Abstract
1. Glycogen depletion pattern in human skeletal muscle fibres was studied after bicycle exercise of varying intensity performed at different pedalling rates. Work intensities studied were equivalent to 30-150% of V̇O2 max. with pedalling rates of 30-120 rev/min.
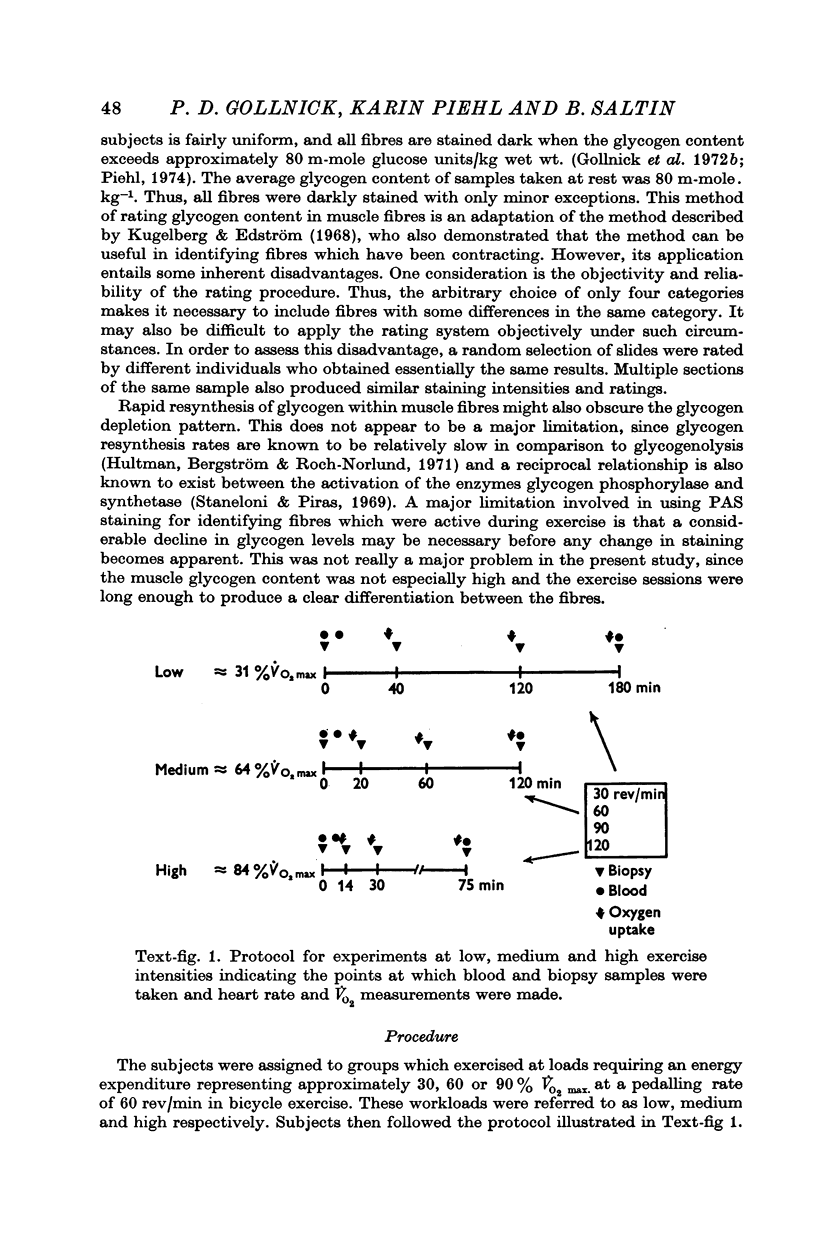
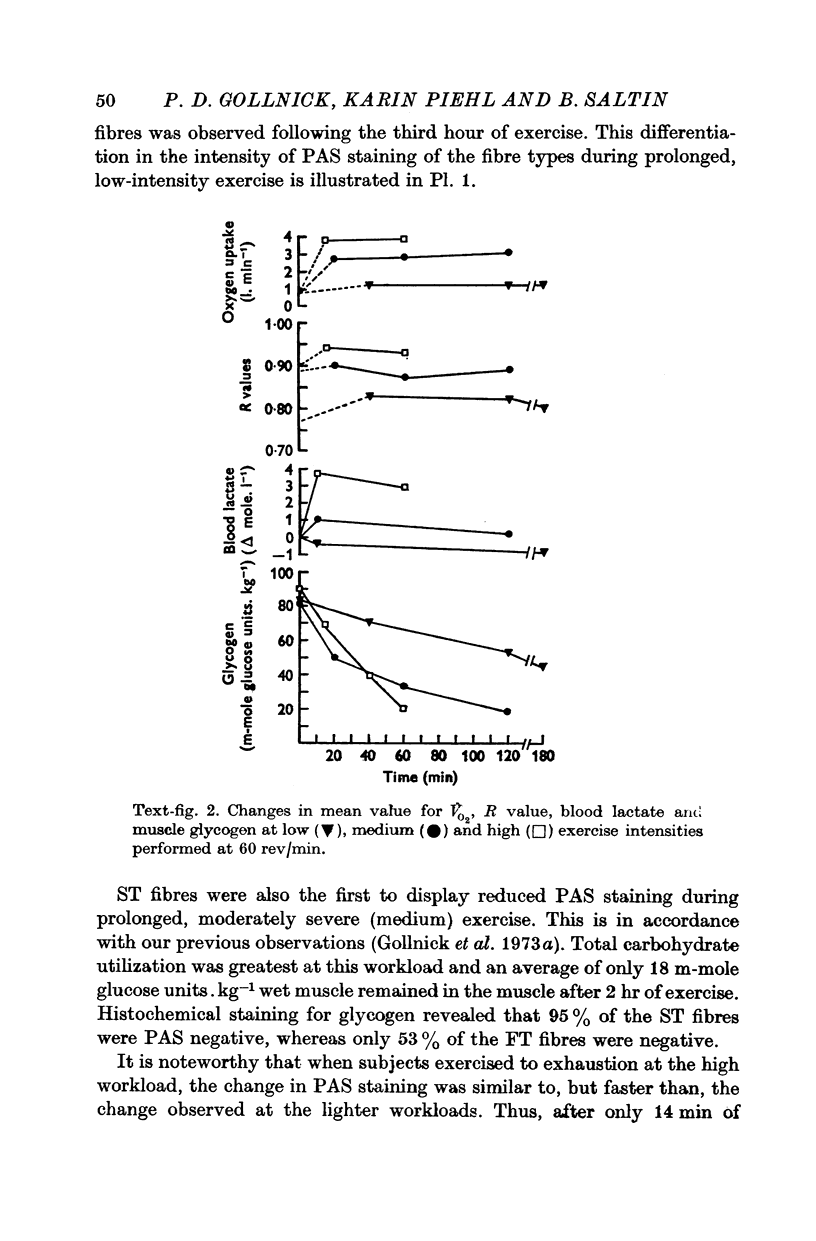
