Abstract
1. A new technique has been developed for making serial measurements of water and solute absorption from the lumen of isolated small intestine.
2. The isolated intestine is perfused in a single pass with a segmented flow of slugs of liquid separated by bubbles of oxygen-carbon dioxide mixture. Simultaneous collections are made of effluent from the lumen and of the fluid which is transported across the mucosa. This latter fluid appears to be a fair sample of the tissue fluid.
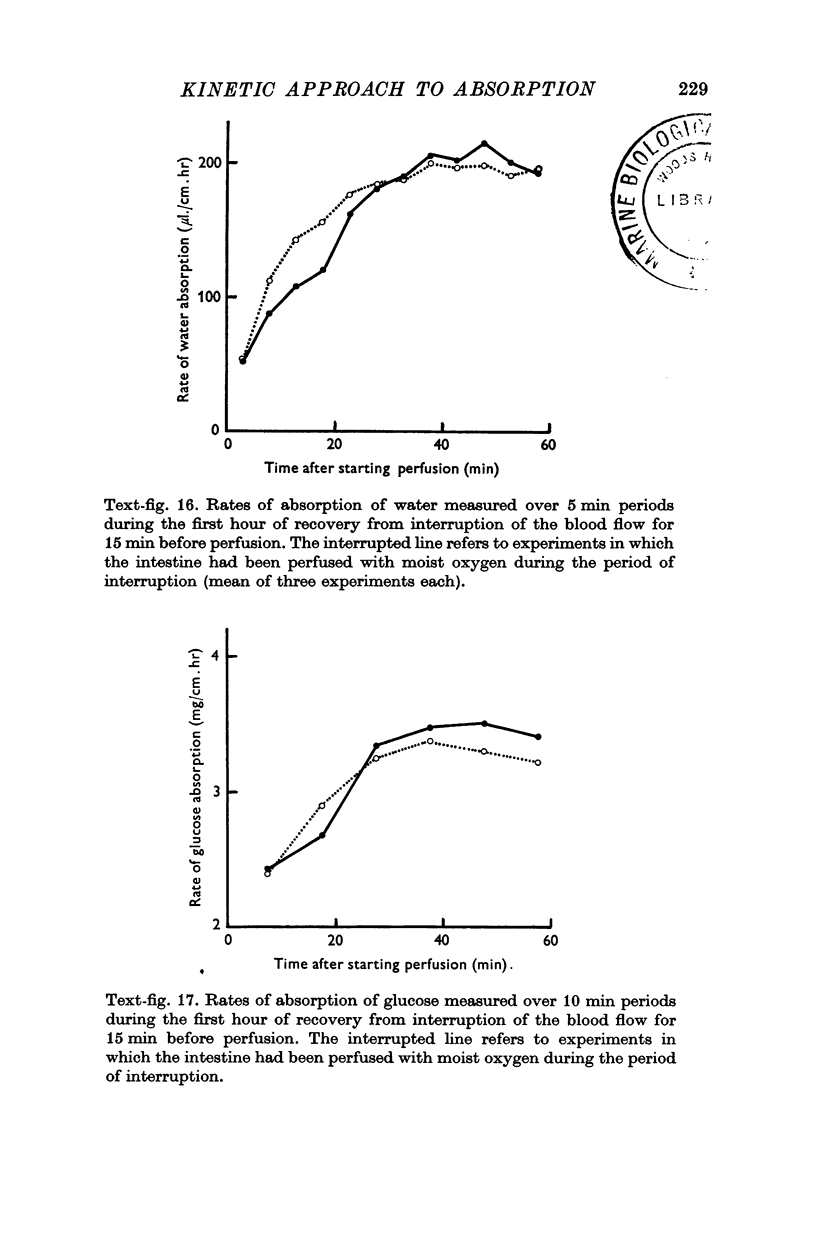
