Abstract
1. It is shown that a single presumably cholinergic presynaptic neurone can mediate, monosynaptically, multicomponent responses in a given cell and different responses in different cells.
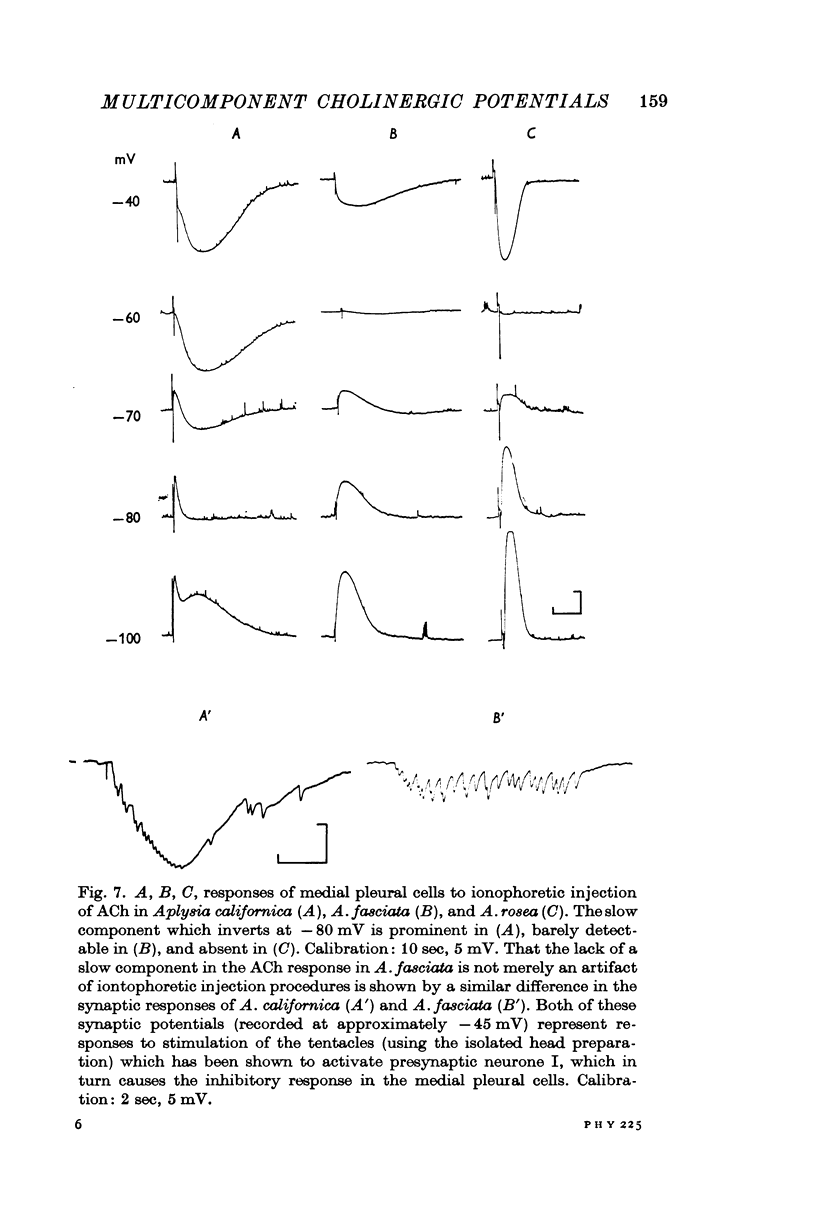
2. Complex responses (whether evoked synaptically or by ACh injection) are shown to be the result of the coexistence on a given post-synaptic neurone of more than one of three cholinergic receptor types previously described. Likewise, different responses in different cells are due to the fact that different post-synaptic neurones bear different combinations of these three receptors.
