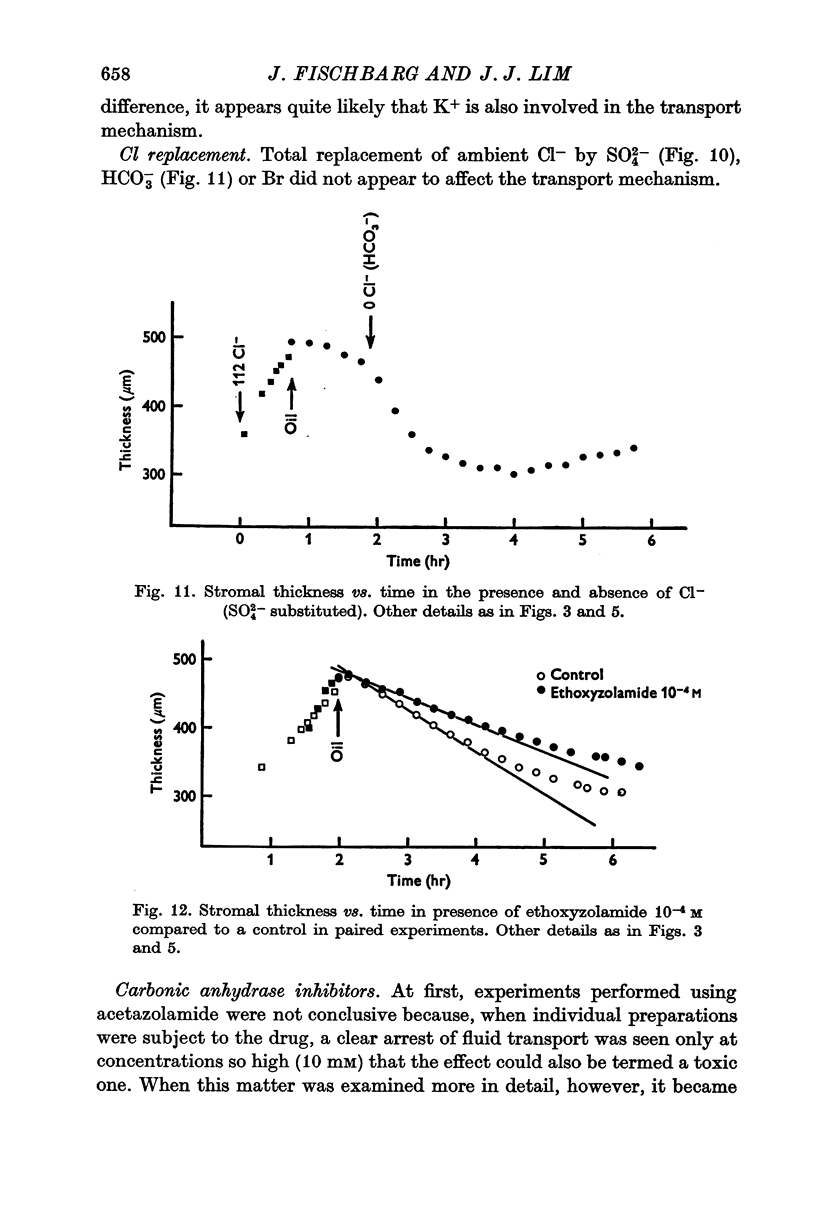
4. Total replacement of Cl- by HCO3- or SO42- produced no impairment on either fluid transport or potential difference.
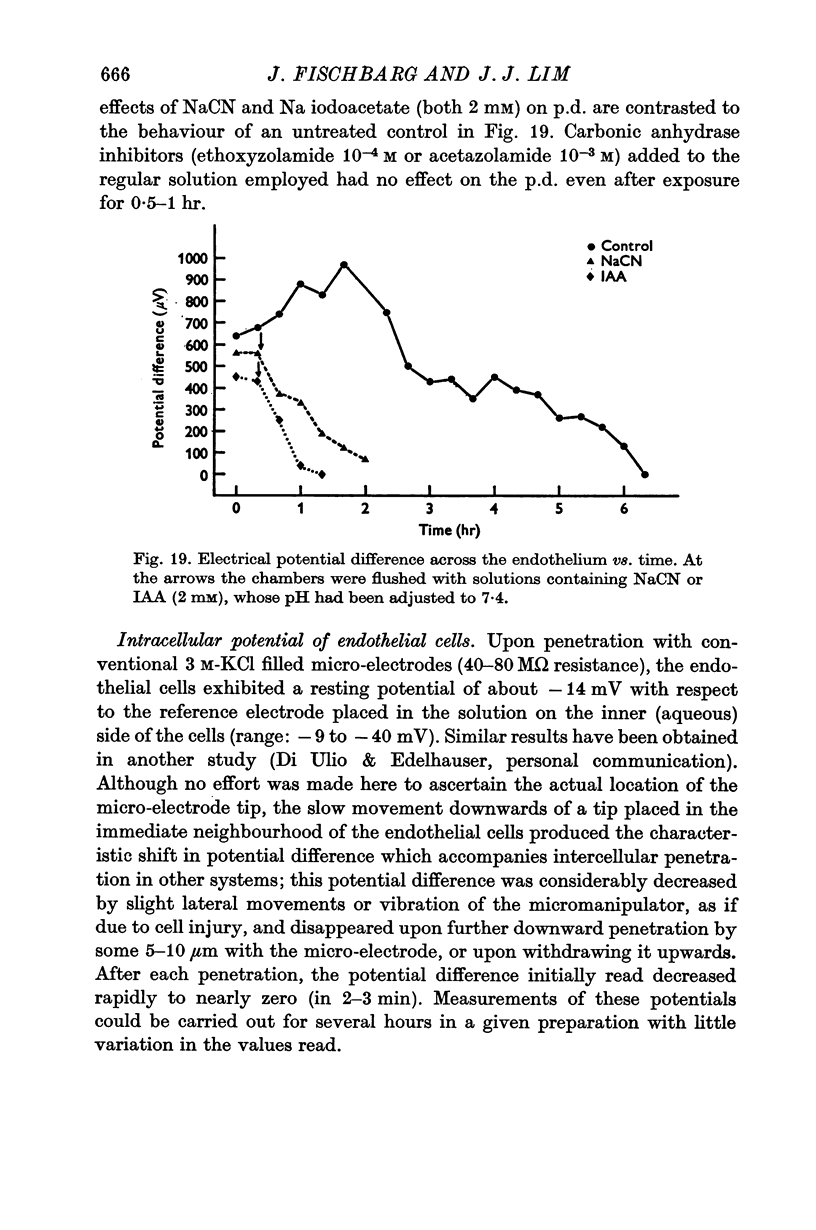
5. Carbonic anhydrase inhibitors (ethoxyzolamide 10-5 or 10-4 M and benzolamide 10-3 M) produced a 40-60% decrease in the rate of fluid pumping. In