Abstract
1. Re-innervation of soleus was studied in the mouse after either crushing the sciatic nerve or re-implanting the nerve to soleus outside the original end-plate region.
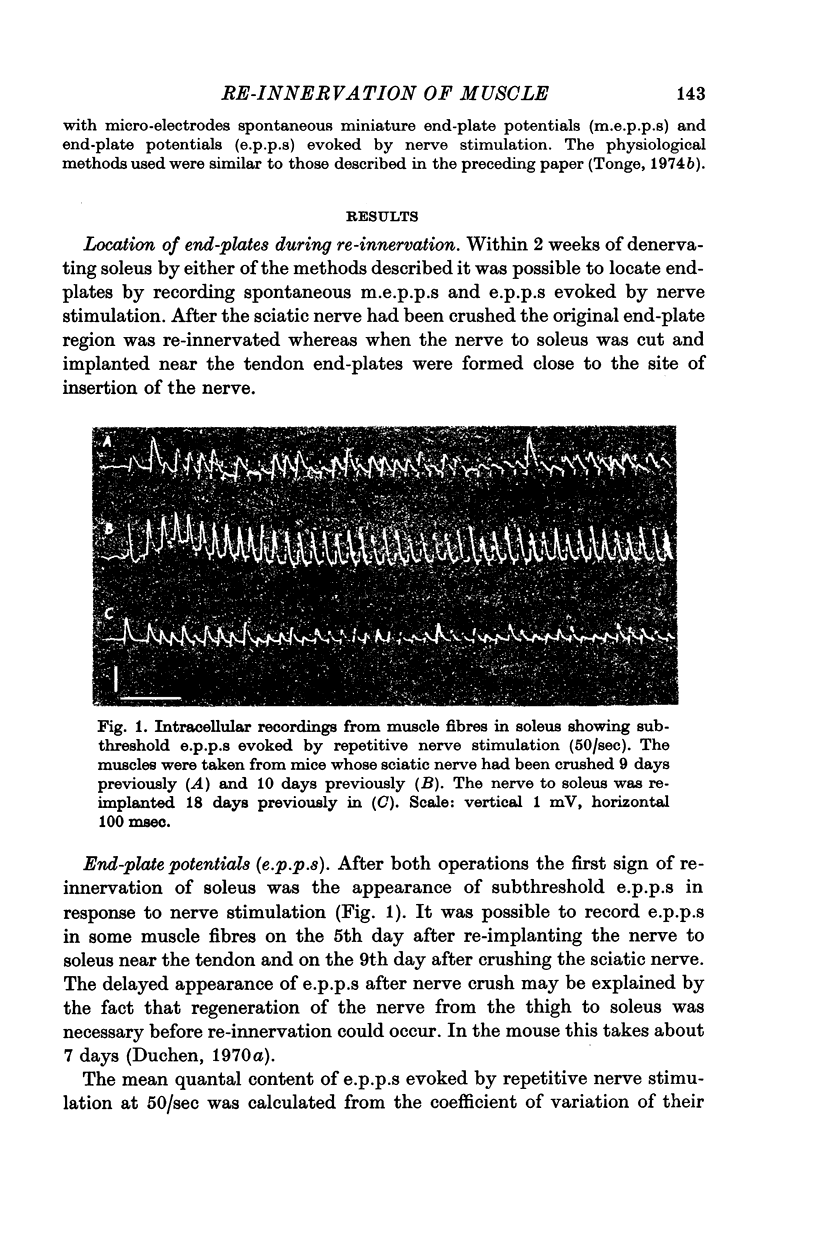
2. During the early stages of re-innervation subthreshold end-plate potentials (e.p.p.s) were recorded in muscle fibres in response to nerve stimulation. Later the e.p.p.s became large enough to evoke action potentials in muscle fibres.