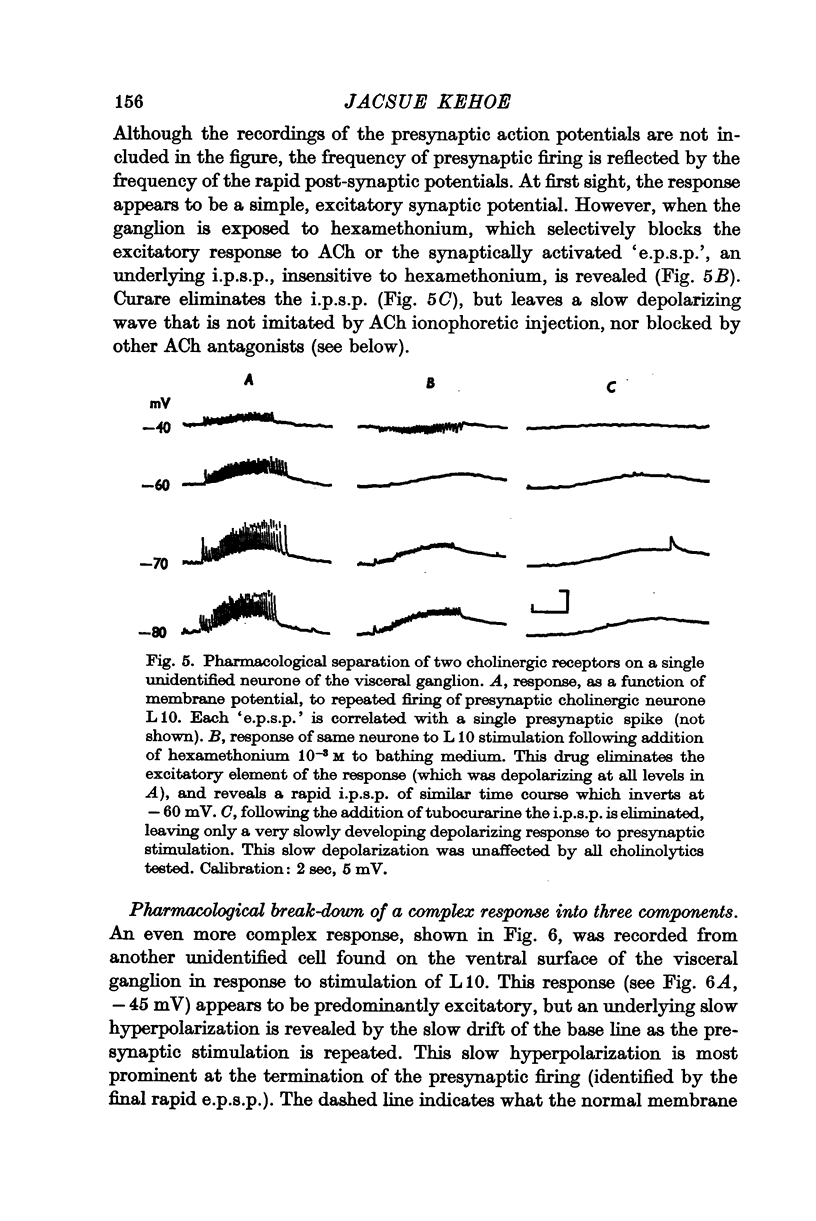
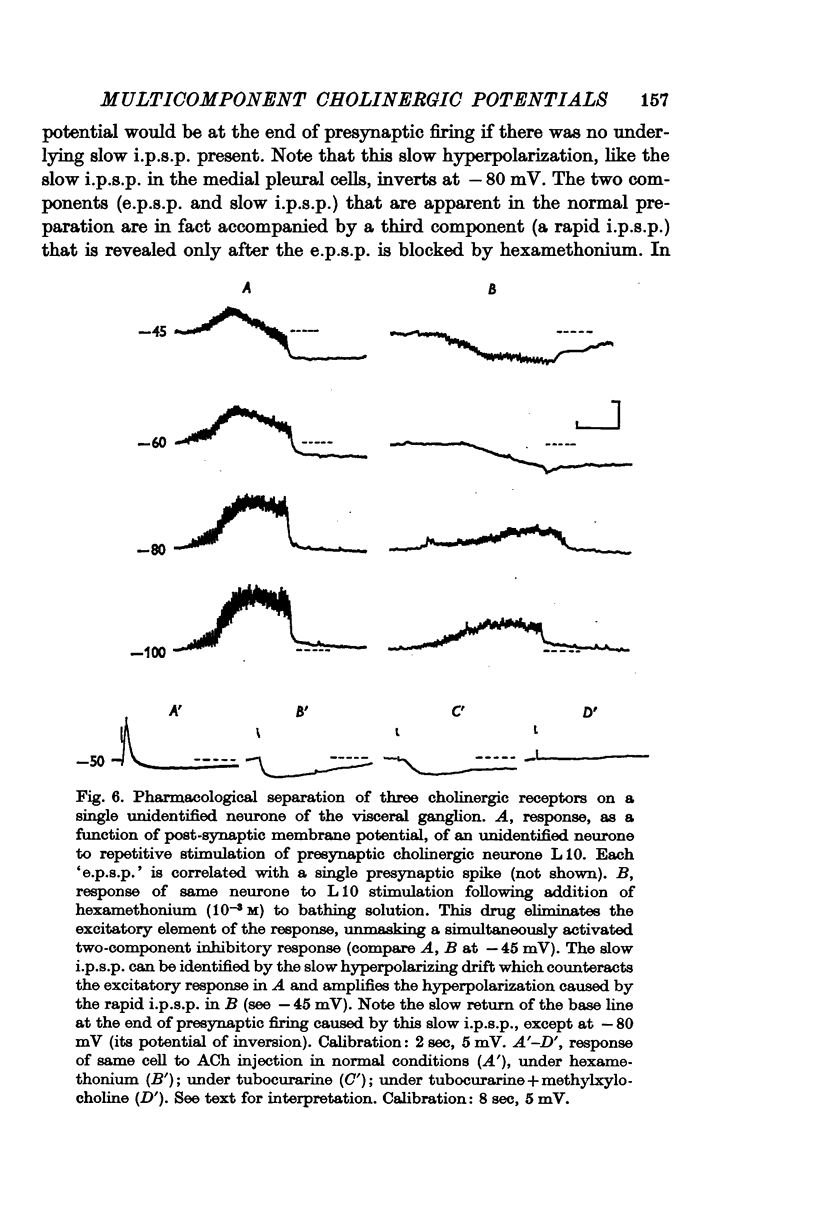
3. Pharmacological analysis shows that the multicomponent nature of many of the responses is not always evident: what appears, under normal conditions, to be a single-component excitatory potential can be shown often to be a complex response consisting of superimposed e.p.s.p.s and rapid i.p.s.p.s which are sometimes, though not always, accompanied by a slow i.p.s.p.