contrast, ethoxyzolamide 10-4 M or acetazolamide 10-3 M did not produce any change in the potential difference. NaCN and Na iodoacetate (both 2 mM) eliminated the potential difference in 1-1·5 hr while in controls it lasted for 5-6 hr.
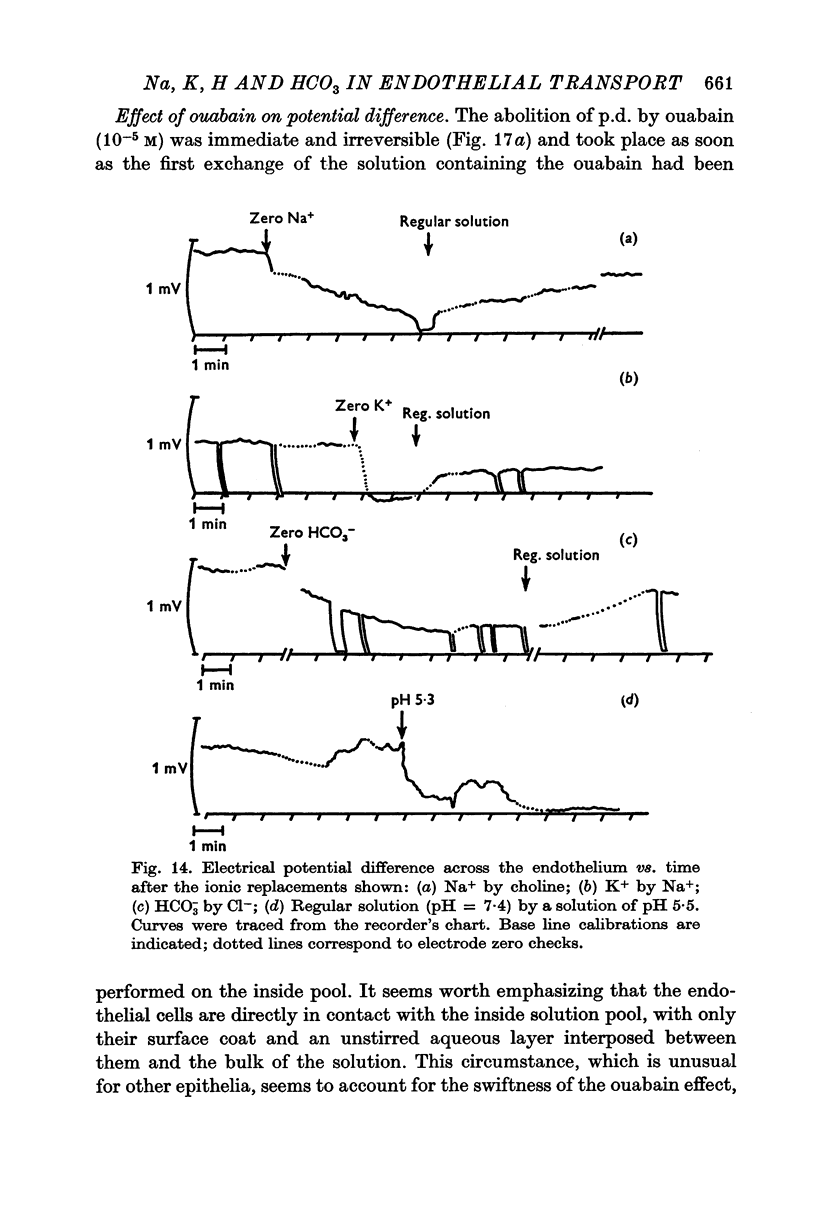
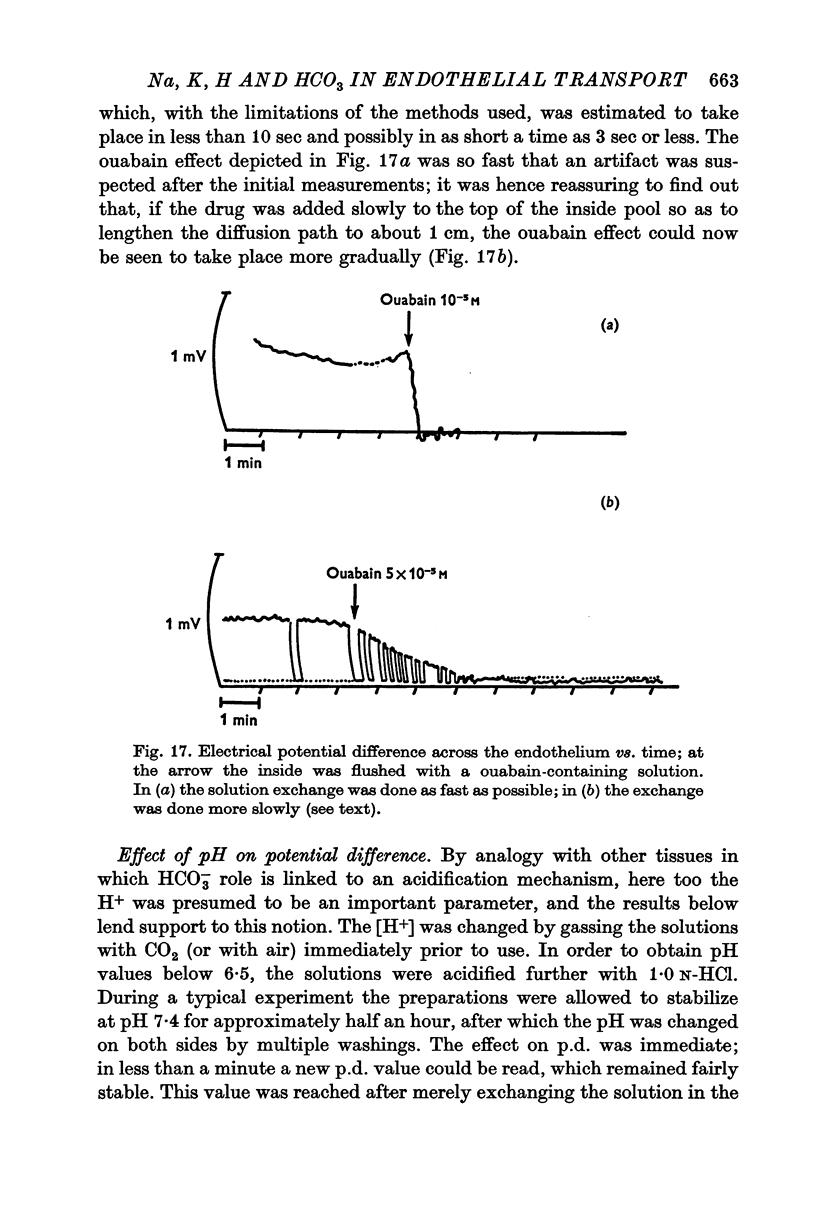
6. Ouabain (10-5 M) abolished the potential difference in less than 10 sec when added to the aqueous side, which suggests the existence of an electrogenic pump. This extremely fast time transient can be accounted for by the accessibility and simple geometry of the present monocellular layer. Ouabain abolished also the reversed potential difference observed at low pH.
