Abstract
1. The development of transmission was studied in chick ciliary ganglia that had been deprived of their periphery during early embryonic development.
2. Peripherally deprived neurones in the ganglion differentiate in normal numbers and send functional axons into the post-ganglionic nerve.
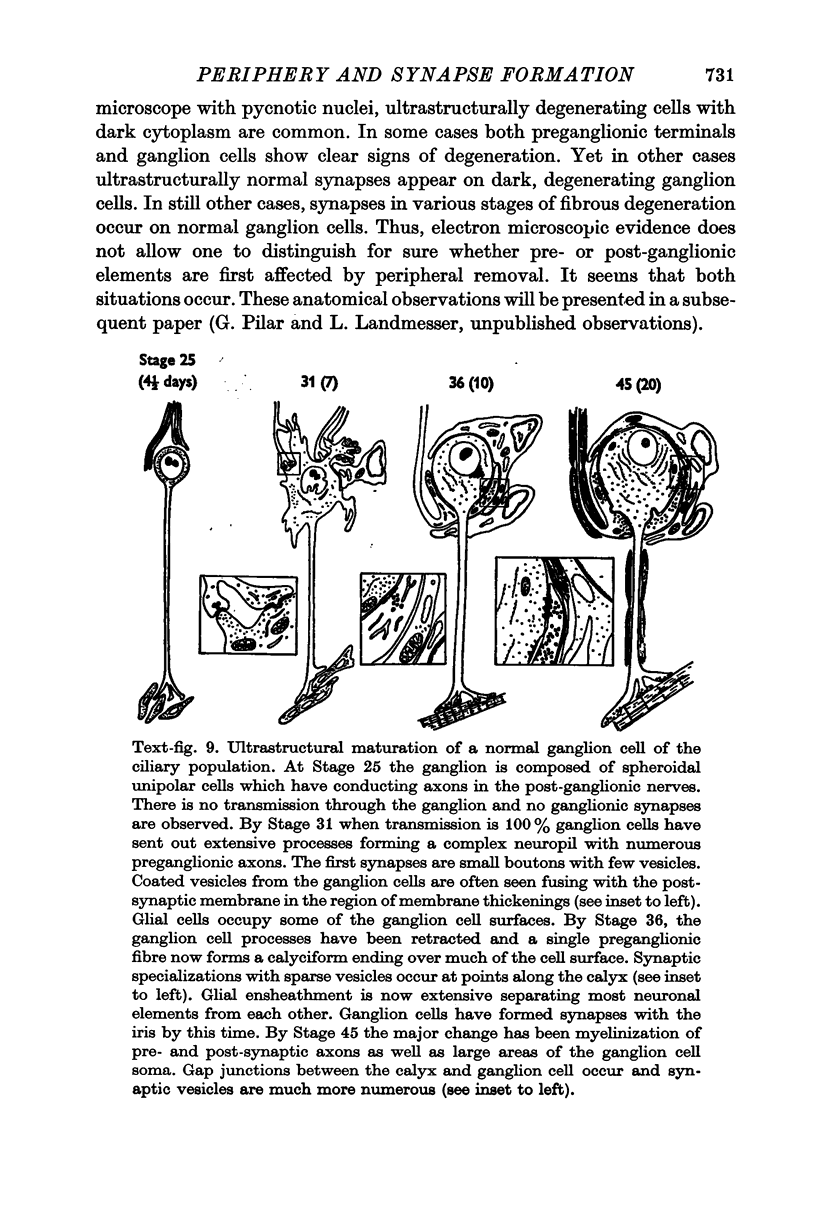
3. Ganglion cells lacking a periphery follow the normal developmental sequence sending out transient dendrites at the time ganglion cell synapses are formed, and later retracting them when calyces appear.
4. Synapses, which appear functionally and ultrastructurally normal, form on all ganglion cells at the normal time and transmission is normal until Stage 34. Therefore information from the periphery is not required for ganglion cell synapse formation per se.
5. From Stages 35 to 38 most cells die, so that only 8% of the original number of cells remain in the operated ganglion. Transmission fails in many cells during this same time, but precedes cell loss by only a short time, so that deafferentation probably does not contribute substantially to cell death.
6. Both ciliary and choroid cells achieve full cytologic differentiation and are distinct from each other, indicating that the periphery is not required for the elaboration of the distinctive characteristics of these cells. Presynaptic fibres also differentiate into typical bouton as well as calyciform endings. Therefore, the type of preganglionic ending does not depend on ganglion cells establishing proper peripheral contacts.