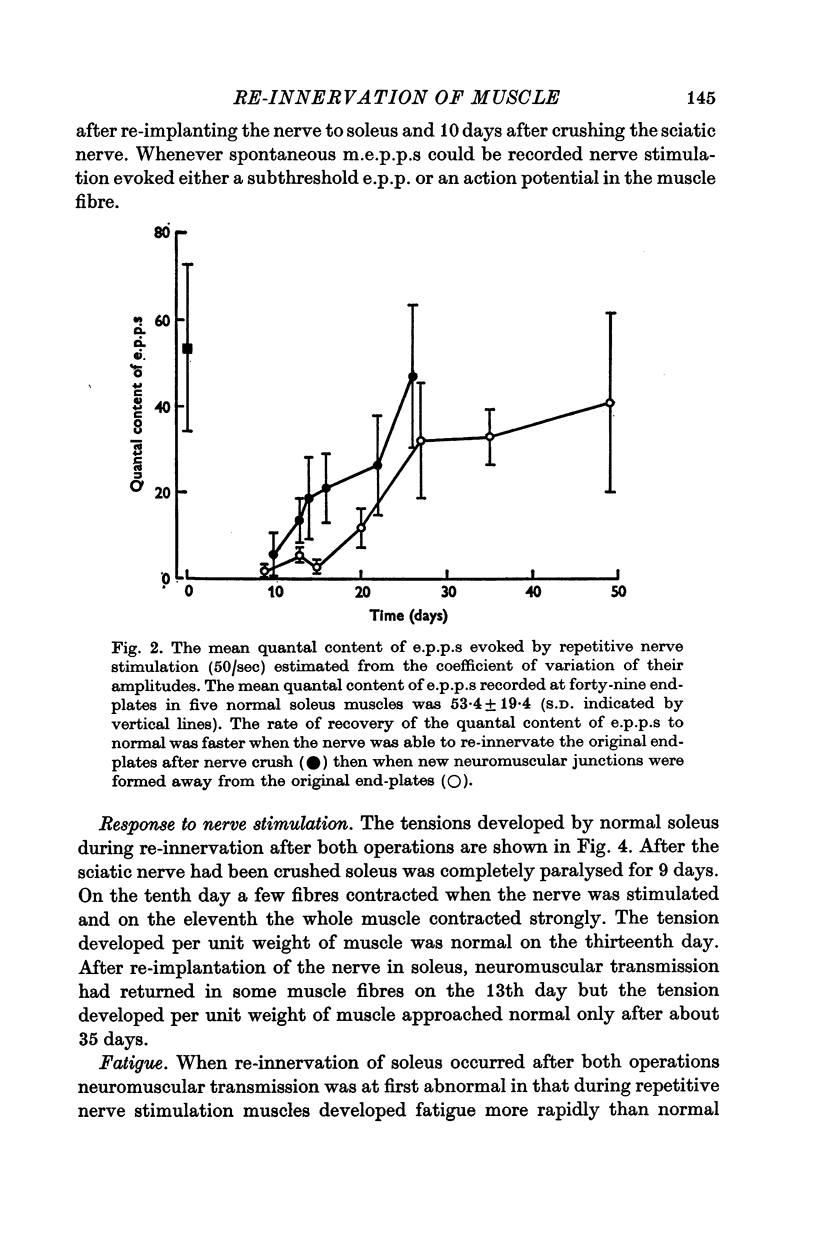
3. The rate of recovery of the release of acetylcholine (ACh) as estimated from the quantal content of e.p.p.s was faster when nerves re-innervated the old end-plate region after nerve crush than after re-implantation so that new neuromuscular junctions were formed.
