Abstract
1. Paired frog sartorius muscles were exposed to Ringer solutions labelled with 22Na+ for about 20 min. At the end of this exposure one of them was stimulated supramaximally one hundred to two hundred times. Immediately after the stimulation both members of the pair were washed in a series of tubes filled with a Na+-free medium containing 3 × 10-5 M strophanthidin.
2. Under the above conditions the intracellular component of the efflux was exponential with an average time constant (τ) of 388 min, that is, approximately four times longer than in the presence of normal Ringer. On the other hand the mean τ for the washout of the interfibre space was 3·2 min.
3. From the extrapolation to time zero of the intracellular component of the washout curve the initial intracellular radioactivity of both muscles was obtained and the resting and extra Na+ influx were calculated.
4. The mean surface membrane area/muscle weight ratio was found to be 552 cm2.g-1 and the mean fibre diameter 53·4 μm for muscles weighing on the average 60 mg.
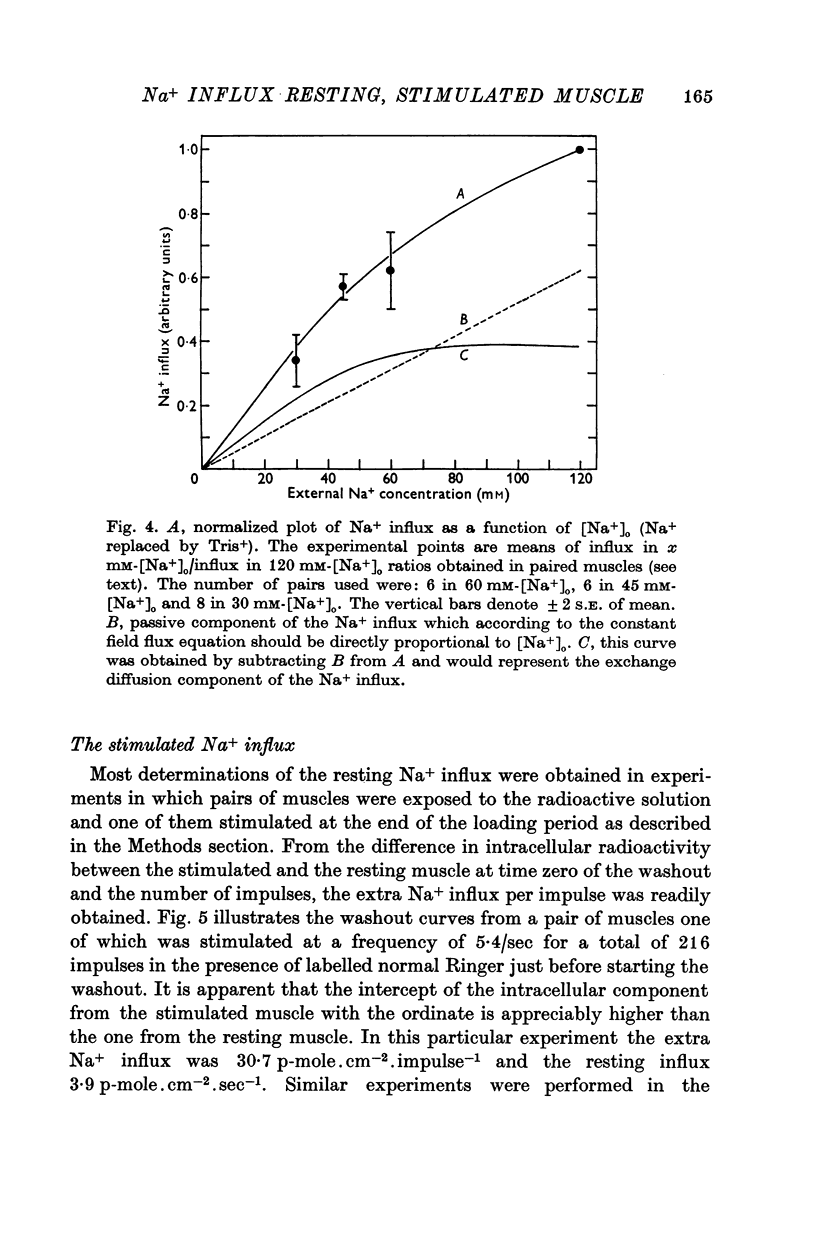
5. The average resting Na+ influx in the presence of normal Ringer was 4·7 p-mole.cm-2.sec-1. As the external Na+ concentration ([Na+]0) was reduced the Na+ influx diminished in a non-linear fashion. This non-linearity could be accounted for by the presence in the influx of a Na+ for Na+ exchange fraction which saturates at low [Na+]0.