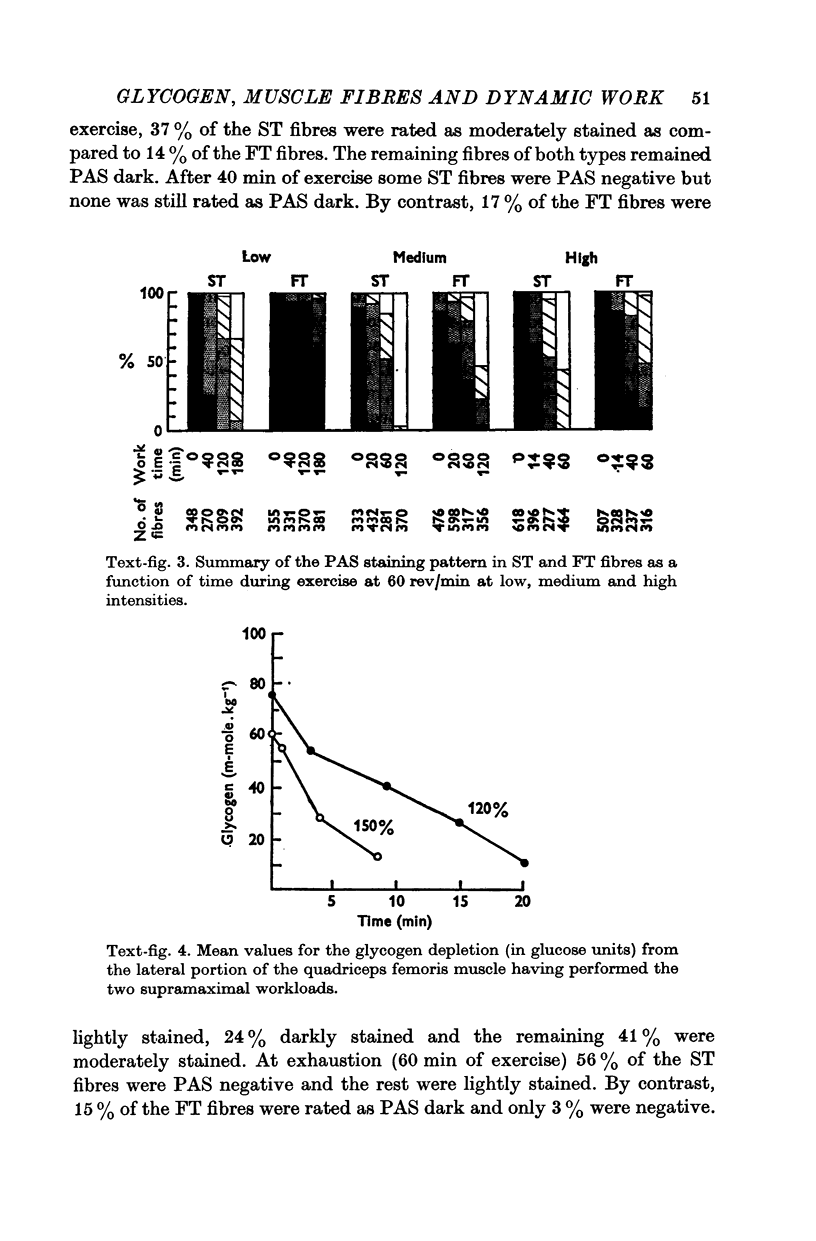
2. Glycogen depletion increased dramatically with increasing exercise intensity; depletion was 2·7 and 7·4 times greater respectively at workloads demanding 64 and 84% V̇O2 max. than at workloads calling for 31% V̇O2 max. Even greater rates of glycogen utilization occurred at supramaximal loads.
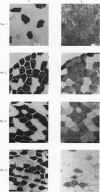
