Abstract
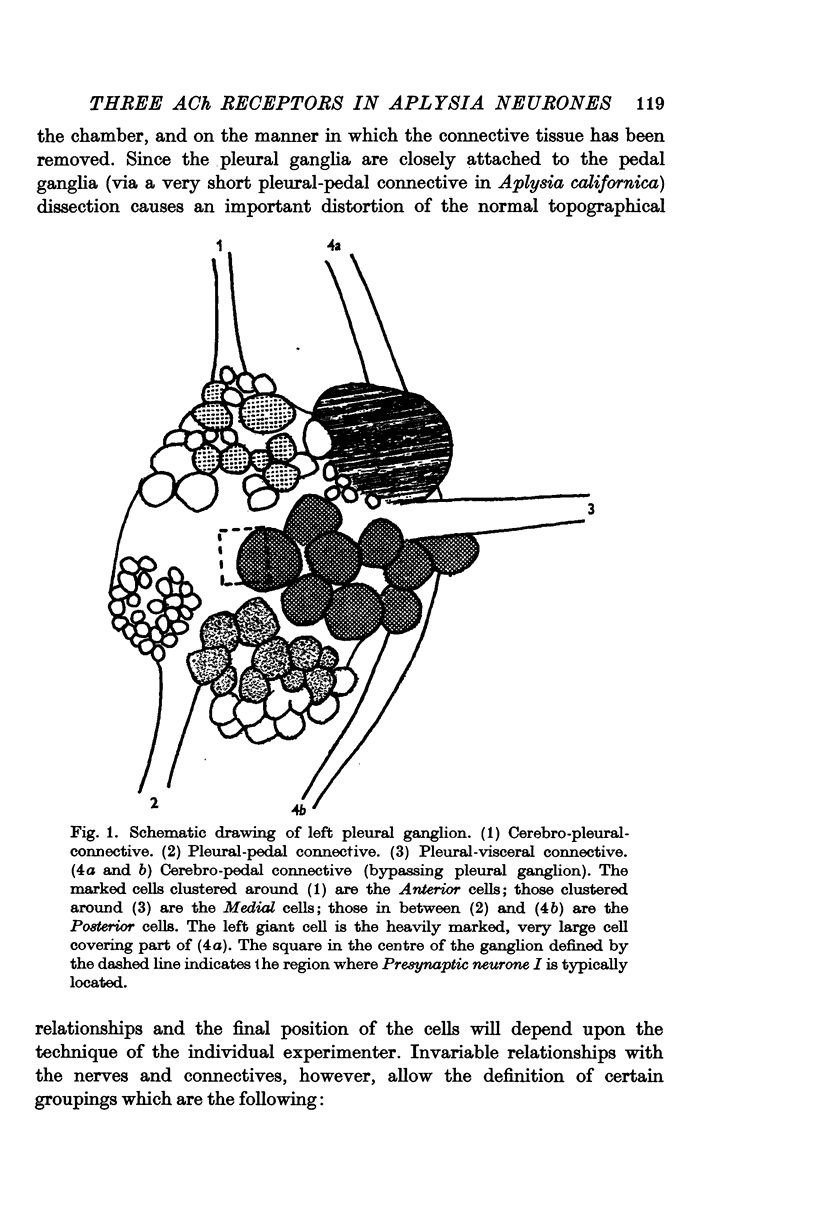
1. In the pleural ganglion of Aplysia californica, groups of cells were identified that respond differently to an iontophoretic injection of ACh. The anterior neurones are excited by ACh, whereas the medial cells are inhibited. The inhibitory response is biphasic, consisting of a short-latency, rapid component and a longer-latency, slow component. Homologous responses (e.p.s.p.s in the anterior cells and a two-component inhibitory response in the medial cells) are evoked in these same cell groups by stimulation of an identifiable presynaptic neurone.