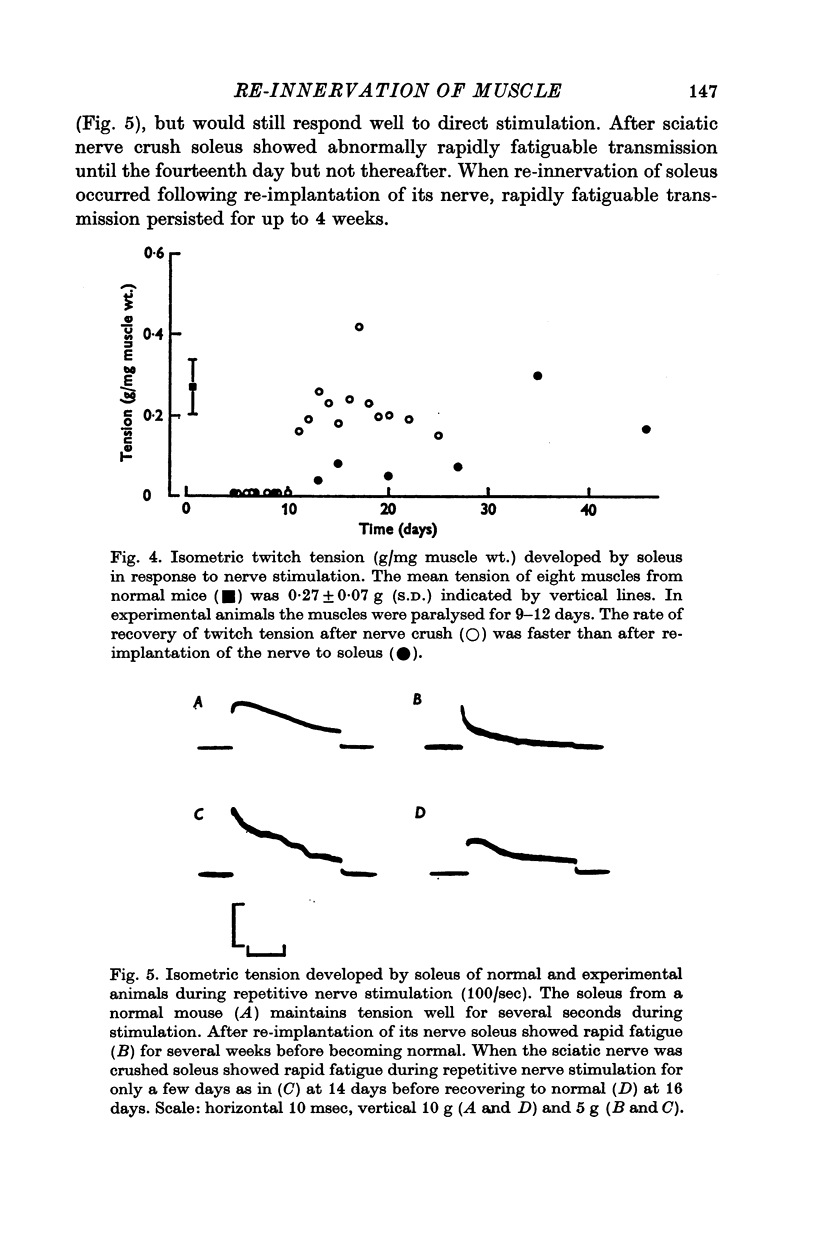
4. During re-innervation soleus fatigued more rapidly than normal during repetitive nerve stimulation. The fatigue was due to failure of neuromuscular transmission associated with an impaired release of ACh.
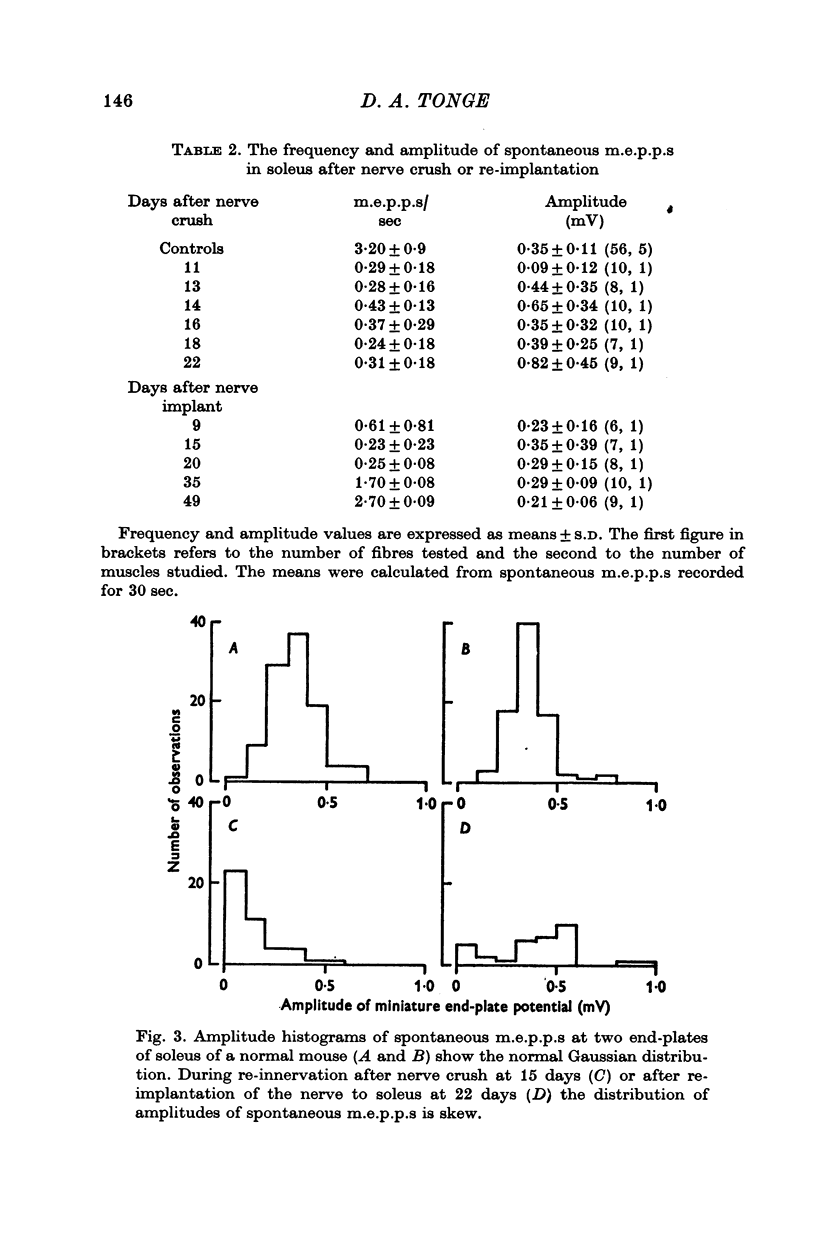
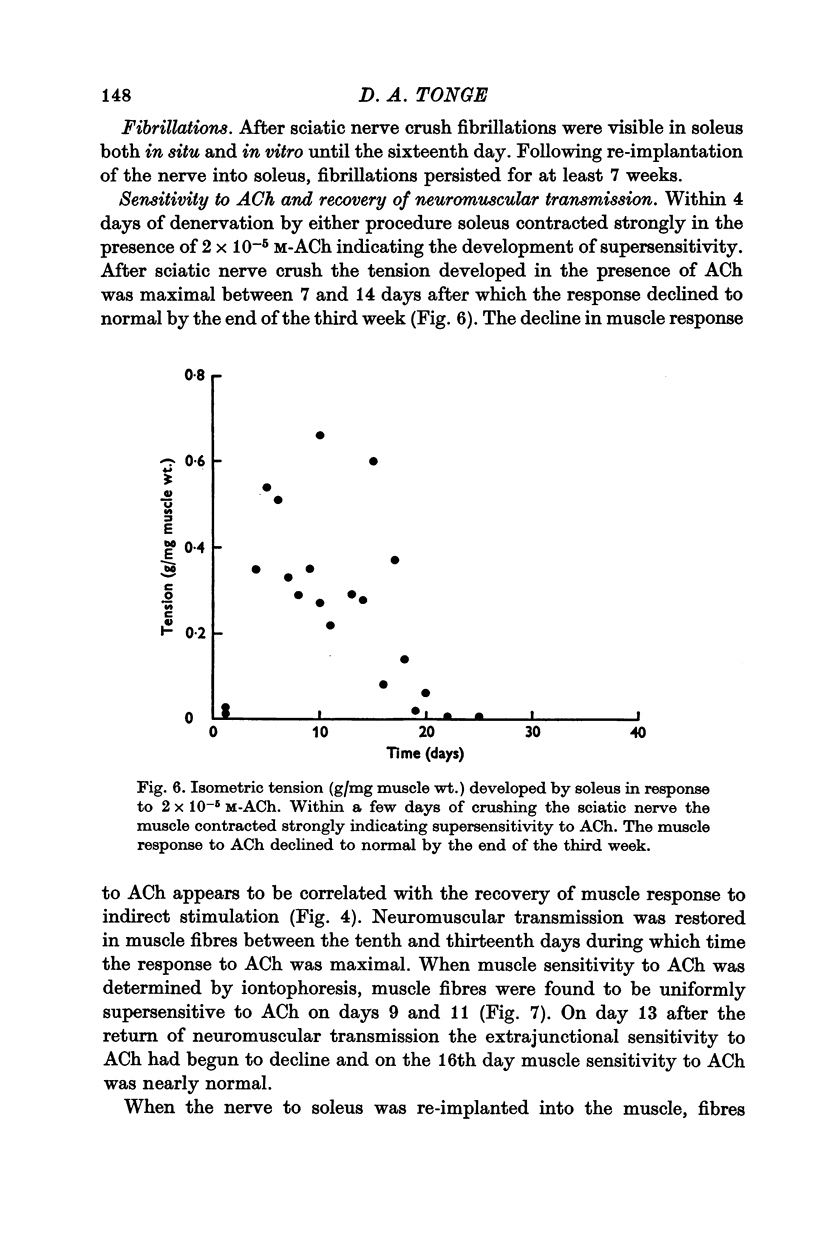
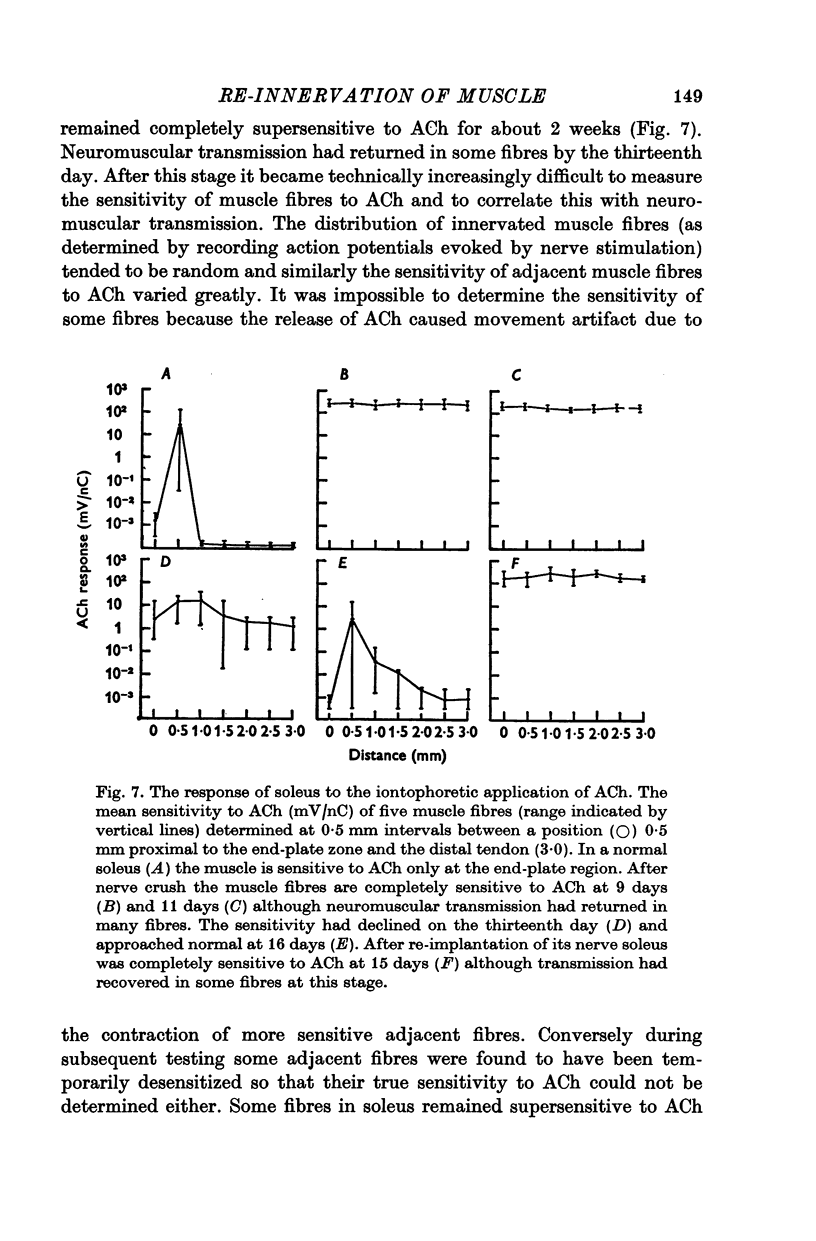
5. During re-innervation soleus was supersensitive to ACh until nerve stimulation was capable of evoking action potentials in muscle fibres.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G., Taylor R. S. The formation of synapses in reinnervated and cross-reinnervated adult avian muscle. J Physiol. 1973 Apr;230(2):331–357. doi: 10.1113/jphysiol.1973.sp010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M., Miledi R. Lack of correspondence between the amplitudes of spontaneous potentials and unit potentials evoked by nerve impulses at regenerating neuromuscular junctions. Nat New Biol. 1971 Jul 28;232(30):126–128. doi: 10.1038/newbio232126a0. [DOI] [PubMed] [Google Scholar]