3. Conditions in the lumen can be changed within less than 5 min. The effects of two or more treatments applied to the same segment of intestine can be determined and the time course of a change in luminal conditions.
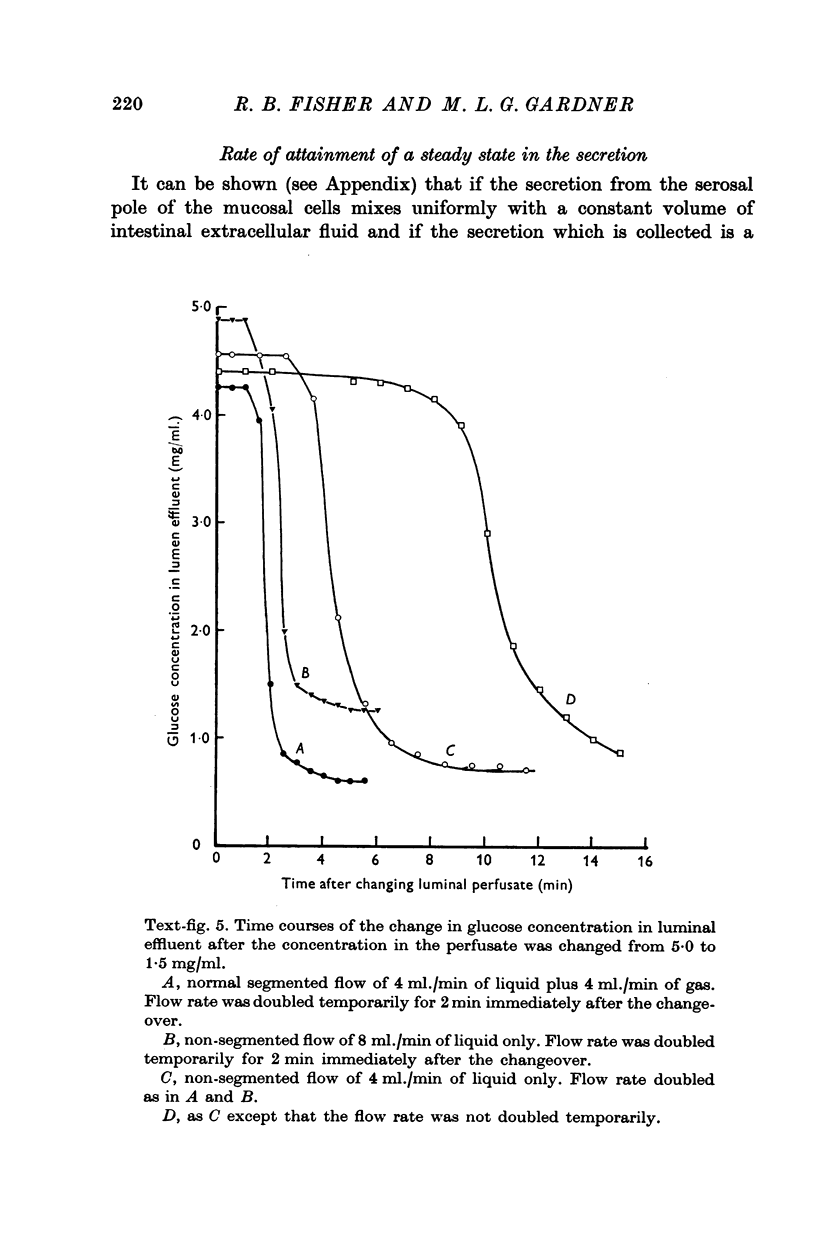
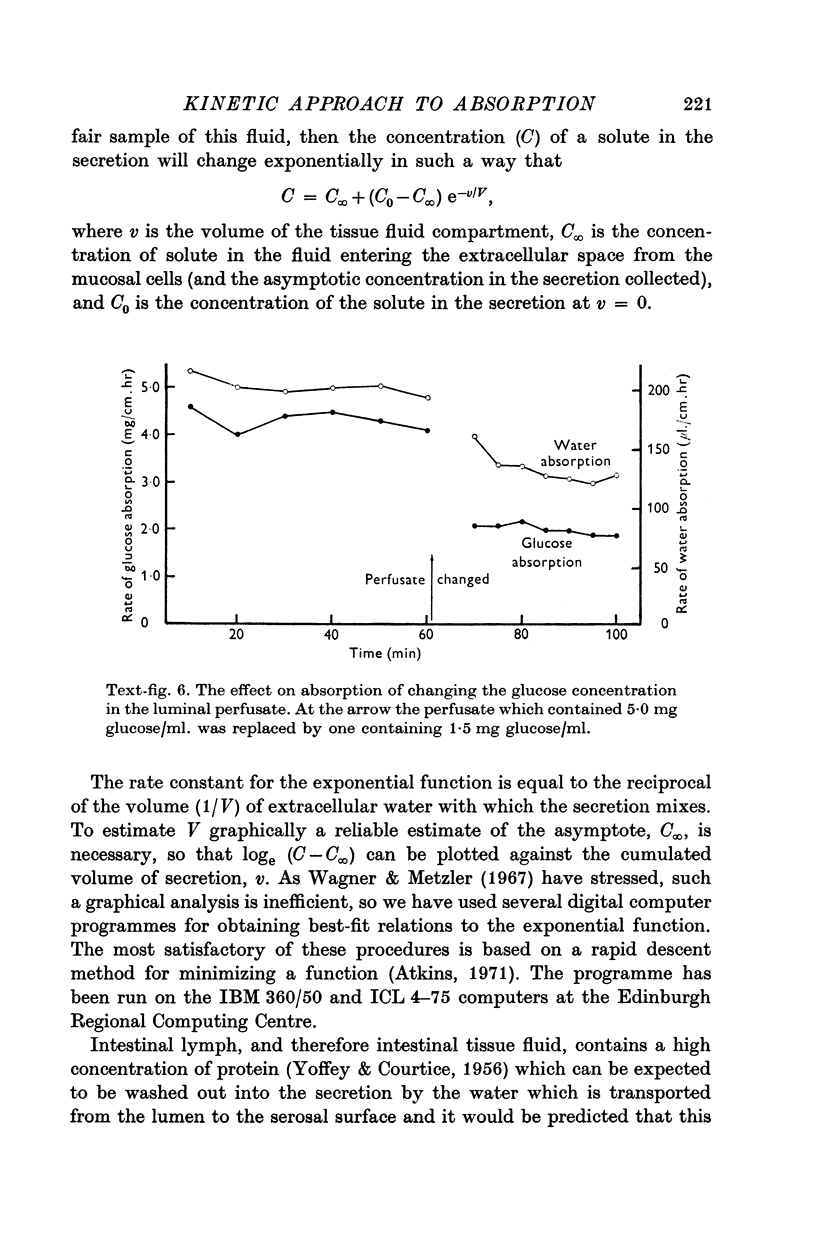
4. The rate of appearance of solutes on the serosal side depends on the rate of water absorption, and changes exponentially towards a steady state. The rate constant is a function of tissue fluid volume.