7. It has not been possible to ascertain whether ganglion cell specificity is affected by the periphery.
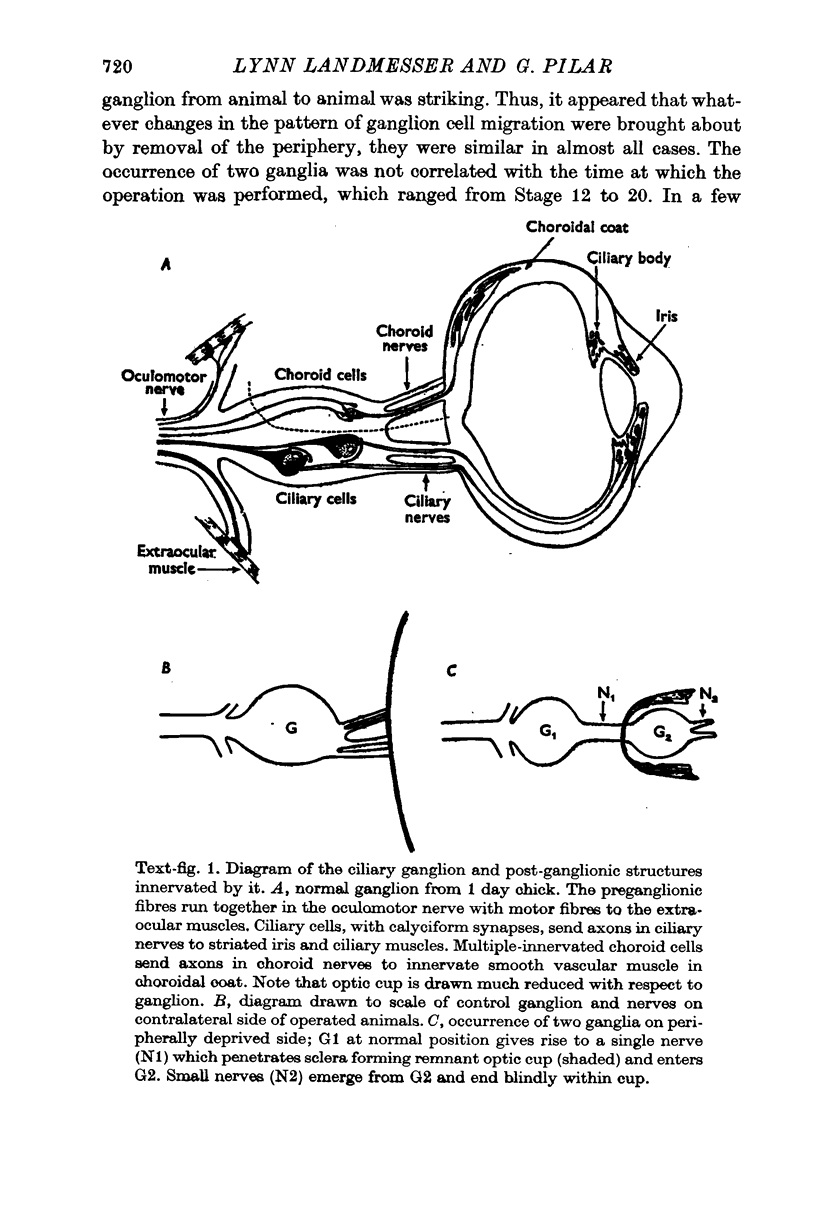
8. Peripheral removal affects ganglion cell migration, so that two ganglia are formed. Approximately half of the cells migrate into the remnant optic cup forming a second misplaced ganglion. Ciliary and choroid cells occur in both ganglia and these cells go through the typical sequence of events described above.
