Abstract
1. HeLa cells were grown in normal and altered growth solutions; the ion contents, volumes, K sensitive ouabain binding, the Na-K-ATPase and the Na and K transport measured.
2. Cells grown in 1 × 10-4 M ethacrynate or low-K media for 24 hr have a raised [Na]i, a decreased [K]i, and an increased ouabain binding. Those grown in low-K also have an increased Na-K-ATPase activity.
3. When cells are put into low-K solutions the [Na]i initially rises to a high value, and then starts to fall some 8 hours later as the ouabain binding increases, suggesting that these additional sites represent working Na pumps. Flux measurements on low-K cells provide some support for this view.
4. Experiments in which sorbitol replaced [Na]o showed that the increased ouabain binding and Na-K-ATPase was related to the increase in [Na]i rather than the decrease in [K]i and was not due to a non-specific effect of [K]o change.
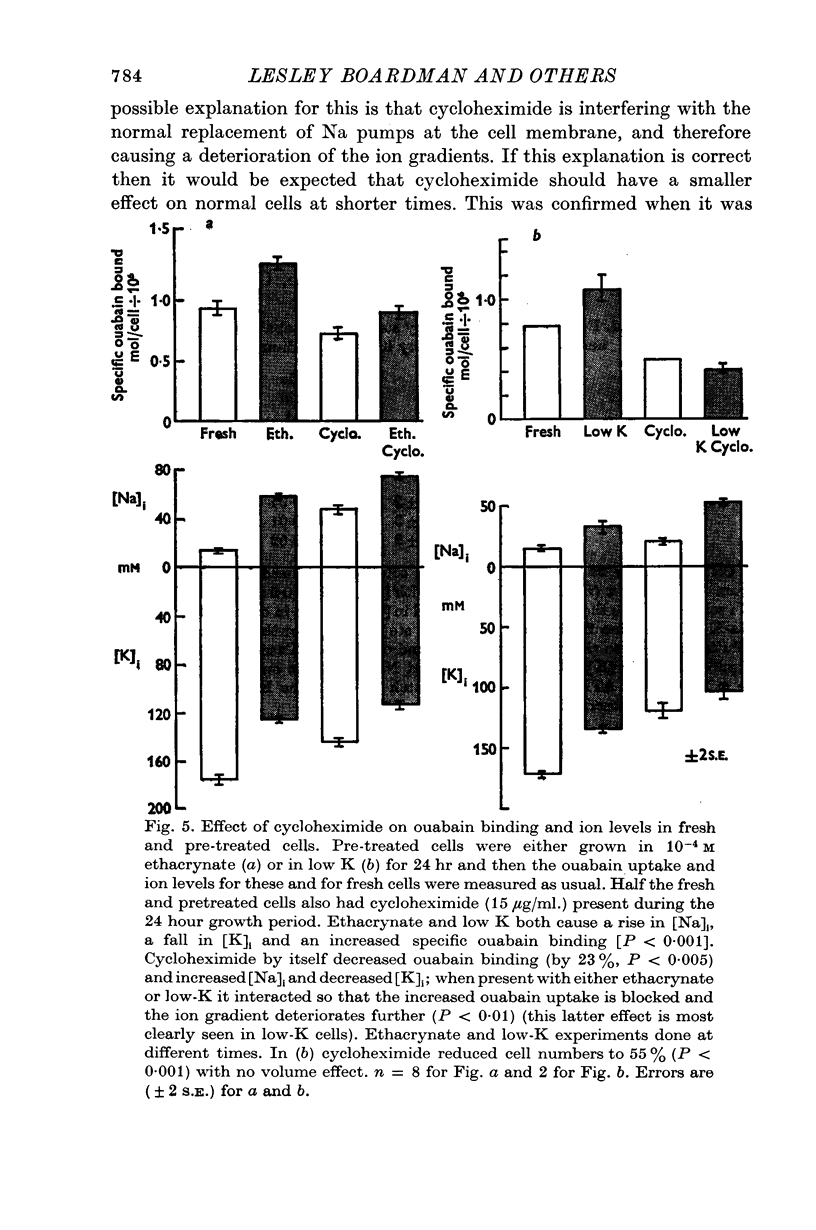
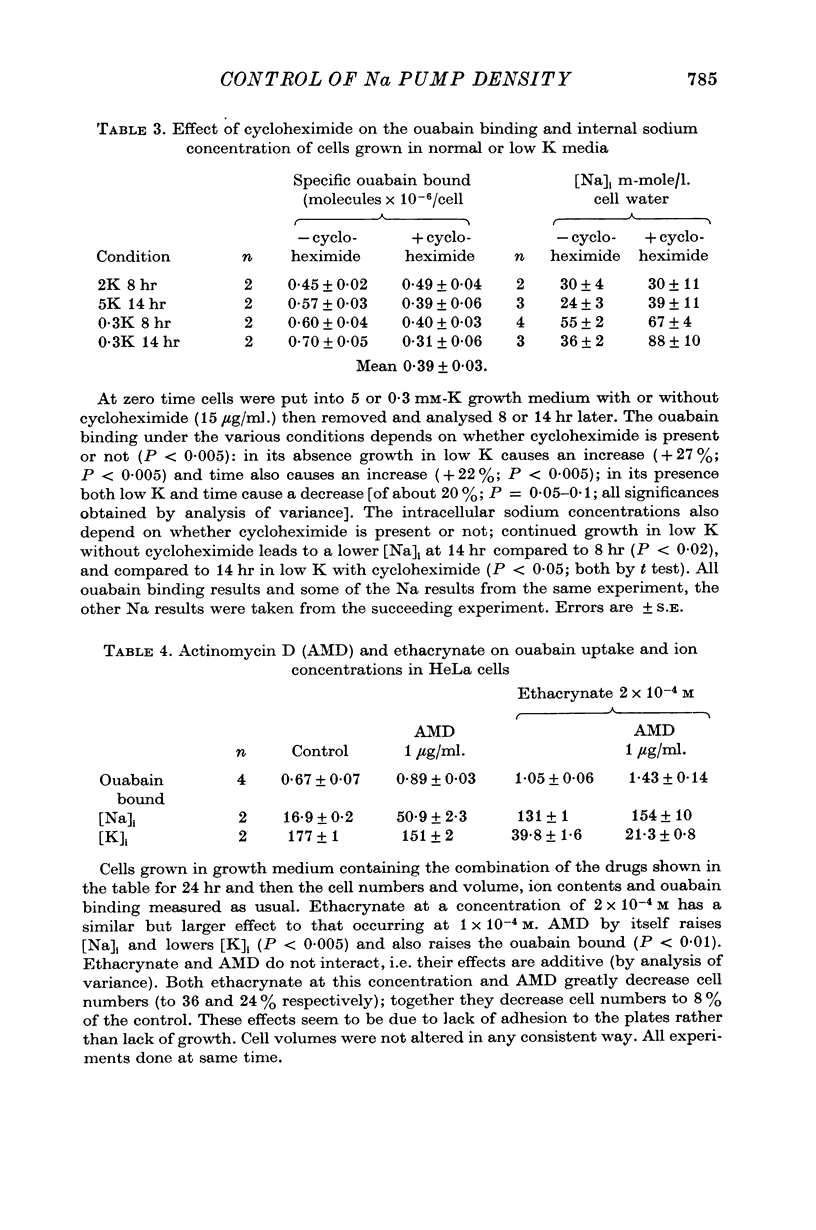
5. The protein synthesis inhibitors cycloheximide and puromycin stopped the effect of ethacrynate and low-K solutions on increased ouabain binding. They also decreased the ouabain binding and K influx in normal cells over 24 hr. Cycloheximide had similar effects on Na-K-ATPase in low-K treated and normal cells. These results suggest that protein synthesis is required for the appearance of more ouabain sensitive sites in the cell membrane, both in response to ethacrynate and low-K treatment and for normal replacement during the cell's life.