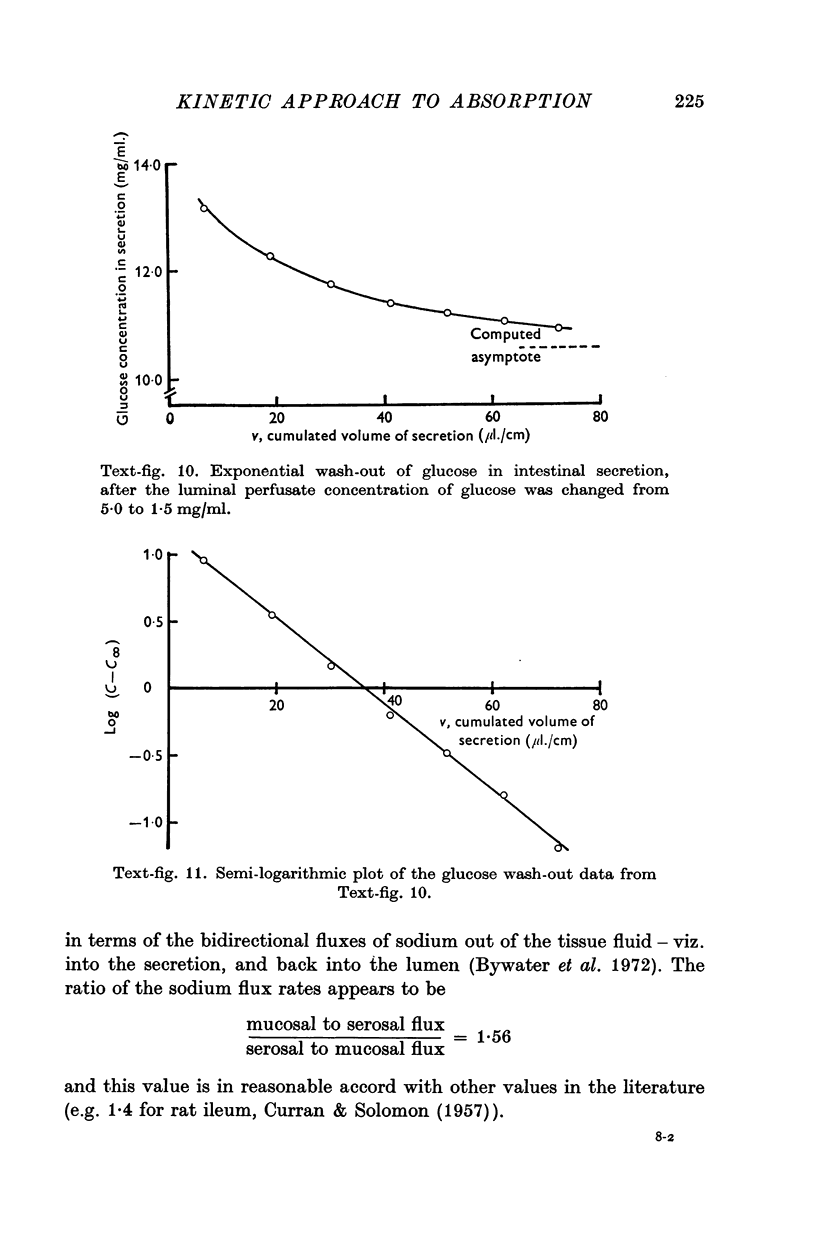
5. In the steady state the concentration of glucose in the tissue fluid is 71 mM when the luminal concentration is 28 mM, and is 45 mM when the luminal concentration is 8·3 mM.
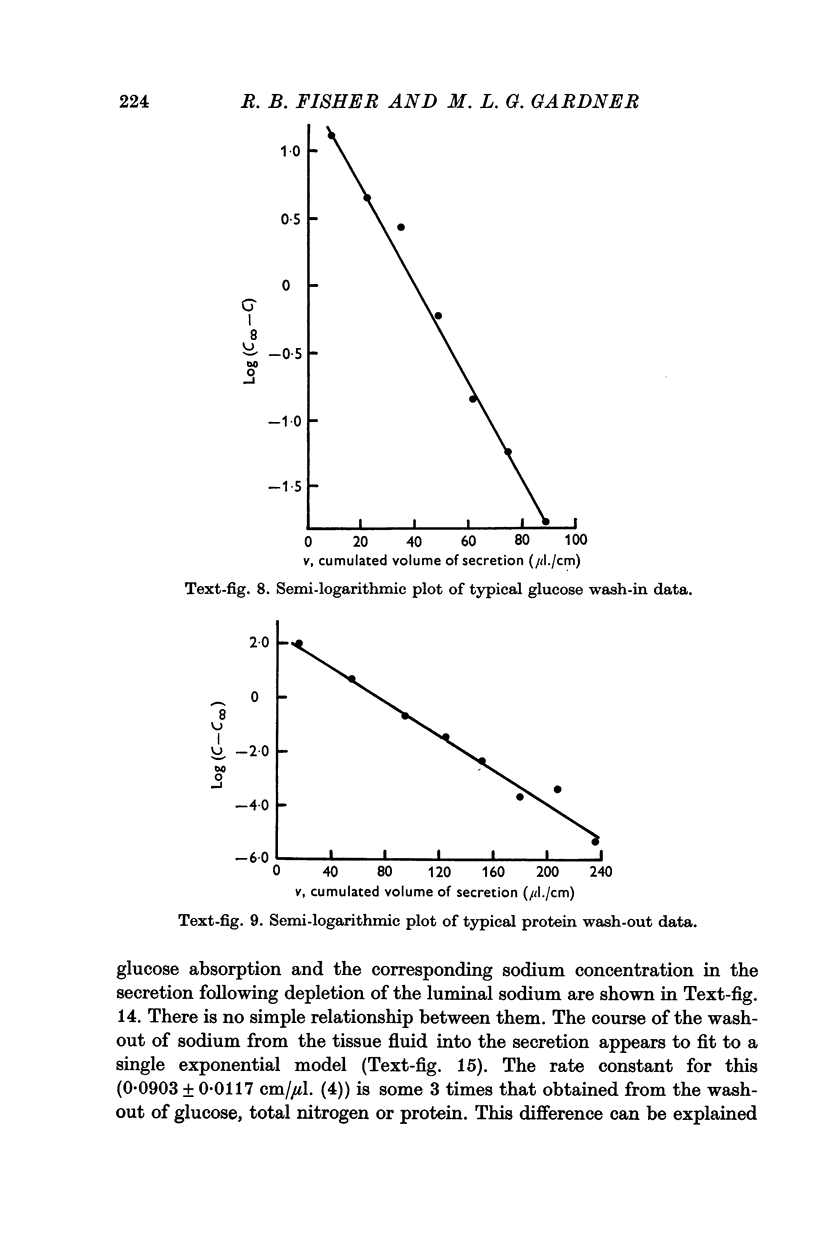
6. For solutes such as glucose for which reflux from tissue fluid to lumen is small relative to flux from lumen to tissue fluid, the time of attainment of a steady state in secretion is usually 50-60 min.