Full text
PDF





















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cowan W. M., Wenger E. Degeneration in the nucleus of origin of the preganglionic fibers to the chick ciliary ganglion following early removal of the optic vesicle. J Exp Zool. 1968 May;168(1):105–123. doi: 10.1002/jez.1401680109. [DOI] [PubMed] [Google Scholar]
- Guth L. "Trophic" influences of nerve on muscle. Physiol Rev. 1968 Oct;48(4):645–687. doi: 10.1152/physrev.1968.48.4.645. [DOI] [PubMed] [Google Scholar]
- HAMBURGER V. Regression versus peripheral control of differentiation in motor hypoplasia. Am J Anat. 1958 May;102(3):365–409. doi: 10.1002/aja.1001020303. [DOI] [PubMed] [Google Scholar]
- HAMMOND W. S., YNTEMA C. L. Origin of ciliary ganglia in the chick. J Comp Neurol. 1958 Dec;110(3):367–389. doi: 10.1002/cne.901100304. [DOI] [PubMed] [Google Scholar]
- HUGHES A. Cell degeneration in the larval ventral horn of Xenopus laevis (Daudin). J Embryol Exp Morphol. 1961 Jun;9:269–284. [PubMed] [Google Scholar]
- HUGHES A., TSCHUMI P. A. The factors controlling the development of the dorsal root ganglia and ventral horn in Xenopus laevis (Daud.). J Anat. 1958 Oct;92(4):498–527. [PMC free article] [PubMed] [Google Scholar]
- Hess A. Developmental changes in the structure of the synapse on the myelinated cell bodies of the chicken ciliary ganglion. J Cell Biol. 1965 Jun;25(3 Suppl):1–19. doi: 10.1083/jcb.25.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M., Baker R. E. Development of neuronal connections with skin grafts in frogs: behavioral and electrophysiological studies. J Comp Neurol. 1969 Oct;137(2):121–141. doi: 10.1002/cne.901370202. [DOI] [PubMed] [Google Scholar]
- Koenig H. L. Relations entre la distribution de l'activité acétylcholinestérasique et celle de l'ergastoplasme dans les neurones du ganglion ciliaire du poulet. Arch Anat Microsc Morphol Exp. 1965 Oct-Dec;54(4):937–963. [PubMed] [Google Scholar]
- LEVI-MONTALCINI R. The development to the acoustico-vestibular centers in the chick embryo in the absence of the afferent root fibers and of descending fiber tracts. J Comp Neurol. 1949 Oct;91(2):209-41, illust, incl 3 pl. doi: 10.1002/cne.900910204. [DOI] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Selective reinnervation of two cell populations in the adult pigeon ciliary ganglion. J Physiol. 1970 Nov;211(1):203–216. doi: 10.1113/jphysiol.1970.sp009275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. Synaptic transmission and cell death during normal ganglionic development. J Physiol. 1974 Sep;241(3):737–749. doi: 10.1113/jphysiol.1974.sp010681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Pilar G. The onset and development of transmission in the chick ciliary ganglion. J Physiol. 1972 May;222(3):691–713. doi: 10.1113/jphysiol.1972.sp009822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwitt R., Pilar G., Weakly J. N. Characterization of two ganglion cell populations in avian ciliary ganglia. Brain Res. 1971 Jan 22;25(2):317–334. doi: 10.1016/0006-8993(71)90441-0. [DOI] [PubMed] [Google Scholar]
- Morest D. K. The differentiation of cerebral dendrites: A study of the post-migratory neuroblast in the medial nucleus of the trapezoid body. Z Anat Entwicklungsgesch. 1969;128(4):271–289. doi: 10.1007/BF00522528. [DOI] [PubMed] [Google Scholar]
- PRESTIGE M. C. CELL TURNOVER IN THE SPINAL GANGLIA OF XENOPUS LAEVIS TADPOLES. J Embryol Exp Morphol. 1965 Feb;13:63–72. [PubMed] [Google Scholar]
- Pilar G., Landmesser L. Axotomy mimicked by localized colchicine application. Science. 1972 Sep 22;177(4054):1116–1118. doi: 10.1126/science.177.4054.1116. [DOI] [PubMed] [Google Scholar]
- Prestige M. C. The control of cell number in the lumbar spinal ganglia during the development of Xenopus laevis tadpoles. J Embryol Exp Morphol. 1967 Jun;17(3):453–471. [PubMed] [Google Scholar]
- Prestige M. C. The control of cell number in the lumbar ventral horns during the development of Xenopus laevis tadpoles. J Embryol Exp Morphol. 1967 Dec;18(3):359–387. [PubMed] [Google Scholar]
- Rogers L. A., Cowan W. M. The development of the mesencephalic nucleus of the trigeminal nerve in the chick. J Comp Neurol. 1973 Feb 1;147(3):291–320. doi: 10.1002/cne.901470302. [DOI] [PubMed] [Google Scholar]