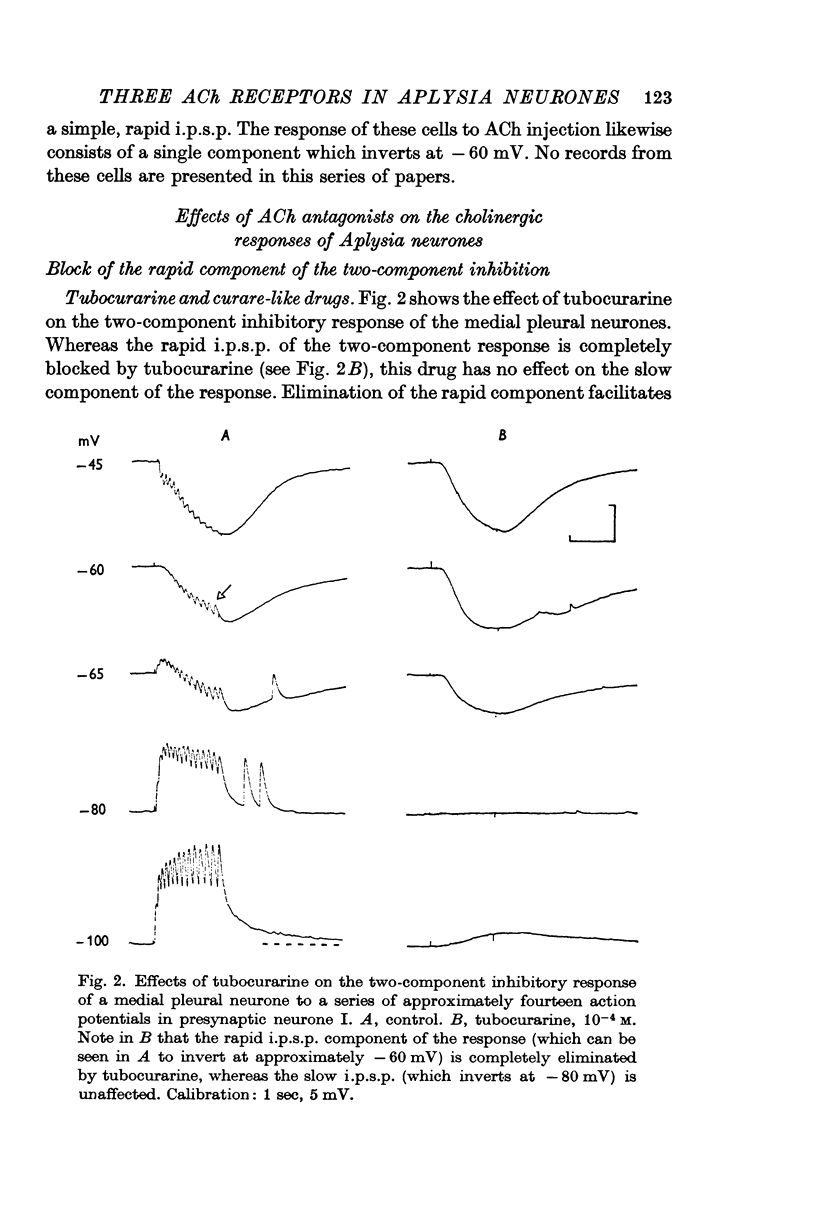
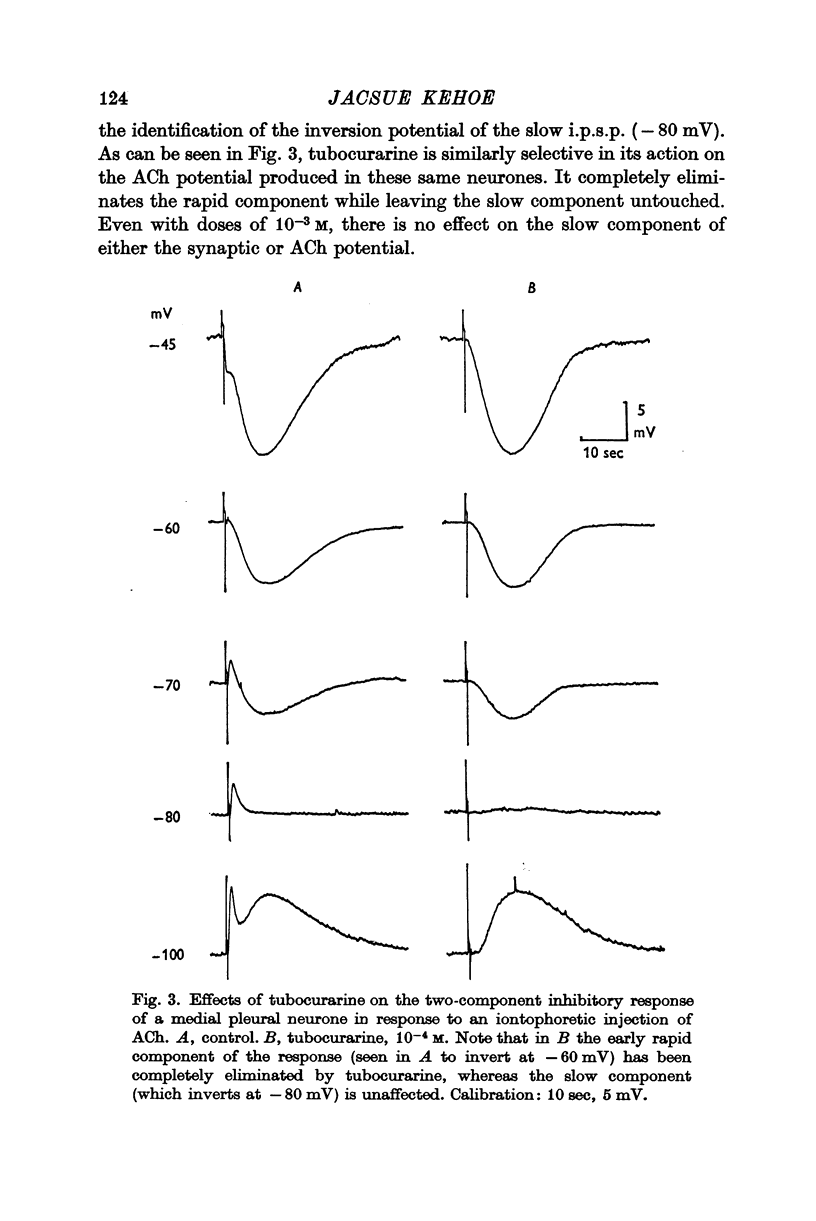
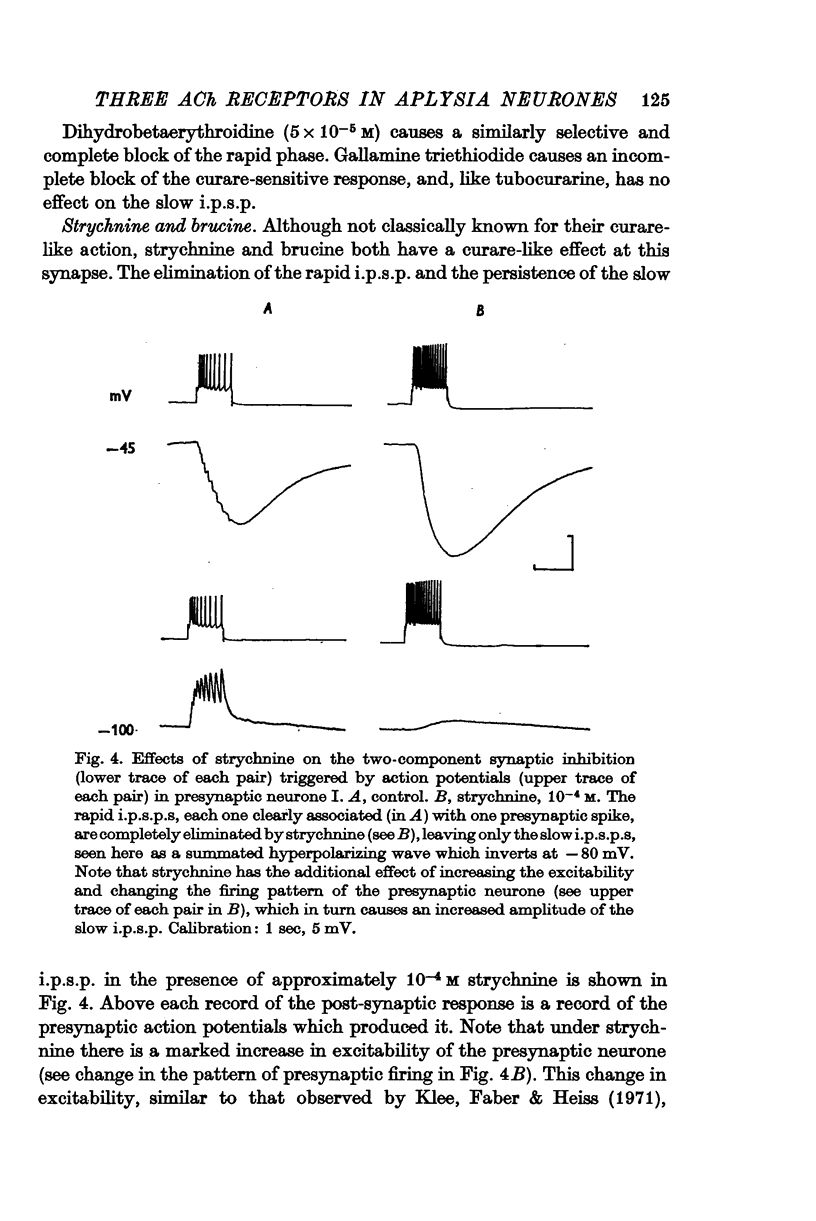
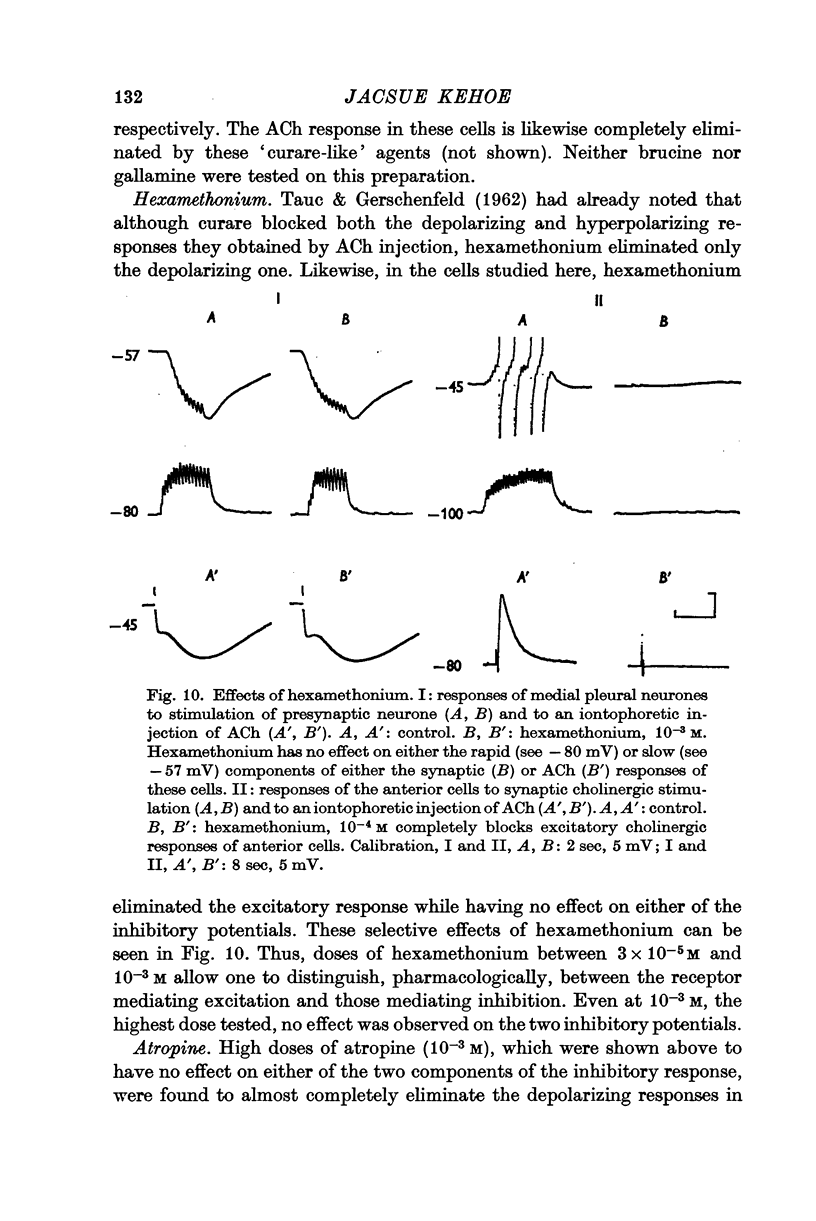
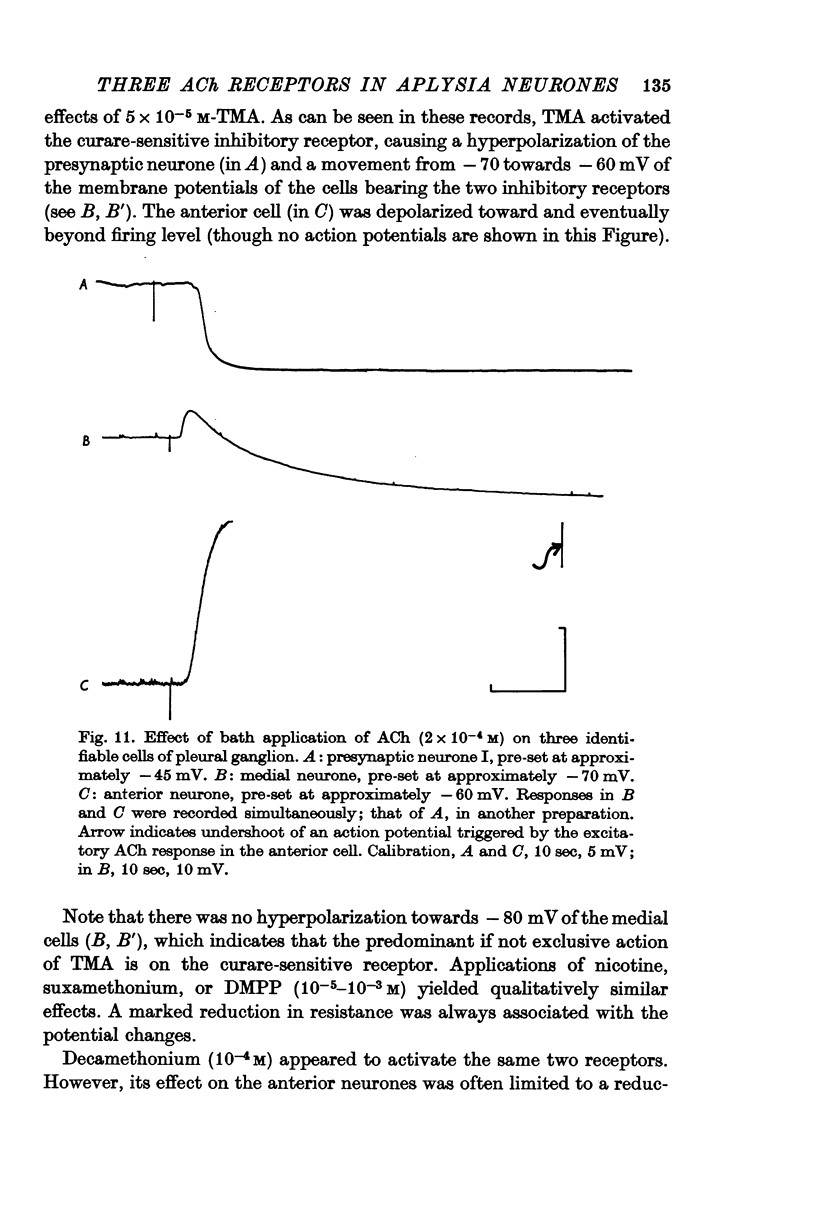