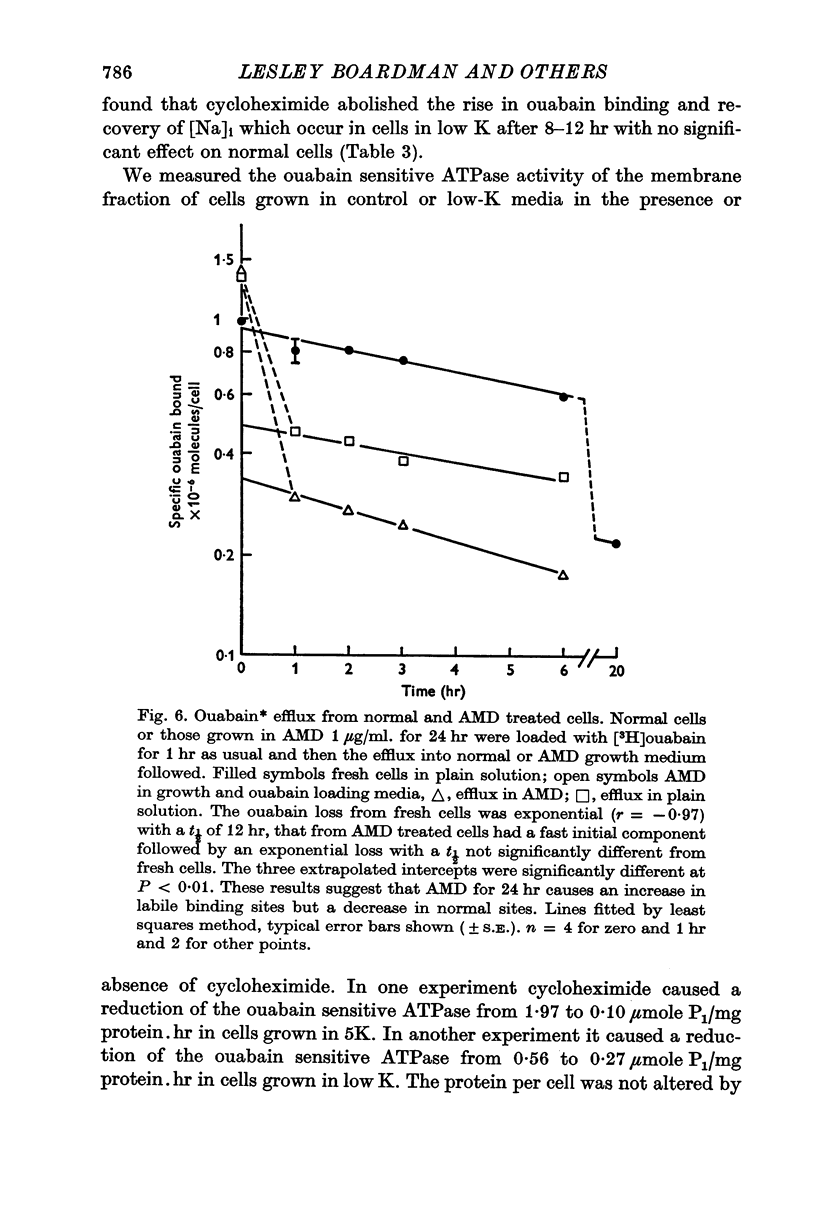
6. The RNA synthesis inhibitors actinomycin D (AMD) and cordycepin had complex effects on ouabain binding in fresh and ethacrynate treated cells. These inhibitors increased the ouabain binding but decreased the K influx. This discrepancy was due to the appearance of ouabain binding sites with different characteristics from normal sites. A limited investigation of this phenomenon was carried out. Probably AMD stops the normal replacement of sites in the membrane.
7. These results are consistent with the hypothesis that HeLa cells have a system for controlling the number of Na pumps in their membranes. This system responds to the level of [Na]i within the cell and involves protein synthesis. It is not clear to what extent the nucleus is normally involved in this process.