7. For solutes such as sodium for which the reflux is relatively high, the steady state may be reached in 15-20 min.
8. The Km for glucose absorption (14-19 mM) is much lower than is found with unsegmented flow perfusion.
9. These findings emphasize problems in interpreting results from other types of intestinal preparation.
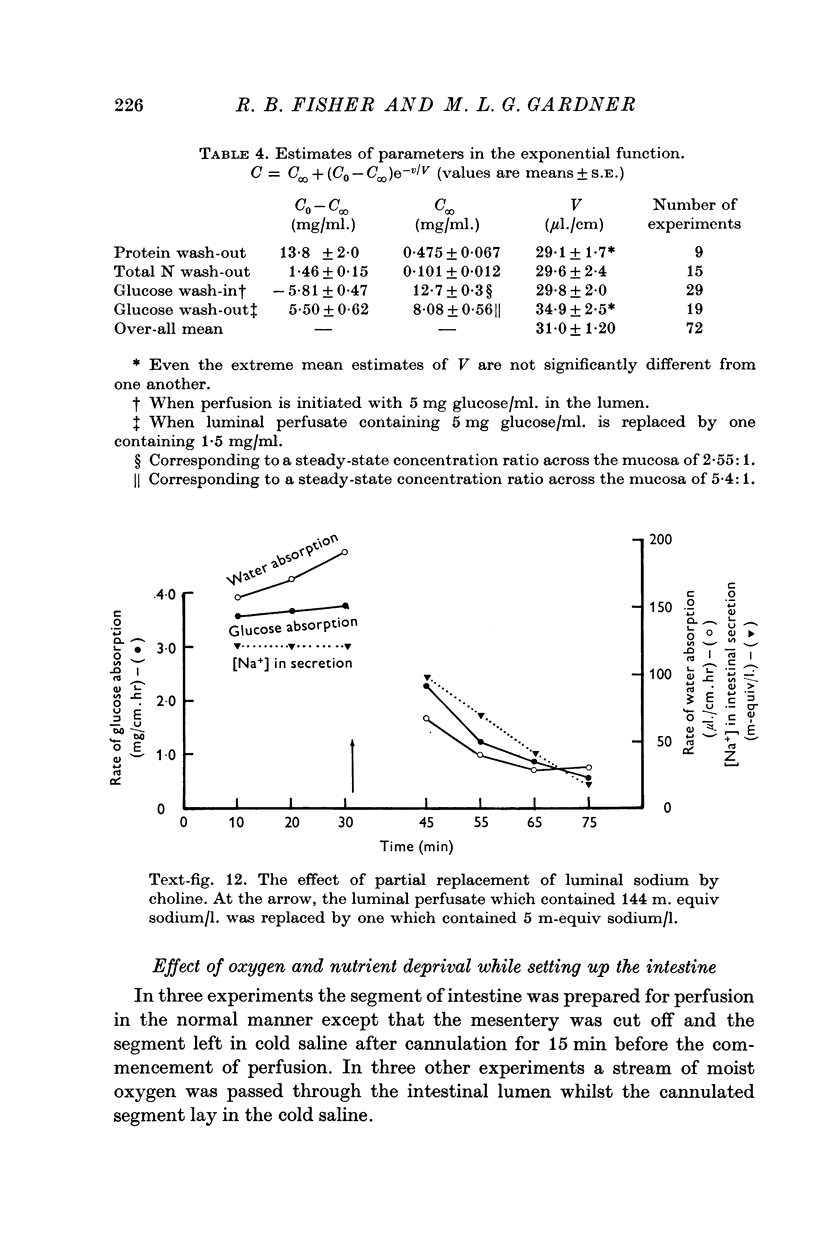
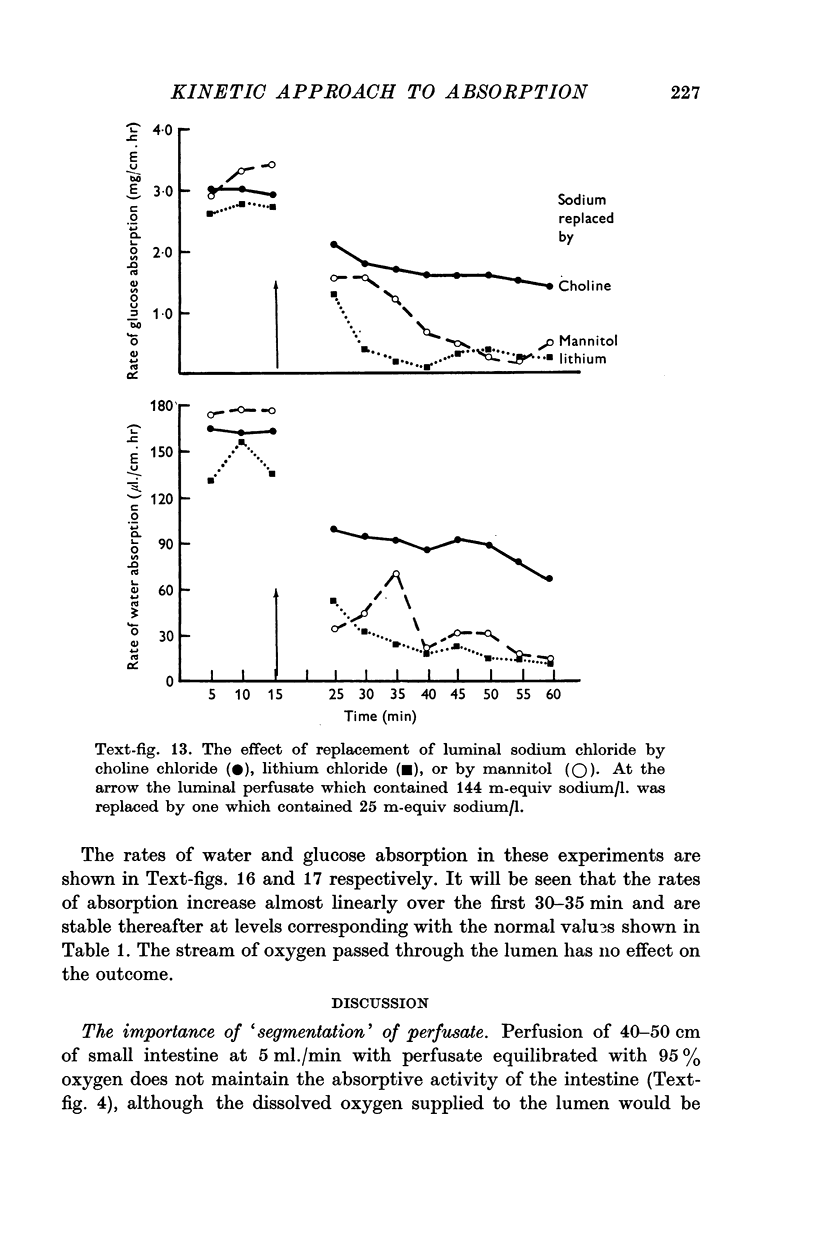