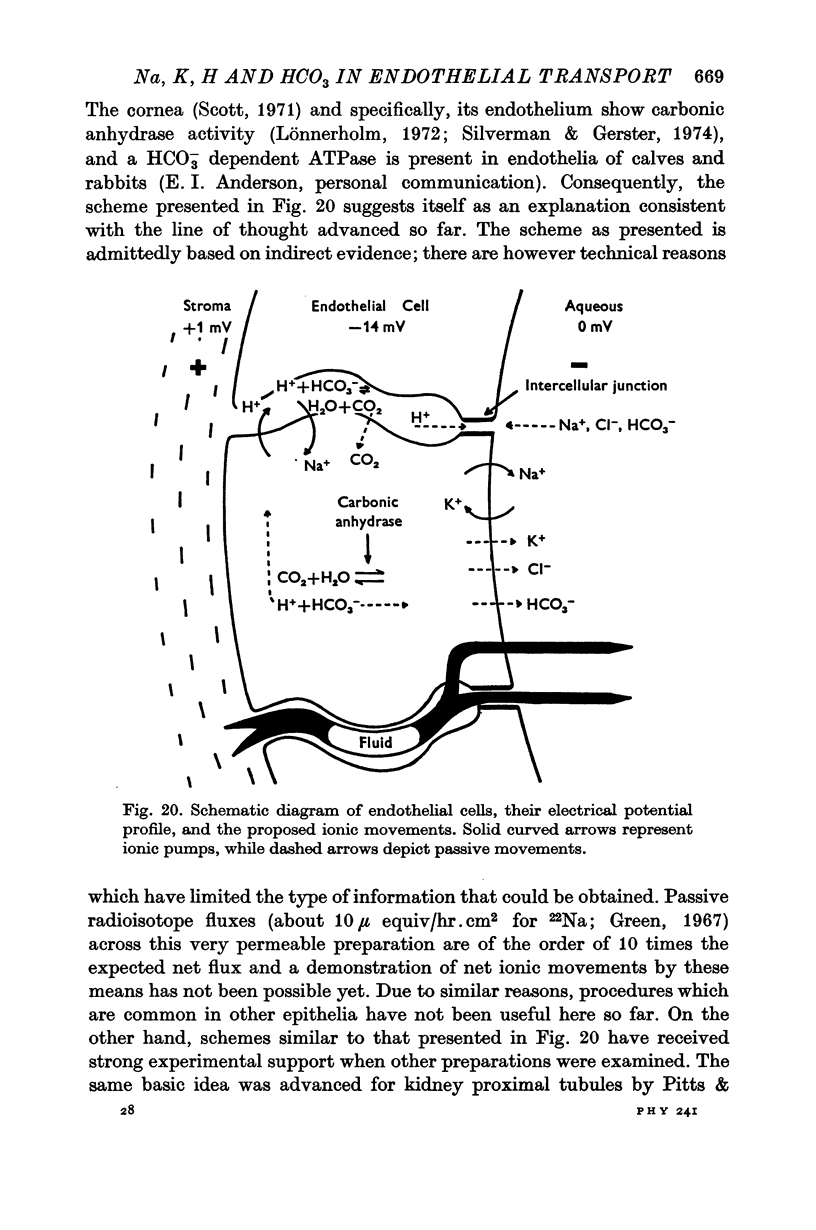
7. The data are interpreted in terms of a scheme similar to that advanced for other epithelia and in which (a) H+ would be pumped into the intercellular spaces, while Na+ and CO2 would enter into the cells, and (b) Na+ would be subsequently pumped into the aqueous humour, producing as a result the fluid movement observed. The actual origin of the potential difference is further discussed in terms of two contrasting possibilities: (i) one or more electrogenic pumps, and (ii) a neutral pump which would create a diffusion potential across `leaky' intercellular junctions.