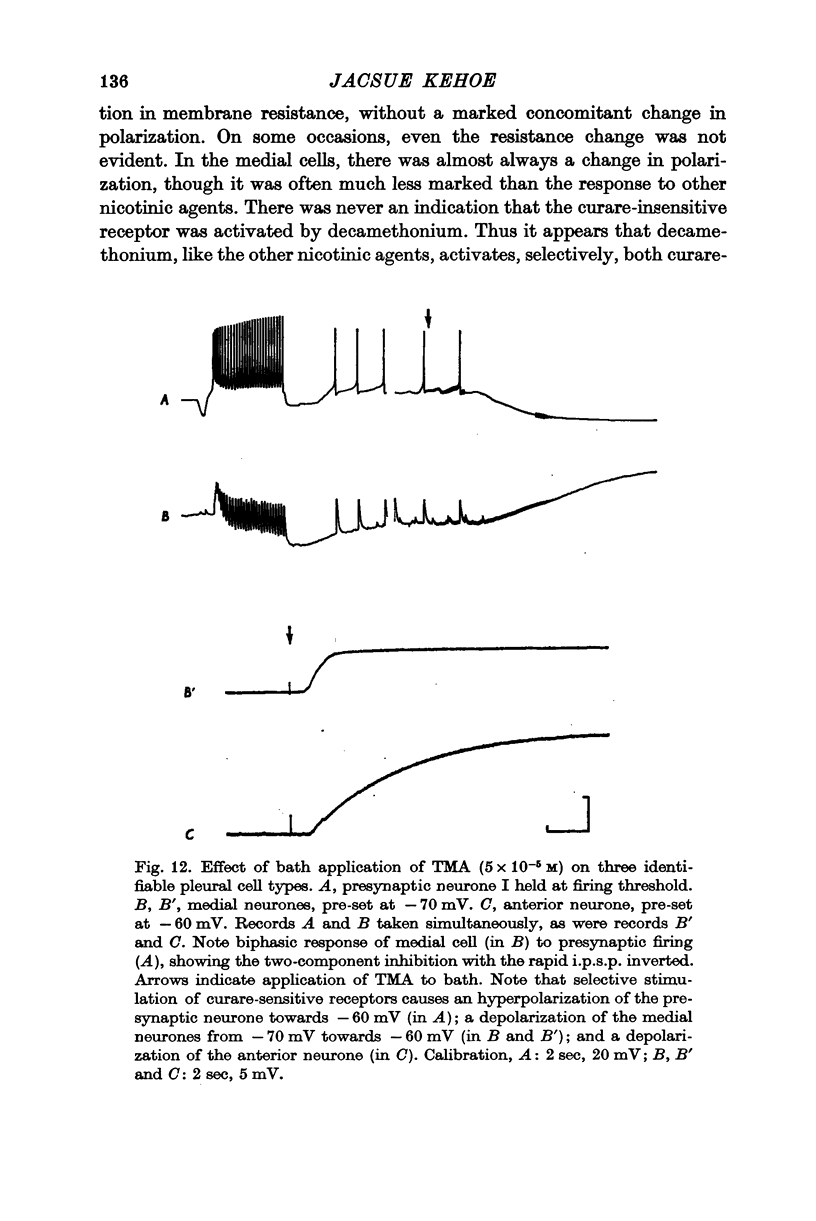
2. The e.p.s.p. and the corresponding ACh potential are completely eliminated by hexamethonium which has no effect on either of the inhibitory potentials. Both the e.p.s.p. and the rapid i.p.s.p. (and the corresponding ACh potentials) are blocked by tubocurarine, dihydro-β-erythroidine, strychnine and brucine. These drugs have no effect on the slow inhibitory potential, whether elicited synaptically or by ACh injection. The slow response can be selectively blocked by methylxylocholine, tetraethylammonium (TEA), and phenyltrimethylammonium (PTMA). Since the three types of potentials were found to be differentially affected by ACh antagonists, it was concluded that the various responses are due to activation of three different ACh receptors.