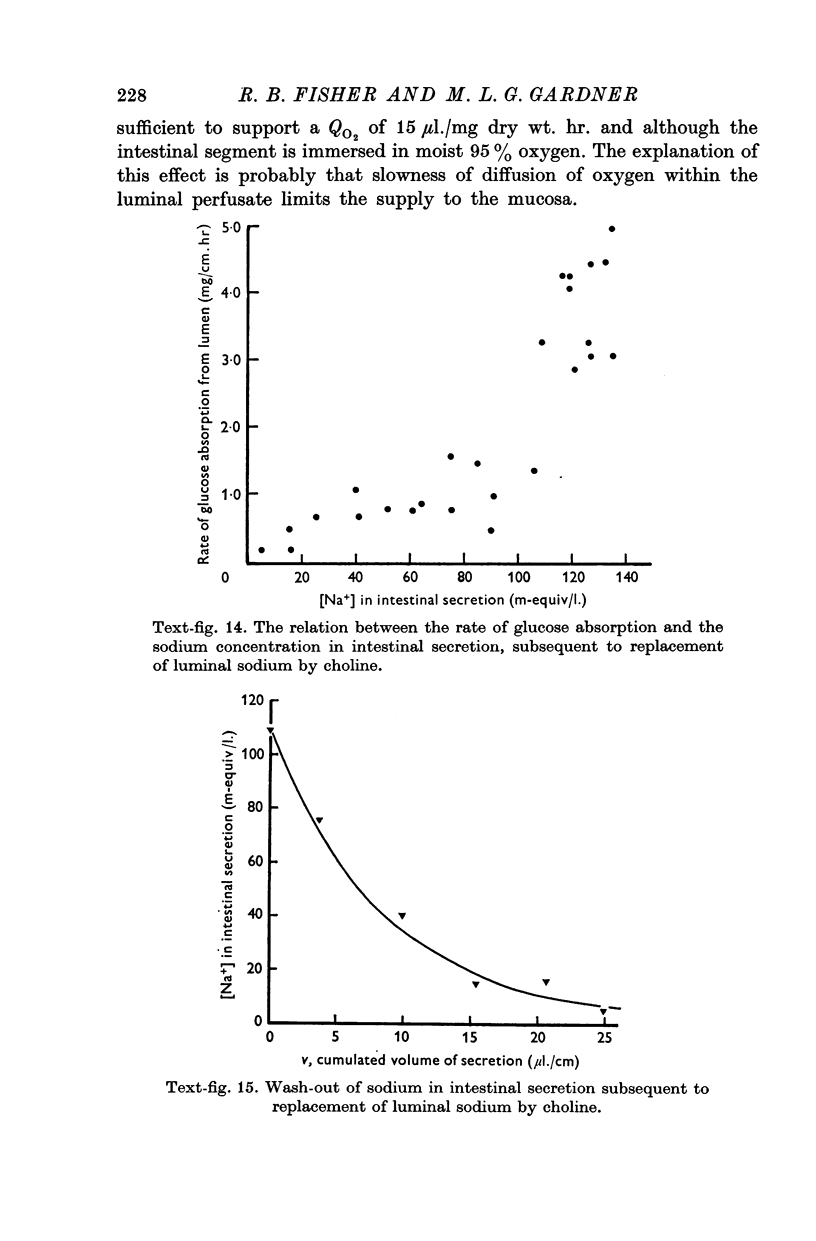
10. The rate of glucose absorption from the lumen falls only gradually when the luminal sodium concentration is reduced abruptly. In contrast the rate of glucose absorption falls suddenly when the luminal glucose concentration is reduced abruptly. This suggests that glucose absorption is not directly dependent on luminal sodium ions.