Full text
PDF


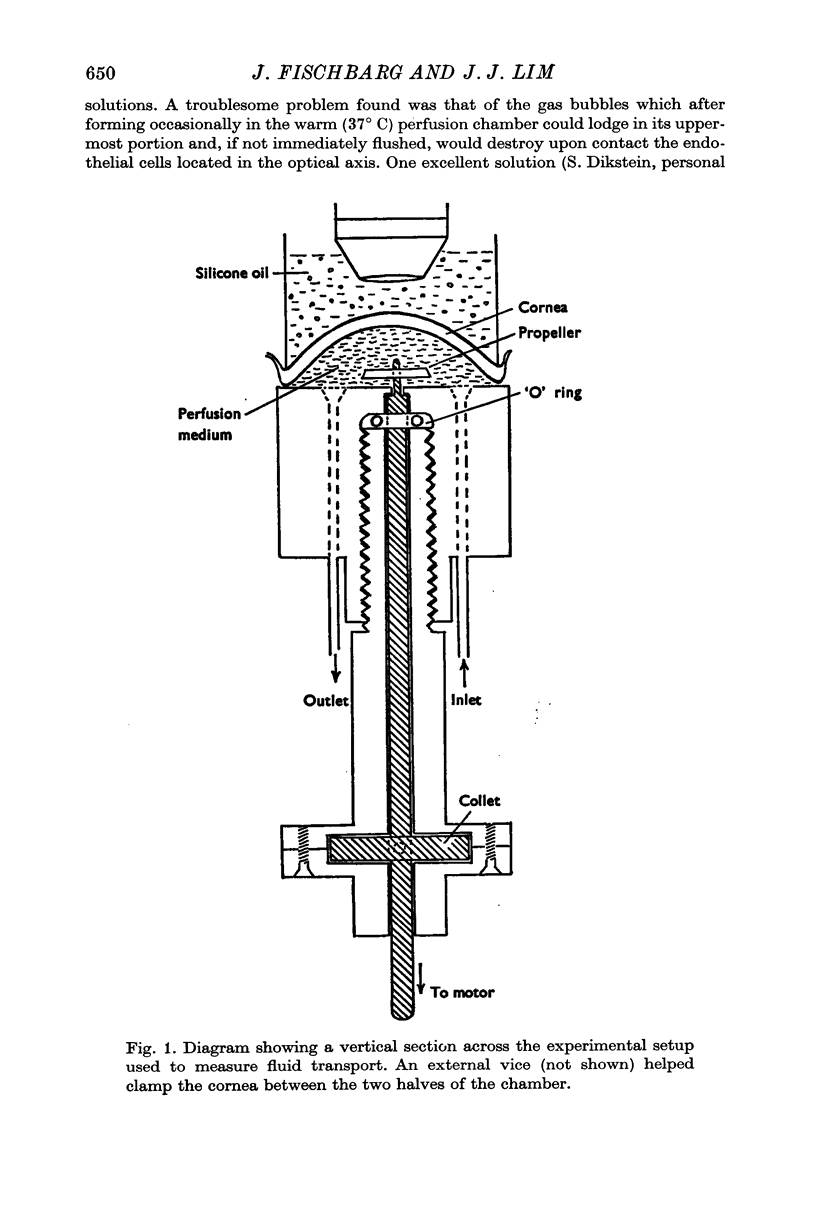
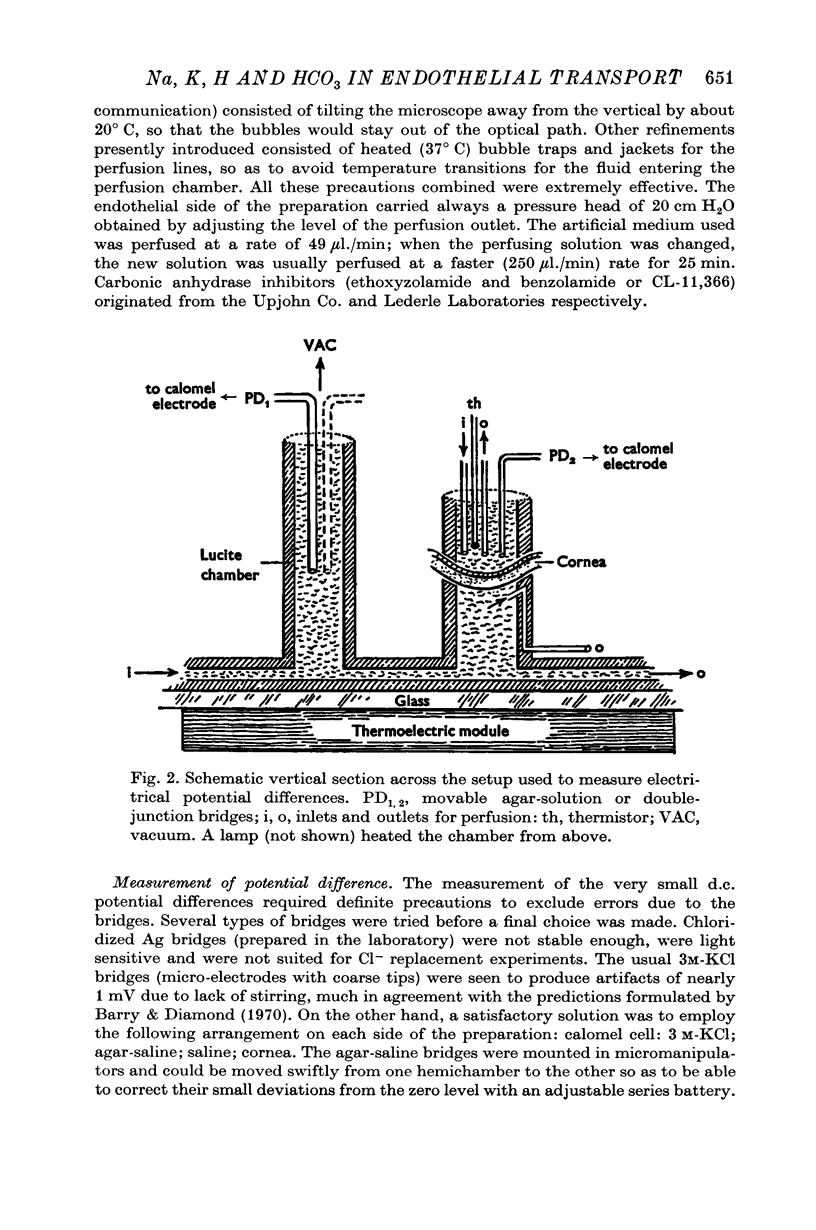











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER B. Carbonic anhydrase and the formation of aqueous humor. Am J Ophthalmol. 1959 Jan;47(1 Pt 2):342–361. doi: 10.1016/s0002-9394(14)78041-9. [DOI] [PubMed] [Google Scholar]
- BERGGREN L. DIRECT OBSERVATION OF SECRETORY PUMPING IN VITRO OF THE RABBIT EYE CILIARY PROCESSES. INFLUENCE OF ION MILIEU AND CARBONIC ANHYDRASE INHIBITION. Invest Ophthalmol. 1964 Jun;3:266–272. [PubMed] [Google Scholar]
- CLAPP J. R., WATSON J. F., BERLINER R. W. EFFECT OF CARBONIC ANHYDRASE INHIBITION ON PROXIMAL TUBULAR BICARBONATE REABSORPTION. Am J Physiol. 1963 Oct;205:693–696. doi: 10.1152/ajplegacy.1963.205.4.693. [DOI] [PubMed] [Google Scholar]
- Cole D. F. ELECTROCHEMICAL CHANGES ASSOCIATED WITH THE FORMATION OF THE AQUEOUS HUMOUR. Br J Ophthalmol. 1961 Mar;45(3):202–217. doi: 10.1136/bjo.45.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVSON H. Some considerations on the salt content of fresh and old ox corneae. Br J Ophthalmol. 1949 Mar;33(3):175–182. doi: 10.1136/bjo.33.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVSON H. The hydration of the cornea. Biochem J. 1955 Jan;59(1):24–28. doi: 10.1042/bj0590024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. M. TRANSPORT OF SALT AND WATER IN RABBIT AND GUINEA PIG GALL BLADDER. J Gen Physiol. 1964 Sep;48:1–14. doi: 10.1085/jgp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. M. The reabsorptive function of the gall-bladder. J Physiol. 1962 May;161:442–473. doi: 10.1113/jphysiol.1962.sp006898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Bossert W. H. Functional consequences of ultrastructural geometry in "backwards" fluid-transporting epithelia. J Cell Biol. 1968 Jun;37(3):694–702. doi: 10.1083/jcb.37.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J., Lim J. J. Determination of the impedance locus of rabbit corneal endothelium. Biophys J. 1973 Jun;13(6):595–599. doi: 10.1016/S0006-3495(73)86009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J. Potential difference and fluid transport across rabbit corneal endothelium. Biochim Biophys Acta. 1972 Nov 2;288(2):362–366. doi: 10.1016/0005-2736(72)90257-x. [DOI] [PubMed] [Google Scholar]