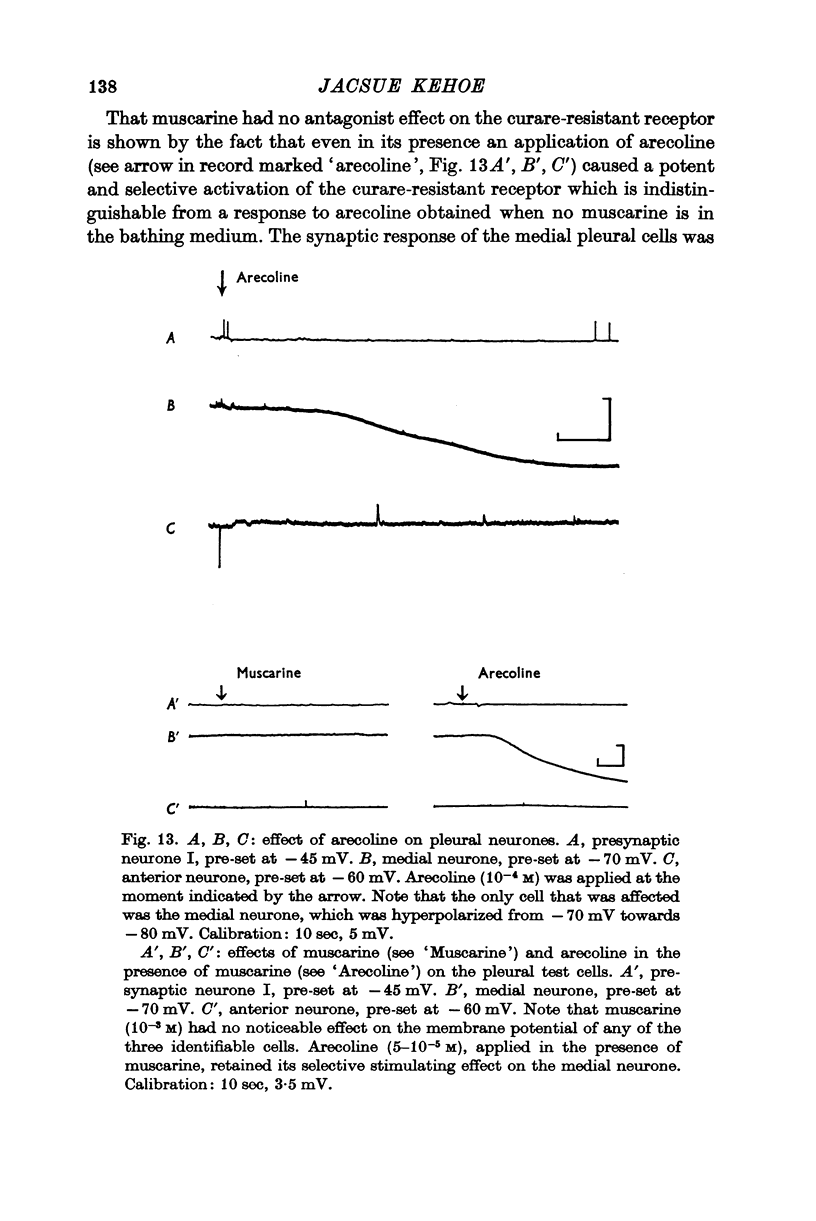
3. Of the cholinomimetics tested, only carbamylcholine imitates all three actions of ACh. Nicotinic agents, which were shown to activate the two curare-sensitive receptors, have no stimulating effect on the curare-insensitive receptor. This latter receptor can be selectively stimulated by arecoline. The cholinomimetics were shown to have a secondary blocking effect on the receptor(s) they stimulate.
4. Muscarine, even at high doses, is ineffective as either a stimulating or a blocking agent on any of the three receptor types. The muscarinelike drugs oxotremorine, methacholine, and pilocarpine have only weak and non-specific cholinomimetic action on these receptors. Their blocking effects are likewise negligible.