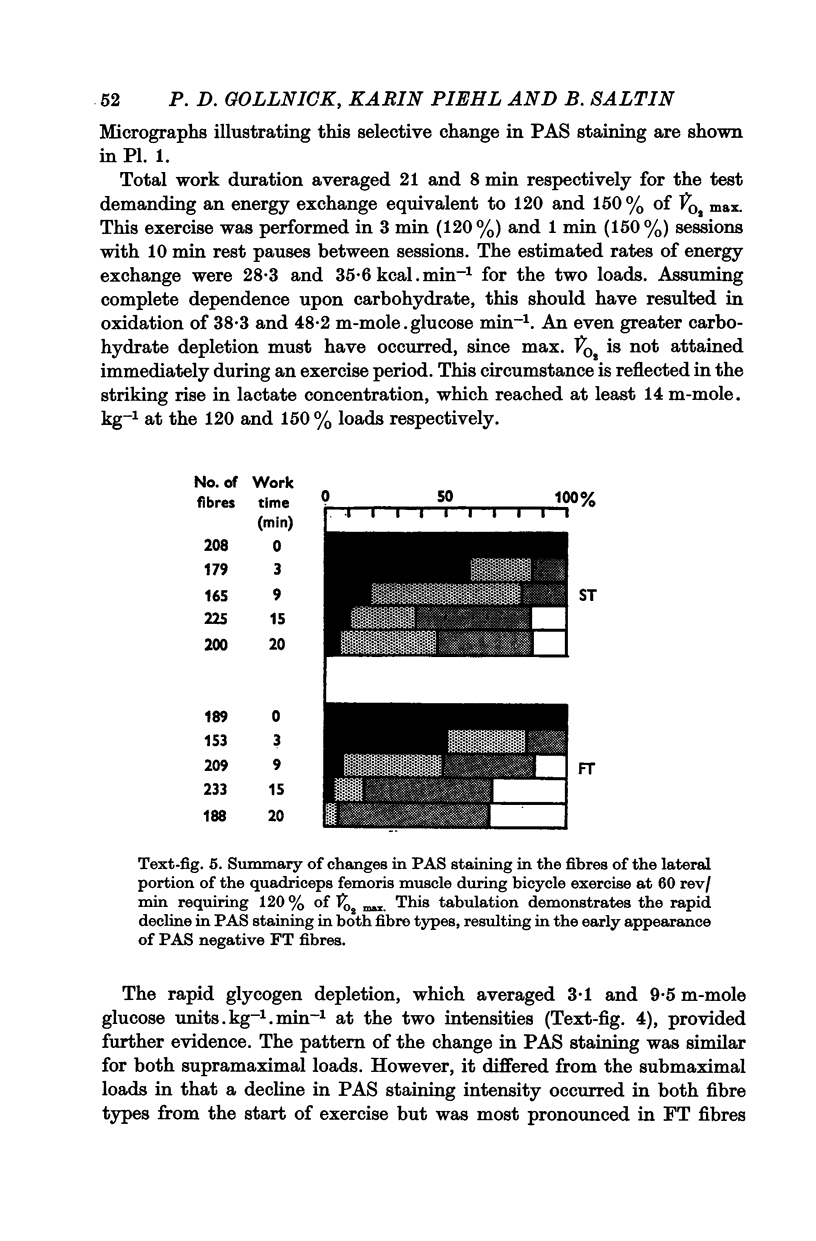
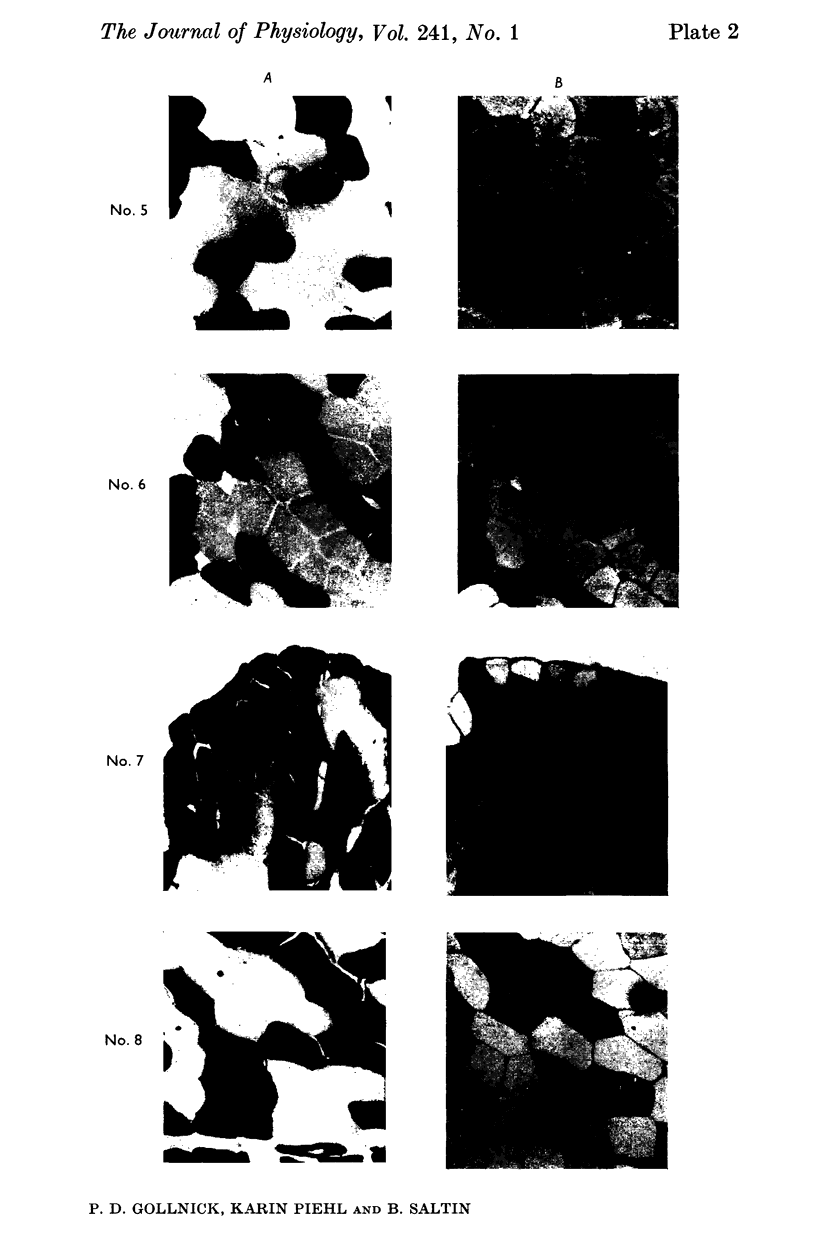
3. Slow twitch, high oxidative (ST) fibres were the first to lose glycogen (reduced PAS staining) at all workloads below V̇O2 max. Progressive glycogen depletion occurred in fast twitch (FT) fibres as work continued. Large quantities of glycogen remained in the muscle after 3 hr of exercise at low exercise intensity. This was almost exclusively found in FT fibres. At workloads exceeding maximal aerobic power, there was an initial depletion of glycogen in both fibre types. Varying the pedalling rate and, thus, the total force exerted in each pedal thrust had no effect on the pattern of glycogen depletion in the fibres.