- Green K. Dependence of corneal thickness on epithelial ion transport and stromal sodium. Am J Physiol. 1969 Oct;217(4):1169–1177. doi: 10.1152/ajplegacy.1969.217.4.1169. [DOI] [PubMed] [Google Scholar]
- Green K., Otori T. Direct measurements of membrane unstirred layers. J Physiol. 1970 Mar;207(1):93–102. doi: 10.1113/jphysiol.1970.sp009050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. Solute movement across the constituent membranes of the cornea. Exp Eye Res. 1967 Apr;6(2):79–92. doi: 10.1016/s0014-4835(67)80058-7. [DOI] [PubMed] [Google Scholar]
- Hedbys B. O., Mishima S. The thickness-hydration relationship of the cornea. Exp Eye Res. 1966 Jul;5(3):221–228. doi: 10.1016/s0014-4835(66)80010-6. [DOI] [PubMed] [Google Scholar]
- Hodson S. Evidence for a bicarbonate-dependent sodium pump in corneal endothelium. Exp Eye Res. 1971 Jan;11(1):20–29. doi: 10.1016/s0014-4835(71)80060-x. [DOI] [PubMed] [Google Scholar]
- KINSEY V. E., REDDY D. V. Turnover of total carbon dioxide in the aqueous humors and the effect thereon of acetazolamide. AMA Arch Ophthalmol. 1959 Jul;62(1):78–83. doi: 10.1001/archopht.1959.04220010082009. [DOI] [PubMed] [Google Scholar]
- Kaye G. I., Fenoglio C. M., Hoefle F. B., Fischbarg J. Studies on the cornea. IX. Physiologic and morphologic effects of cytochalasin B on endothelium of rabbit corneas perfused in vitro. J Cell Biol. 1974 May;61(2):537–543. doi: 10.1083/jcb.61.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyce S. D., Neufeld A. H., Zadunaisky J. A. The activation of chloride transport by epinephrine and Db cyclic-AMP in the cornea of the rabbit. Invest Ophthalmol. 1973 Feb;12(2):127–139. [PubMed] [Google Scholar]
- Kunau R. T., Jr The influence of the carbonic anhydrase inhibitor, benzolamide (CL-11,366), on the reabsorption of chloride, sodium, and bicarbonate in the proximal tubule of the rat. J Clin Invest. 1972 Feb;51(2):294–306. doi: 10.1172/JCI106814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGHAM M. E., TAYLOR I. S. Factors affecting the hydration of the cornea in the excised eye and the living animal. Br J Ophthalmol. 1956 Jun;40(6):321–340. doi: 10.1136/bjo.40.6.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOESCHCKE H. H. Uber Bestandspotentiale im Gebiete der Medulla oblongata. Pflugers Arch. 1956;262(6):517–531. doi: 10.1007/BF00362114. [DOI] [PubMed] [Google Scholar]