Full text
PDF


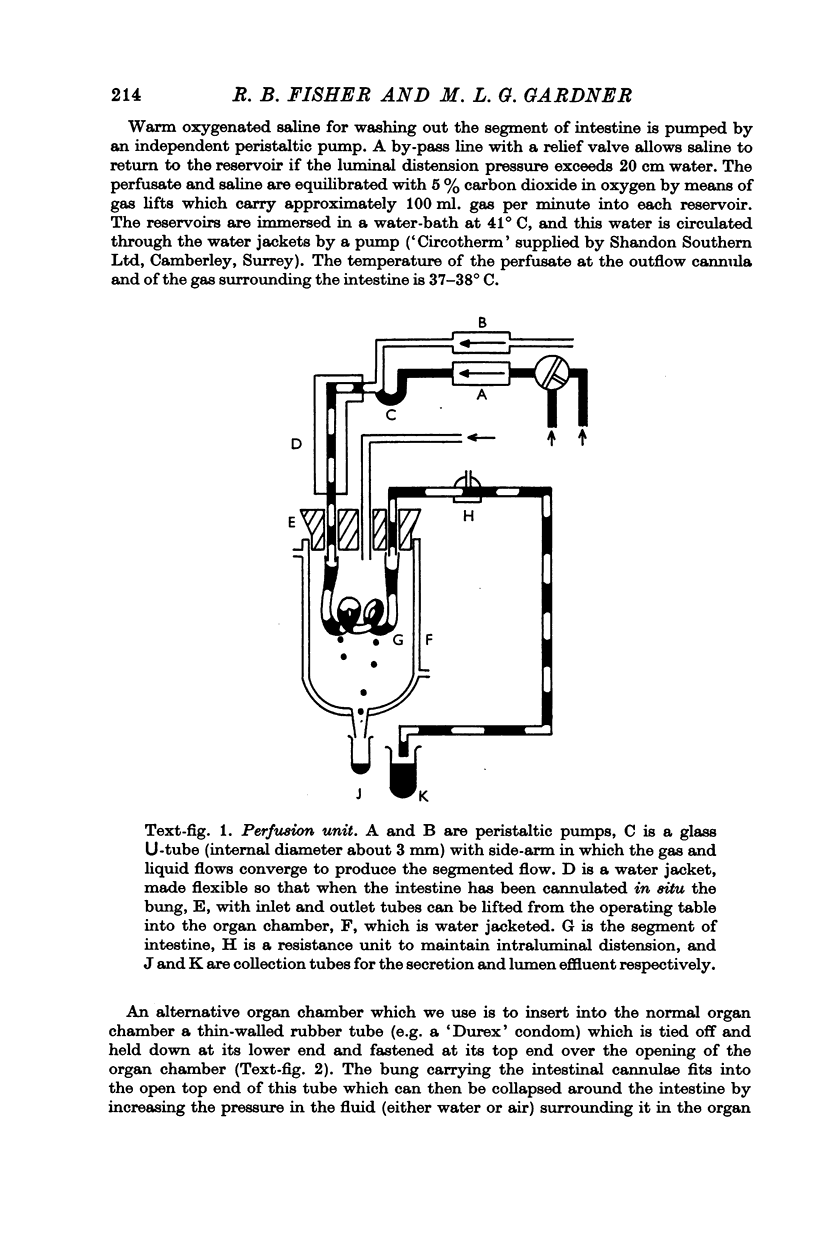
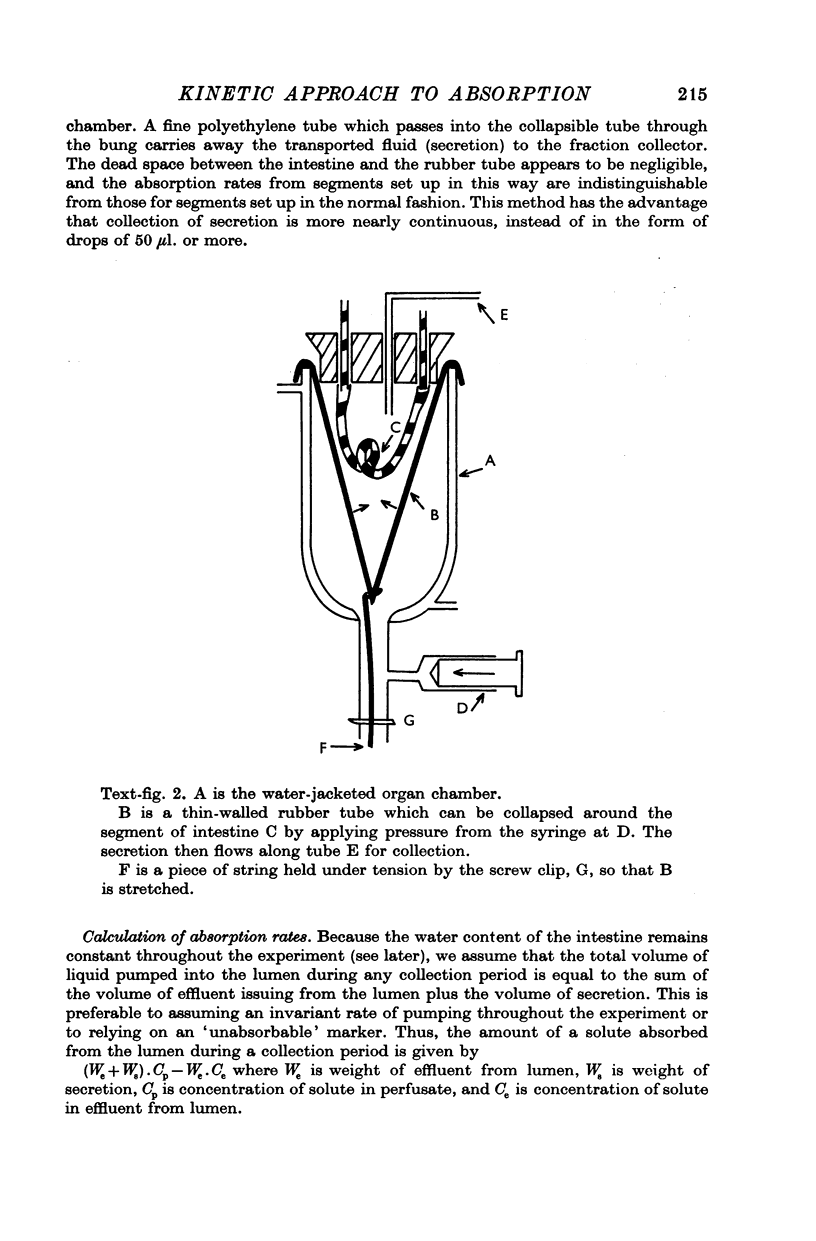
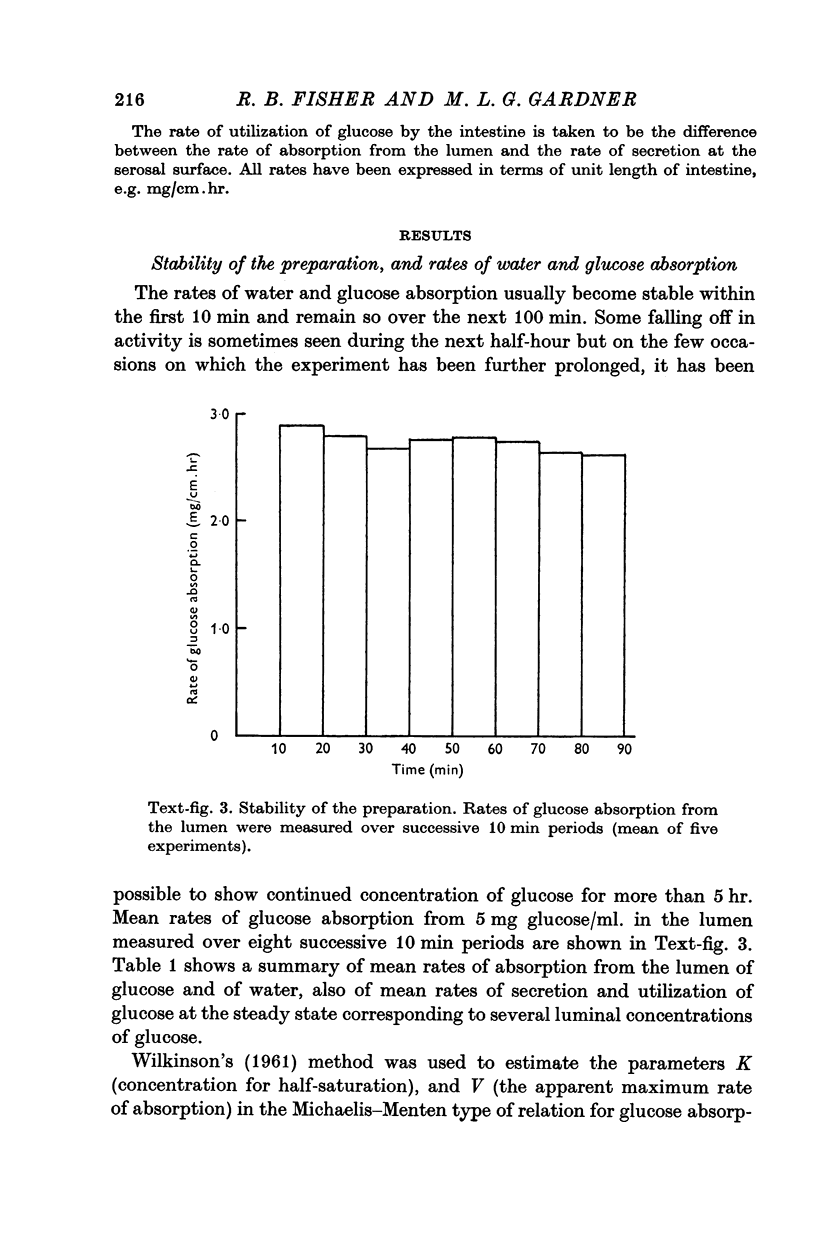
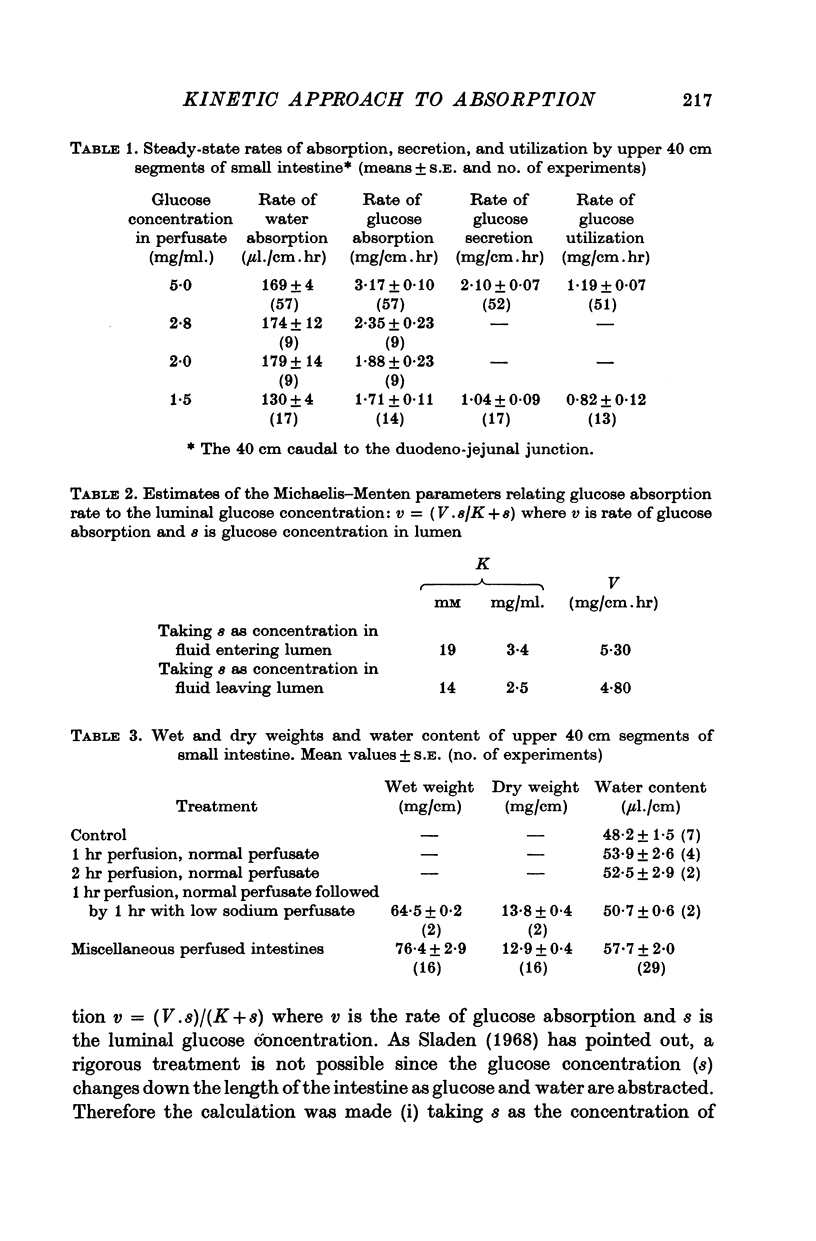







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins G. L. A versatile digital computer program for non-linear regression analysis. Biochim Biophys Acta. 1971 Dec 21;252(3):405–420. doi: 10.1016/0304-4165(71)90142-5. [DOI] [PubMed] [Google Scholar]
- Bywater R. J., Fisher R. B., Gardner M. L. Deuterium oxide as a water flux tracer in rat small intestine. J Physiol. 1972 Dec;227(2):55P–56P. [PubMed] [Google Scholar]
- CURRAN P. F., SOLOMON A. K. Ion and water fluxes in the ileum of rats. J Gen Physiol. 1957 Sep 20;41(1):143–168. doi: 10.1085/jgp.41.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane R. K. Na+ -dependent transport in the intestine and other animal tissues. Fed Proc. 1965 Sep-Oct;24(5):1000–1006. [PubMed] [Google Scholar]