4. Results point to primary reliance upon ST fibres during submaximal endurance exercise, FT fibres being recruited after ST fibres are depleted of glycogen. During exertion requiring energy expenditure greater than the maximal aerobic power, both fibre types appeared to be continuously involved in carrying out the exercise.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard R. J., Edgerton V. R., Furukawa T., Peter J. B. Histochemical, biochemical, and contractile properties of red, white, and intermediate fibers. Am J Physiol. 1971 Feb;220(2):410–414. doi: 10.1152/ajplegacy.1971.220.2.410. [DOI] [PubMed] [Google Scholar]
- Costill D. L., Gollnick P. D., Jansson E. D., Saltin B., Stein E. M. Glycogen depletion pattern in human muscle fibres during distance running. Acta Physiol Scand. 1973 Nov;89(3):374–383. doi: 10.1111/j.1748-1716.1973.tb05532.x. [DOI] [PubMed] [Google Scholar]
- DUBOWITZ V., PEARSE A. G. A comparative histochemical study of oxidative enzyme and phosphorylase activity in skeletal muscle. Z Zellforch Microsk Anat Histochem. 1960;2:105–117. doi: 10.1007/BF00744575. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Armstrong R. B., Saubert C. W., 4th, Piehl K., Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol. 1972 Sep;33(3):312–319. doi: 10.1152/jappl.1972.33.3.312. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Armstrong R. B., Sembrowich W. L., Shepherd R. E., Saltin B. Glycogen depletion pattern in human skeletal muscle fibers after heavy exercise. J Appl Physiol. 1973 May;34(5):615–618. doi: 10.1152/jappl.1973.34.5.615. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Piehl K., Saubert C. W., 4th, Armstrong R. B., Saltin B. Diet, exercise, and glycogen changes in human muscle fibers. J Appl Physiol. 1972 Oct;33(4):421–425. doi: 10.1152/jappl.1972.33.4.421. [DOI] [PubMed] [Google Scholar]
- Karlsson J., Diamant B., Saltin B. Muscle metabolites during submaximal and maximal exercise in man. Scand J Clin Lab Invest. 1970 Dec;26(4):385–394. doi: 10.3109/00365517009046250. [DOI] [PubMed] [Google Scholar]
- Karlsson J., Saltin B. Lactate, ATP, and CP in working muscles during exhaustive exercise in man. J Appl Physiol. 1970 Nov;29(5):596–602. doi: 10.1152/jappl.1970.29.5.598. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Edström L. Differential histochemical effects of muscle contractions on phosphorylase and glycogen in various types of fibres: relation to fatigue. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):415–423. doi: 10.1136/jnnp.31.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955 May;3(3):170–195. doi: 10.1177/3.3.170. [DOI] [PubMed] [Google Scholar]
- Piehl K. Time course for refilling of glycogen stores in human muscle fibres following exercise-induced glycogen depletion. Acta Physiol Scand. 1974 Feb;90(2):297–302. doi: 10.1111/j.1748-1716.1974.tb05592.x. [DOI] [PubMed] [Google Scholar]
- Staneloni R., Piras R. Changes in glycogen synthetase and phosphorylase during muscular contraction. Biochem Biophys Res Commun. 1969 Sep 10;36(6):1032–1038. doi: 10.1016/0006-291x(69)90308-8. [DOI] [PubMed] [Google Scholar]