5. The two curare-sensitive receptors, which are presumably the same as those described by Tauc & Gerschenfeld (1962), respond like vertebrate nicotinic receptors to both cholinomimetics and cholinolytics. The third receptor type, on the other hand, has a unique pharmacological profile. It is unaffected by both nicotine and muscarine, and is blocked neither by curare nor by atropine. Knowing that it can be stimulated by arecoline and blocked by methylxylocholine, TEA and PTMA does not facilitate its incorporation into the classical scheme of cholinergic receptors.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVING B. O. The action of strychnine at cholinergic junctions. Arch Int Pharmacodyn Ther. 1961 Apr 1;131:123–150. [PubMed] [Google Scholar]
- Ascher P. Inhibitory and excitatory effects of dopamine on Aplysia neurones. J Physiol. 1972 Aug;225(1):173–209. doi: 10.1113/jphysiol.1972.sp009933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner J., Tauc L. Long-lasting phenomena in the molluscan nervous system. Symp Soc Exp Biol. 1966;20:457–475. [PubMed] [Google Scholar]
- GINSBORG B. L., GUERRERO S. ON THE ACTION OF DEPOLARIZING DRUGS ON SYMPATHETIC GANGLION CELLS OF THE FROG. J Physiol. 1964 Aug;172:189–206. doi: 10.1113/jphysiol.1964.sp007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmitters in invertebrate nervous systems. Symp Soc Exp Biol. 1966;20:299–323. [PubMed] [Google Scholar]
- HUGHES G. M., TAUC L. The path of the giant cell axons in Aplysia depilans. Nature. 1961 Jul 22;191:404–405. doi: 10.1038/191404a0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Ionic mechanisms of a two-component cholinergic inhibition in Aplysia neurones. J Physiol. 1972 Aug;225(1):85–114. doi: 10.1113/jphysiol.1972.sp009930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Multiple actions of tetraethylammonium at a two-component inhibitory synapse in Aplysia. J Physiol. 1969 Sep;204(1):11P–12P. [PubMed] [Google Scholar]
- Kehoe J. Pharmacological characteristics and ionic bases of a 2 component postsynaptic inhibition. Nature. 1967 Sep 30;215(5109):1503–1505. doi: 10.1038/2151503b0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Single presynaptic neurone mediates a two component postsynaptic inhibition. Nature. 1969 Mar 1;221(5183):866–868. doi: 10.1038/221866a0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. The physiological role of three acetylcholine receptors in synaptic transmission in Aplysia. J Physiol. 1972 Aug;225(1):147–172. doi: 10.1113/jphysiol.1972.sp009932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan H., Tauc L. Acetylcholine receptors: topographic distribution and pharmacological properties of two receptor types on a single molluscan neurone. J Physiol. 1972 May;222(3):537–558. doi: 10.1113/jphysiol.1972.sp009813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEAN R. A., GEUS R. J., PASTERNACK J., MATTIS P. A., ULLYOT G. E. Pharmacology of trimethyl [2-2,6-dimethylphenoxy)propyl]-trimethylammonium chloride, monohydrate; compound 6890 or betaTM 10. J Pharmacol Exp Ther. 1960 May;129:17–23. [PubMed] [Google Scholar]
- Sakharov D. A. Cellular aspects of invertebrate neuropharmacology. Annu Rev Pharmacol. 1970;10:335–352. doi: 10.1146/annurev.pa.10.040170.002003. [DOI] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. A cholinergic mechanism of inhibitory synaptic transmission in a molluscan nervous system. J Neurophysiol. 1962 Mar;25:236–262. doi: 10.1152/jn.1962.25.2.236. [DOI] [PubMed] [Google Scholar]
- TAUC L. Identification of active membrane areas in the giant neuron of Aplysia. J Gen Physiol. 1962 Jul;45:1099–1115. doi: 10.1085/jgp.45.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. J., Hedges A. The effect of cholinergic antagonists on the response to acetylcholine, acetyl-beta-methylcholine and nicotine of neurones of Helix aspersa. Comp Biochem Physiol. 1967 Dec;23(3):977–989. doi: 10.1016/0010-406x(67)90358-1. [DOI] [PubMed] [Google Scholar]
- Walker R. J., Woodruff G. N., Glaizner B., Sedden C. B., Kerkut G. A. The pharmacology of Helix dopamine receptor of specific neurones in the snail, Helix aspersa. Comp Biochem Physiol. 1968 Feb;24(2):455–469. doi: 10.1016/0010-406x(68)90997-3. [DOI] [PubMed] [Google Scholar]