Full text
PDF


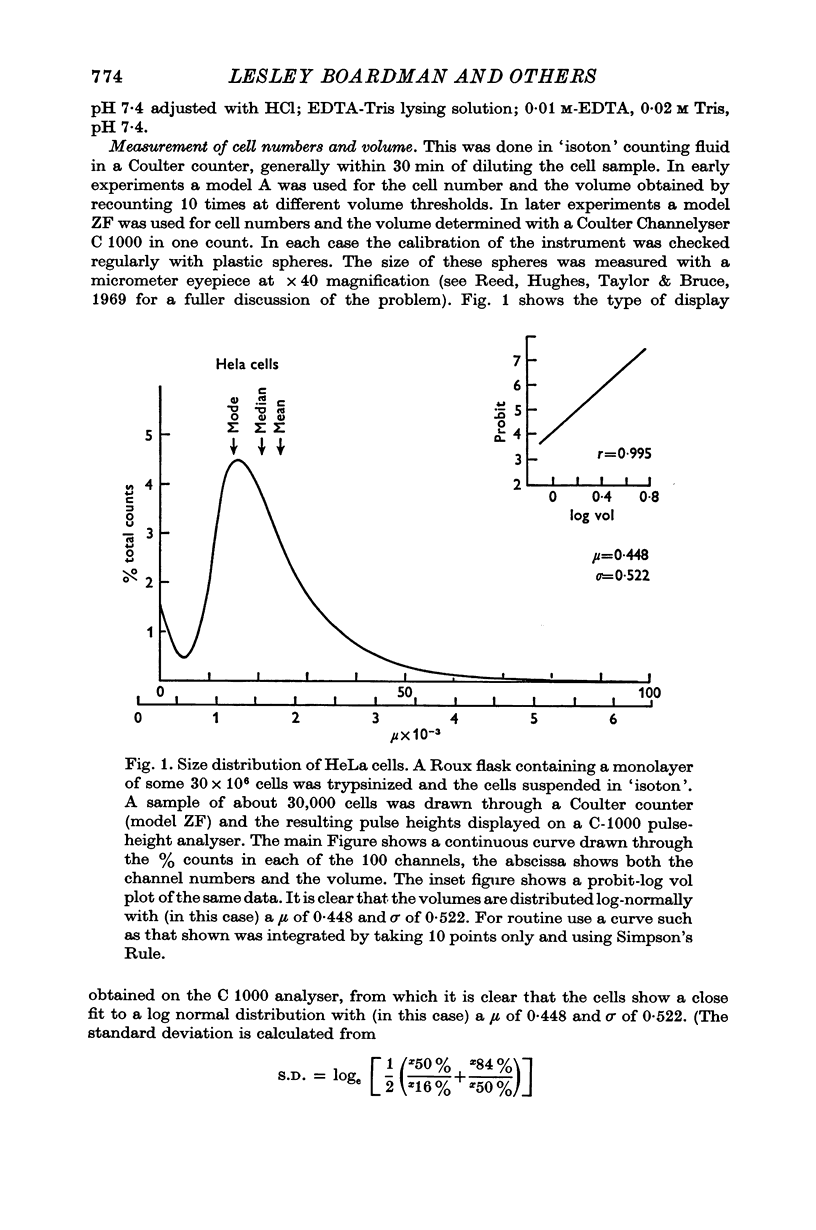



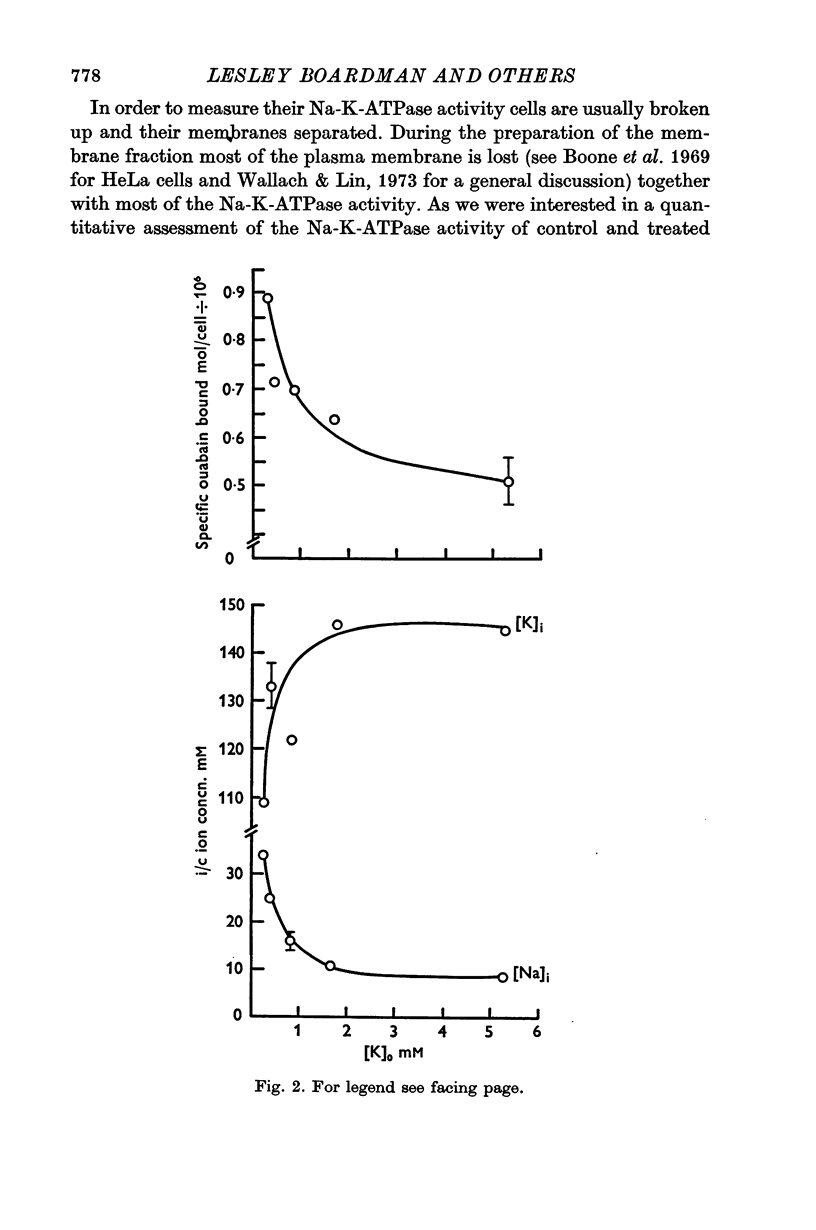

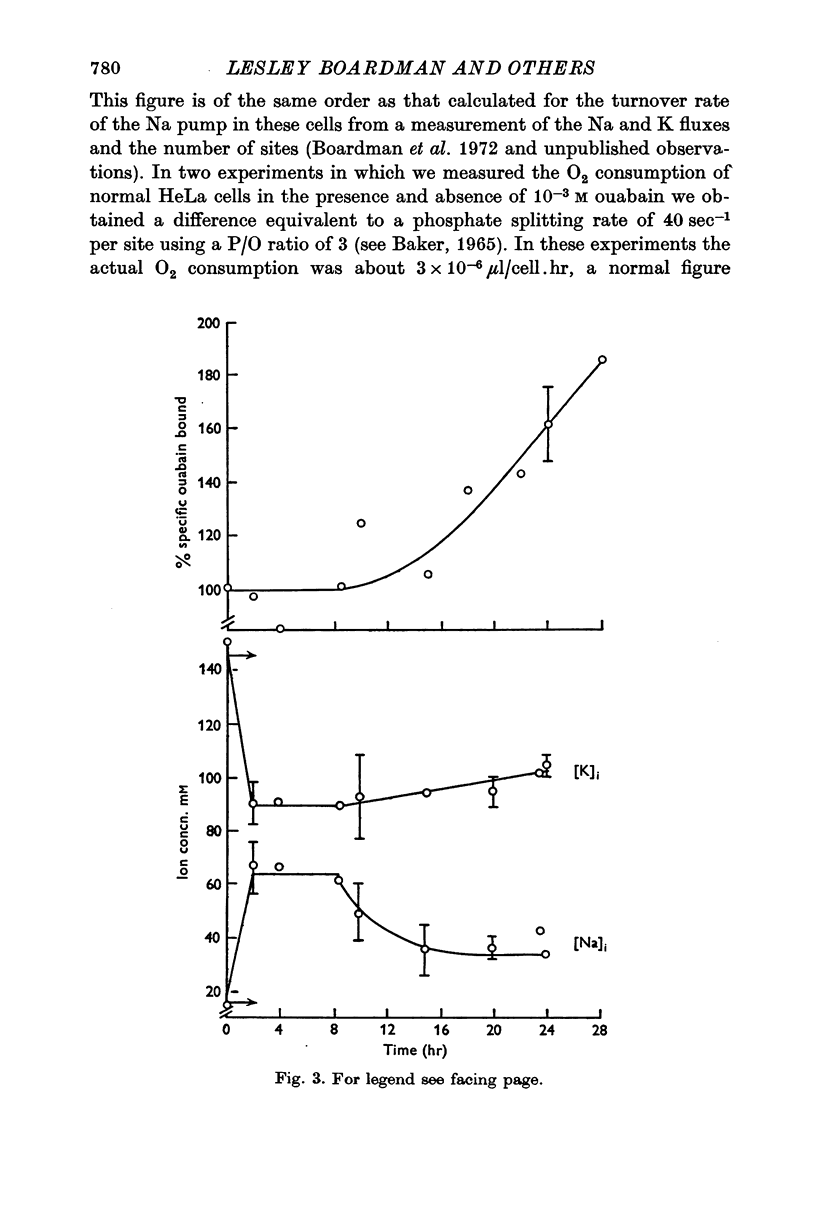
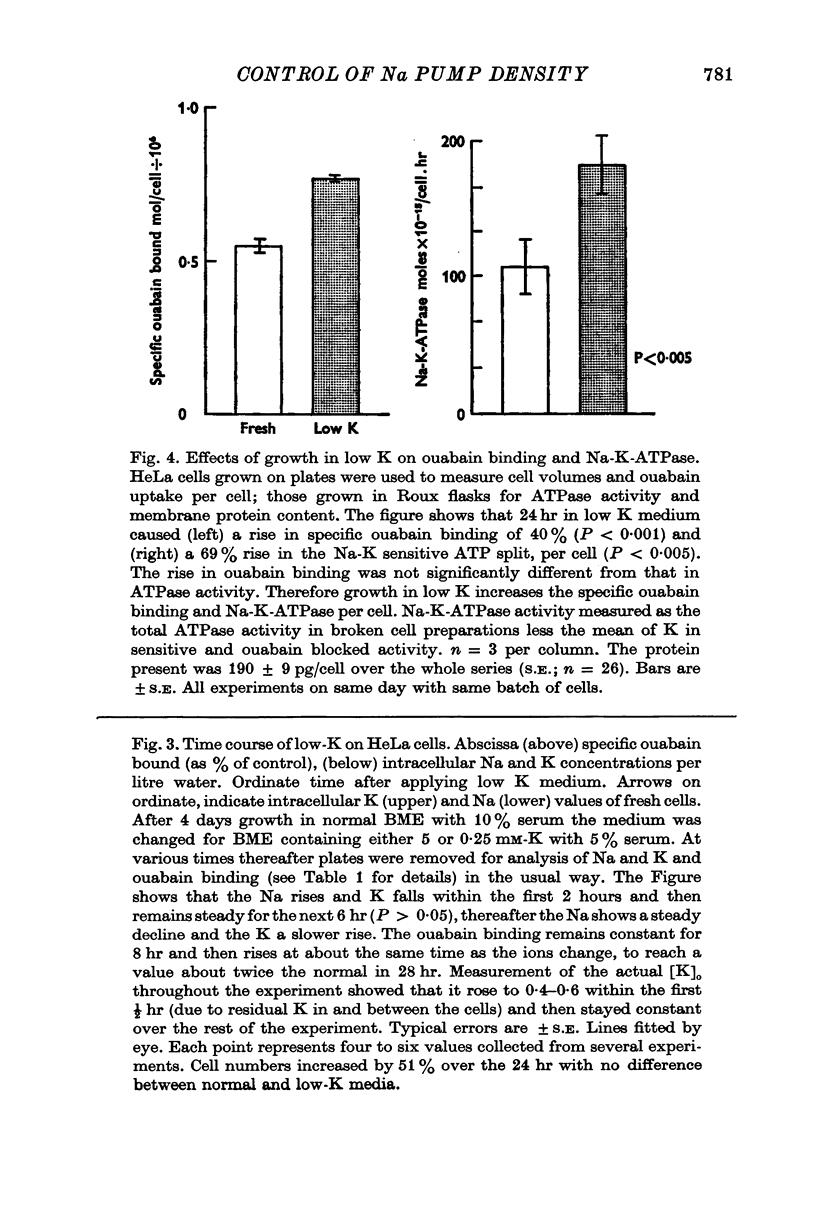










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNSTEIN R. E. Alterations in metabolic energetics and cation transport during aging of red cells. J Clin Invest. 1959 Sep;38:1572–1586. doi: 10.1172/JCI103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONTING S. L., CARAVAGGIO L. L. Studies on sodium-potassium-activated adenosinetriphosphatase. V. Correlation of enzyme activity with cation flux in six tissues. Arch Biochem Biophys. 1963 Apr;101:37–46. doi: 10.1016/0003-9861(63)90531-9. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Phosphorus metabolism of intact crab nerve and its relation to the active transport of ions. J Physiol. 1965 Sep;180(2):383–423. doi: 10.1113/jphysiol.1965.sp007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Willis J. S. Binding of the cardiac glycoside ouabain to intact cells. J Physiol. 1972 Jul;224(2):441–462. doi: 10.1113/jphysiol.1972.sp009904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E., Dick D. A., Fry D. J. The action of ethacrynic acid on sodium efflux from single toad oocytes. J Physiol. 