- MACROBBIE E. A., USSING H. H. Osmotic behaviour of the epithelial cells of frog skin. Acta Physiol Scand. 1961 Nov-Dec;53:348–365. doi: 10.1111/j.1748-1716.1961.tb02293.x. [DOI] [PubMed] [Google Scholar]
- Maurice D. M. The location of the fluid pump in the cornea. J Physiol. 1972 Feb;221(1):43–54. doi: 10.1113/jphysiol.1972.sp009737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, CARTER N. W., SELDIN D. W. THE MECHANISM OF BICARBONATE REABSORPTION IN THE PROXIMAL AND DISTAL TUBULES OF THE KIDNEY. J Clin Invest. 1965 Feb;44:278–290. doi: 10.1172/JCI105142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIKLIS E., QUASTEL J. H. Effects of cations on sugar absorption by isolated surviving guinea pig intestine. Can J Biochem Physiol. 1958 Mar;36(3):347–362. [PubMed] [Google Scholar]
- SCHATZMANN H. J., WINDHAGER E. E., SOLOMON A. K. Single proximal tubules of the Necturus kidney. II. Effect of 2, 4-dinitro-phenol and ouabain on water reabsorption. Am J Physiol. 1958 Dec;195(3):570–574. doi: 10.1152/ajplegacy.1958.195.3.570. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. I. SHORT-CIRCUIT CURRENT AND NA FLUXES. J Gen Physiol. 1964 Jan;47:567–584. doi: 10.1085/jgp.47.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ B., DANES B., LEINFELDER P. J. The role of metabolism in the hydration of the isolated lens and cornea. Am J Ophthalmol. 1954 Jul;38(12):182–193. doi: 10.1016/0002-9394(54)90023-9. [DOI] [PubMed] [Google Scholar]
- Scott W. N., Shamoo Y. E., Brodsky W. A. Carbonic anhydrase content of turtle urinary bladder mucosal cells. Biochim Biophys Acta. 1970;219(1):248–250. doi: 10.1016/0005-2736(70)90084-2. [DOI] [PubMed] [Google Scholar]
- Silverman D. N., Gerster R. The detection and localization of carbonic anhydrase in the rabbit cornea. Exp Eye Res. 1973 Oct 24;17(2):129–136. doi: 10.1016/0014-4835(73)90202-9. [DOI] [PubMed] [Google Scholar]
- TSCHIRGI R. D., FROST R. W., TAYLOR J. L. Inhibition of cerebrospinal fluid formation by a carbonic anhydrase inhibitor, 2-acetylamino-1,3,4-thiadiazole-5-sulfonamide (diamox). Proc Soc Exp Biol Med. 1954 Nov;87(2):373–376. doi: 10.3181/00379727-87-21386. [DOI] [PubMed] [Google Scholar]
- Trenberth S. M., Mishima S. The effect of ouabain on the rabbit corneal endothelium. Invest Ophthalmol. 1968 Feb;7(1):44–52. [PubMed] [Google Scholar]