6. The mean extra Na+ influx in the presence of normal Ringer was 27·4 p-mole.cm-2.impulse-1 and was not significantly affected either by halving [Na+]0 or by varying the frequency of stimulation. When [Na+]0 was reduced to 45 mM by partial replacement of Na+ by Tris+ the extra influx was significantly higher than when choline+ instead of Tris+ was used to substitute for Na+.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Noda K. Effect of the local anesthetic quatacaine on the membrane potential and sodium conductance of frog muscle fibers. Jpn J Physiol. 1972 Jun;22(3):281–293. doi: 10.2170/jjphysiol.22.281. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Caputo C., Gonzalez-Serratos H., Venosa R. A. Sodium dependence of the inward spread of activation in isolated twitch muscle fibres of the frog. J Physiol. 1972 Jun;223(2):507–523. doi: 10.1113/jphysiol.1972.sp009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESMEDT J. E. Electrical activity and intracellular sodium concentration in frog muscle. J Physiol. 1953 Jul;121(1):191–205. doi: 10.1113/jphysiol.1953.sp004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS C., RITCHIE J. M., WILKIE D. R. The effect of some cations on the active state of muscle. J Physiol. 1956 Aug 28;133(2):412–419. doi: 10.1113/jphysiol.1956.sp005596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlij D., Leblanc G. The effects of ethacrynic acid and other sulphydryl reagents on sodium fluxes in frog muscle. J Physiol. 1971 Apr;214(2):327–347. doi: 10.1113/jphysiol.1971.sp009435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferroni A., Blanchi D. Maximum rate of depolarization of single muscle fiber in normal and low sodium solutions. J Gen Physiol. 1965 Sep;49(1):17–25. doi: 10.1085/jgp.49.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Movements of Na and K in single muscle fibres. J Physiol. 1959 Mar 3;145(2):405–432. doi: 10.1113/jphysiol.1959.sp006150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. The effect of diameter on the electrical constants of frog skeletal muscle fibres. J Physiol. 1972 Feb;221(1):105–120. doi: 10.1113/jphysiol.1972.sp009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowicz P., Taylor J. W., Waggoner D. M. Fractionation of sodium effux in frog sartorius muscles by strophanthidin and removal of external sodium. J Gen Physiol. 1970 Mar;55(3):401–425. doi: 10.1085/jgp.55.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The resting exchange of radioactive potassium in crab nerve. J Physiol. 1951 Mar;113(1):73–98. doi: 10.1113/jphysiol.1951.sp004557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The effect of external sodium concentration on the sodium fluxes in frog skeletal muscle. J Physiol. 1959 Oct;147:591–625. doi: 10.1113/jphysiol.1959.sp006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASHIMA H., MATSUMURA M. Roles of external ions in the excitation-contraction coupling of frog skeletal muscle. Jpn J Physiol. 1962 Dec 15;12:639–653. doi: 10.2170/jjphysiol.12.639. [DOI] [PubMed] [Google Scholar]
- MULLINS L. J., FRUMENTO A. S. The concentration dependence of sodium efflux from muscle. J Gen Physiol. 1963 Mar;46:629–654. doi: 10.1085/jgp.46.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Canessa-Fischer M. Sodium movements in perfused squid giant axons. Passive fluxes. J Gen Physiol. 1968 Aug;52(2):240–257. doi: 10.1085/jgp.52.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin R. A., Beaugé L. A. An analysis of the leakages of sodium ions into and potassium ions out of striated muscle cells. J Gen Physiol. 1973 Feb;61(2):222–250. doi: 10.1085/jgp.61.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan P. C., Howell J. N., Eisenberg R. S. The capacitance of skeletal muscle fibers in solutions of low ionic strength. J Gen Physiol. 1972 Mar;59(3):347–359. doi: 10.1085/jgp.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venosa R. A., Horowicz P. Effects on sodium efflux of treating frog sartorius muscles with hypertonic glycerol solutions. J Membr Biol. 1973 Dec 6;14(1):33–56. doi: 10.1007/BF01868067. [DOI] [PubMed] [Google Scholar]