1968 Jun;196(3):693–701. doi: 10.1113/jphysiol.1968.sp008530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman L. J., Lamb J. F., McCall D. Uptake of ( 3 H)ouabain and Na pump turnover rates in cells cultured in ouabain. J Physiol. 1972 Sep;225(3):619–635. doi: 10.1113/jphysiol.1972.sp009960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone C. W., Ford L. E., Bond H. E., Stuart D. C., Lorenz D. Isolation of plasma membrane fragments from HeLa cells. J Cell Biol. 1969 May;41(2):378–392. doi: 10.1083/jcb.41.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. M., Love R., Studzinski G. P., Ellem K. A. A correlated morphological and functional study of the effects of actinomycin D on HeLa cells. I. Effects on the nucleolar and cytoplasmic ribonucleoproteins. Exp Cell Res. 1967 Jan;45(1):106–119. doi: 10.1016/0014-4827(67)90116-4. [DOI] [PubMed] [Google Scholar]
- DANES B. S., PAUL J. Environmental factors influencing respiration of strain L cells. Exp Cell Res. 1961 Aug;24:344–355. doi: 10.1016/0014-4827(61)90437-2. [DOI] [PubMed] [Google Scholar]
- Franchi-Gazzola R., Gazzola G. C., Ronchi P., Saibene V., Guidotti G. G. Regulation of amino acid transport in chick embryo heart cells. II. Adaptive control sites for the "A mediation". Biochim Biophys Acta. 1973 Jan 26;291(2):545–556. doi: 10.1016/0005-2736(73)90506-3. [DOI] [PubMed] [Google Scholar]