- Duchen L. W. Changes in motor innervation and cholinesterase localization induced by botulinum toxin in skeletal muscle of the mouse: differences between fast and slow muscles. J Neurol Neurosurg Psychiatry. 1970 Feb;33(1):40–54. doi: 10.1136/jnnp.33.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen L. W., Strich S. J. The effects of botulinum toxin on the pattern of innervation of skeletal muscle in the mouse. Q J Exp Physiol Cogn Med Sci. 1968 Jan;53(1):84–89. doi: 10.1113/expphysiol.1968.sp001948. [DOI] [PubMed] [Google Scholar]
- Duchen L. W. The effects in the mouse of nerve crush and regneration on the innervation of skeletal muscles paralysed by Clostridium botulinum toxin. J Pathol. 1970 Sep;102(1):9–14. doi: 10.1002/path.1711020103. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Tonge D. A. The effects of tetanus toxin on neuromuscular transmission and on the morphology of motor end-plates in slow and fast skeletal muscle of the mouse. J Physiol. 1973 Jan;228(1):157–172. doi: 10.1113/jphysiol.1973.sp010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Quastel D. M. A quantitative study of end-plate potentials in isolated human muscle. J Physiol. 1965 Jun;178(3):505–529. doi: 10.1113/jphysiol.1965.sp007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fex S., Jirmanová I. Innervation by nerve implants of "fast" and "slow" skeletal muscles of the rat. Acta Physiol Scand. 1969 Jul;76(3):257–269. doi: 10.1111/j.1748-1716.1969.tb04469.x. [DOI] [PubMed] [Google Scholar]
- Fex S., Thesleff S. The time required for innervation of denervated muscles by nerve implants. Life Sci. 1967 Mar 15;6(6):635–639. doi: 10.1016/0024-3205(67)90100-2. [DOI] [PubMed] [Google Scholar]
- Gwyn D. G., Aitken J. T. The formation of new motor endplates in mammalian skeletal muscle. J Anat. 1966 Jan;100(Pt 1):111–126. [PMC free article] [PubMed] [Google Scholar]
- Koenig J., Pecot-Dechavassine M. Relations entre l'apparition des potentiels miniatures spontanes et l'ultrastructure des plaques motrices en voie de reinnervation et de neoformation chez le rat. Brain Res. 1971 Mar 19;27(1):43–57. doi: 10.1016/0006-8993(71)90371-4. [DOI] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Robbins N., Yonezawa T. Physiological studies during formation and development of rat neuromuscular junctions in tissue culture. J Gen Physiol. 1971 Oct;58(4):467–481. doi: 10.1085/jgp.58.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THESLEFF S. Supersensitivity of skeletal muscle produced by botulinum toxin. J Physiol. 1960 Jun;151:598–607. doi: 10.1113/jphysiol.1960.sp006463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMSON J. D., MORGAN J. A., HINES H. M. Physiologic characteristics of regenerating mammalian nerve and muscle. Am J Physiol. 1950 Apr 1;161(1):142–150. doi: 10.1152/ajplegacy.1950.161.1.142. [DOI] [PubMed] [Google Scholar]
- Tonge D. A. Chronic effects of botulinum toxin on neuromuscular transmission and sensitivity to acetylcholine in slow and fast skeletal muscle of the mouse. J Physiol. 1974 Aug;241(1):127–139. doi: 10.1113/jphysiol.1974.sp010644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge D. A. Proceedings: Reinnervation of skeletal muscle in the mouse. J Physiol. 1974 Jan;236(1):22P–23P. [PubMed] [Google Scholar]