- FISHER R. B., PARSONS D. S. A preparation of surviving rat small intestine for the study of absorption. J Physiol. 1949 Dec 15;110(1-2):36-46, pl. doi: 10.1113/jphysiol.1949.sp004419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. B., O'Brien J. A. The effects of endogenous and added insulin on the time-course of glucose uptake by the isolated perfused rat heart. Q J Exp Physiol Cogn Med Sci. 1972 Apr;57(2):176–191. doi: 10.1113/expphysiol.1972.sp002147. [DOI] [PubMed] [Google Scholar]
- Hill J. B., Cowart D. S. An automated colorimetric mutarotase assay. Anal Biochem. 1966 Aug;16(2):327–337. doi: 10.1016/0003-2697(66)90162-x. [DOI] [PubMed] [Google Scholar]
- Newey H., Rampone A. J., Smyth D. H. The relation between L-methionine uptake and sodium in rat small intestine in vitro. J Physiol. 1970 Dec;211(3):539–549. doi: 10.1113/jphysiol.1970.sp009292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIKLIS E., QUASTEL J. H. Effects of cations on sugar absorption by isolated surviving guinea pig intestine. Can J Biochem Physiol. 1958 Mar;36(3):347–362. [PubMed] [Google Scholar]
- SCHANKER L. S., TOCCO D. J., BRODIE B. B., HOGBEN C. A. Absorption of drugs from the rat small intestine. J Pharmacol Exp Ther. 1958 May;123(1):81–88. [PubMed] [Google Scholar]
- SMYTH D. H., TAYLOR C. B. Transfer of water and solutes by an in vitro intestinal preparation. J Physiol. 1957 May 23;136(3):632–648. doi: 10.1113/jphysiol.1957.sp005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladen G. E. Perfusion studies in relation to intestinal absorption. Gut. 1968 Dec;9(6):624–628. [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. G., Metzler C. M. Estimation of rate constants for absorption and elimination from blood concentration data. J Pharm Sci. 1967 May;56(5):658–659. doi: 10.1002/jps.2600560531. [DOI] [PubMed] [Google Scholar]