- GLYNN I. M. Sodium and potassium movements in human red cells. J Physiol. 1956 Nov 28;134(2):278–310. doi: 10.1113/jphysiol.1956.sp005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautschi J. R., Kern R. M. DNA replication in mammalian cells in the presence of cycloheximide. Exp Cell Res. 1973 Jul;80(1):15–26. doi: 10.1016/0014-4827(73)90270-x. [DOI] [PubMed] [Google Scholar]
- Gracey M., Burke V., Oshin A. Active intestinal transport of D-fructose. Biochim Biophys Acta. 1972 May 9;266(2):397–406. doi: 10.1016/0005-2736(72)90096-x. [DOI] [PubMed] [Google Scholar]
- Hare J. D. Studies on the mechanism of serum stimulation of uridine uptake in serum-less mouse cells. Biochim Biophys Acta. 1972 Mar 17;255(3):905–916. doi: 10.1016/0005-2736(72)90402-6. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The resting exchange of radioactive potassium in crab nerve. J Physiol. 1951 Mar;113(1):73–98. doi: 10.1113/jphysiol.1951.sp004557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney F. T., Lee K. L., Stiles C. D., Fritz J. E. Further evidence against post-transcriptional control of inducible tyrosine aminotransferase synthesis in cultured hepatoma cells. Nat New Biol. 1973 Dec 19;246(155):208–210. doi: 10.1038/newbio246208a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb J. F., Boardman L. J., Newton