- Turnberg L. A., Bieberdorf F. A., Morawski S. G., Fordtran J. S. Interrelationships of chloride, bicarbonate, sodium, and hydrogen transport in the human ileum. J Clin Invest. 1970 Mar;49(3):557–567. doi: 10.1172/JCI106266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnberg L. A., Fordtran J. S., Carter N. W., Rector F. C., Jr Mechanism of bicarbonate absorption and its relationship to sodium transport in the human jejunum. J Clin Invest. 1970 Mar;49(3):548–556. doi: 10.1172/JCI106265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELCH K., SADLER K. ELECTRICAL POTENTIALS OF CHOROID PLEXUS OF THE RABBIT. J Neurosurg. 1965 Apr;22:344–351. doi: 10.3171/jns.1965.22.4.0344. [DOI] [PubMed] [Google Scholar]
- WELCH K. SECRETION OF CEREBROSPINAL FLUID BY CHOROID PLEXUS OF THE RABBIT. Am J Physiol. 1963 Sep;205:617–624. doi: 10.1152/ajplegacy.1963.205.3.617. [DOI] [PubMed] [Google Scholar]
- WHEELER H. O. TRANSPORT OF ELECTROLYTES AND WATER ACROSS WALL OF RABBIT GALL BLADDER. Am J Physiol. 1963 Sep;205:427–438. doi: 10.1152/ajplegacy.1963.205.3.427. [DOI] [PubMed] [Google Scholar]
- WHITLOCK R. T., WHEELER H. O. COUPLED TRANSPORT OF SOLUTE AND WATER ACROSS RABBIT GALLBLADDER EPITHELIUM. J Clin Invest. 1964 Dec;43:2249–2265. doi: 10.1172/JCI105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler H. O., Ross E. D., King K. K. Effect of carbonic anhydrase inhibitors on isolated rabbit gallbladders. Am J Physiol. 1969 Jan;216(1):175–178. doi: 10.1152/ajplegacy.1969.216.1.175. [DOI] [PubMed] [Google Scholar]
- Whitlock R. T., Wheeler H. O. Hydrogen ion transport by isolated rabbit gallbladder. Am J Physiol. 1969 Jul;217(1):310–316. doi: 10.1152/ajplegacy.1969.217.1.310. [DOI] [PubMed] [Google Scholar]
- Wright E. M. Mechanisms of ion transport across the choroid plexus. J Physiol. 1972 Oct;226(2):545–571. doi: 10.1113/jphysiol.1972.sp009997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadunaisky J. A. Active transport of chloride in frog cornea. Am J Physiol. 1966 Aug;211(2):506–512. doi: 10.1152/ajplegacy.1966.211.2.506. [DOI] [PubMed] [Google Scholar]
- de ROUGEMONT, AMES A., 3rd, NESBETT F. B., HOFMANN H. F. Fluid formed by choroid plexus; a technique for its collection and a comparison of its electrolyte composition with serum and cisternal fluids. J Neurophysiol. 1960 Sep;23:485–495. doi: 10.1152/jn.1960.23.5.485. [DOI] [PubMed] [Google Scholar]