J. P., Aiton J. F. Effect of calf serum on sodium pump density in HeLa cells. Nat New Biol. 1973 Mar 28;242(117):115–117. doi: 10.1038/newbio242115a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. F., MacKinnon M. G. Effect of ouabain and metabolic inhibitors on the Na and K movements and nucleotide contents of L cells. J Physiol. 1971 Mar;213(3):665–682. doi: 10.1113/jphysiol.1971.sp009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. F., McCall D. Effect of prolonged ouabain treatment of Na, K, Cl and Ca concentration and fluxes in cultured human cells. J Physiol. 1972 Sep;225(3):599–617. doi: 10.1113/jphysiol.1972.sp009959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. F., McCall D. Uptake of (3H)ouabain and Na pump turnover rates in monolayer cultures of Girardi heart cells. J Physiol. 1971 Mar;213(2):57P–58P. [PubMed] [Google Scholar]
- PRANKERD T. A. The ageing of red cells. J Physiol. 1958 Sep 23;143(2):325–331. doi: 10.1113/jphysiol.1958.sp006062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Rosbash M., Penman M. Messenger and heterogeneous nuclear RNA in HeLa cells: differential inhibition by cordycepin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1878–1885. doi: 10.1073/pnas.67.4.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. D., Hughes T. J., Taylor W. B., Bruce W. R. The electronic determination of the size distribution of L-cell minicolonies. Exp Cell Res. 1969 Aug;56(2):435–442. doi: 10.1016/0014-4827(69)90036-6. [DOI] [PubMed] [Google Scholar]
- Vaughan G. L., Cook J. S. Regeneration of cation-transport capacity in HeLa cell membranes after specific blockade by ouabain. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2627–2631. doi: 10.1073/pnas.69.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. F., Lin P. S. A critical evaluation of plasma membrane fractionation. Biochim Biophys Acta. 1973 Nov;300(3):211–254. doi: 10.1016/0304-4157(73)90005-1. [DOI] [PubMed] [Google Scholar]
- Weiss B. G. The dependence of DNA synthesis on protein synthesis in HeLa S3 cells. J Cell Physiol. 1969 Feb;73(1):85–90. doi: 10.1002/jcp.1040730112. [DOI] [PubMed] [Google Scholar]
- Wheeler K. P., Whittam R. The involvement of phosphatidylserine in adenosine triphosphatase activity of the sodium pump. J Physiol. 1970 Apr;207(2):303–328. doi: 10.1113/jphysiol.1970.sp009063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis A. A., Arimura G. K. Sodium-potassium dependent adenosine triphosphatase of mammalian reticulocytes and mature red blood cells. Proc Soc Exp Biol Med. 1966 Feb;121(2):327–329. doi: 10.3181/00379727-121-30770. [DOI] [PubMed] [Google Scholar]