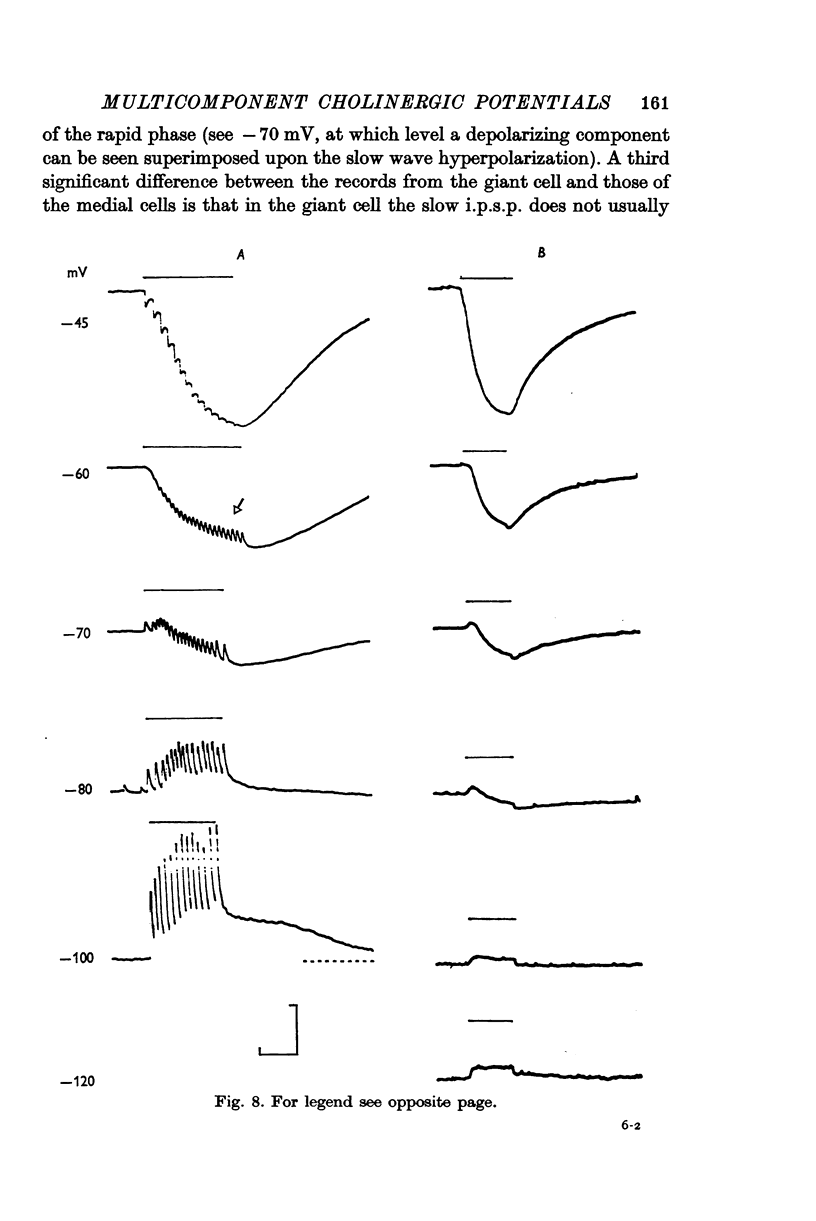
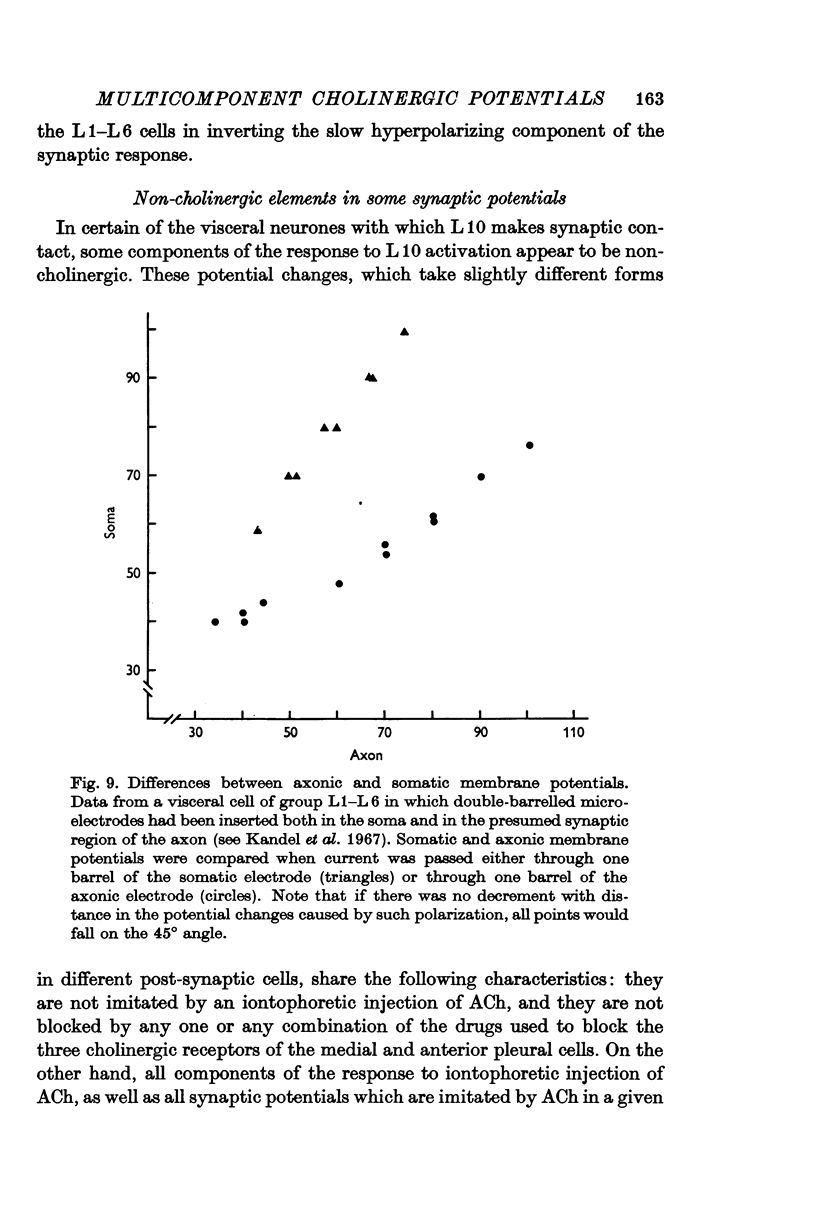
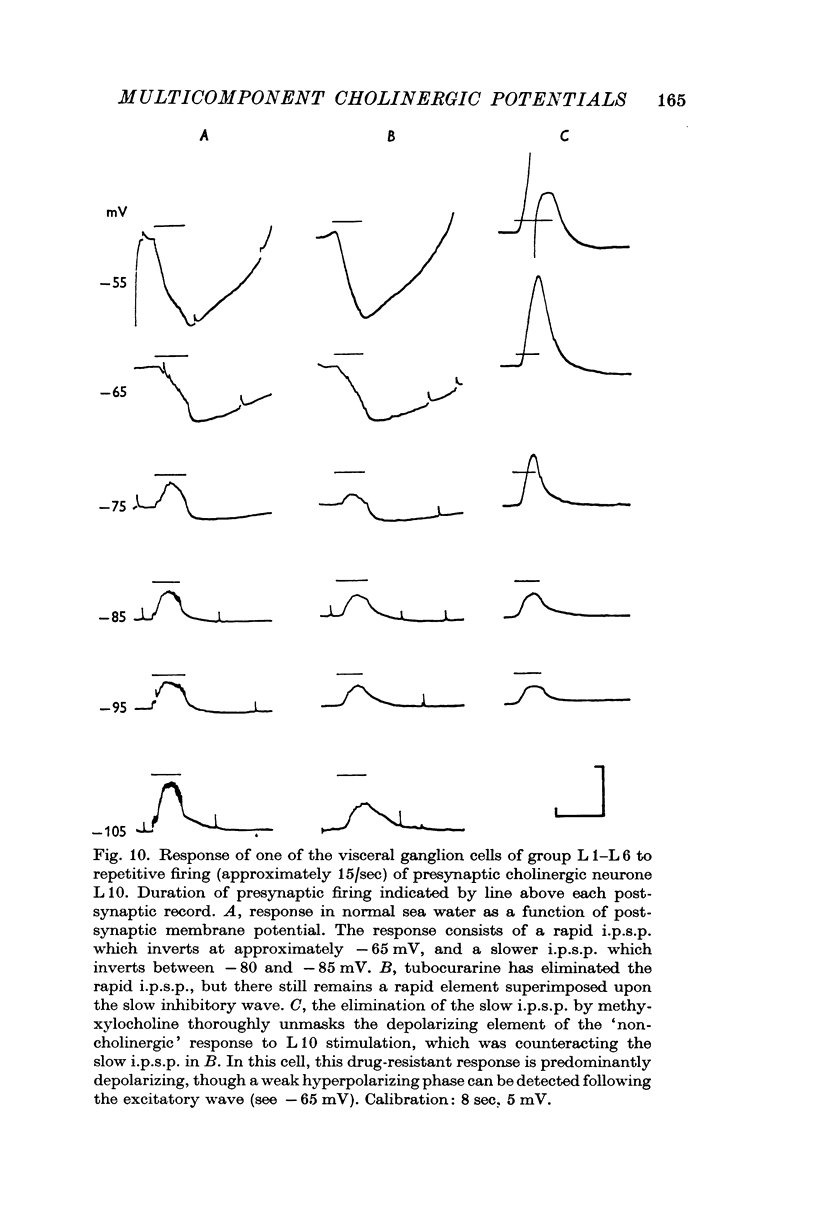
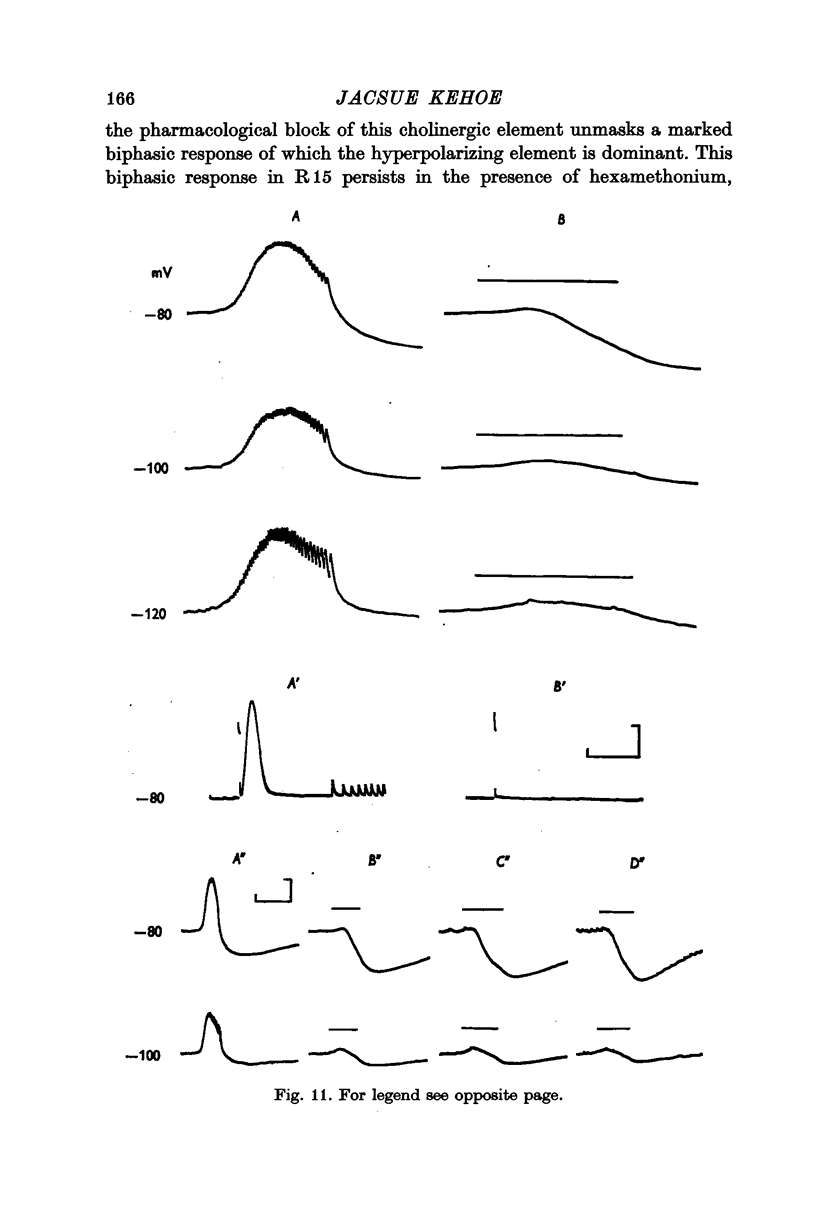
4. Although which and how many of the three receptor types is the major factor contributing to the type of response observed, in the case of some of the synaptic potentials certain other factors were found to contribute to the final response form. First, in the large cells of the visceral ganglion, as well as in the left giant cell of the pleural ganglion, there is a marked `electrical separation' between the region in which the synaptic currents are generated and the point of recording. This `electrical distance' often altered the inversion potential, and sometimes the form of the responses. Secondly, in some visceral neurones, activation of the cholinergic presynaptic neurone L10 causes (either directly or indirectly) a potential change which cannot be accounted for in terms of the activation of cholinergic receptors. This `non-cholinergic' response (not imitated by an ionophoretic injection of ACh) is unmasked by the blocking of all three cholinergic receptors. It contributes differentially in different cells to the total response pattern produced by L10 under normal conditions, but its contribution is often characterized by a late hyperpolarizing phase which appears to be impossible to invert. This phase has been shown, however, to be dependent upon the potassium concentration in the extracellular space surrounding the synapse.
4. It is tentatively suggested that this residual, non-cholinergic element of the synaptic response in some visceral cells be the result of the activation of an electrical synapse.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P. Inhibitory and excitatory effects of dopamine on Aplysia neurones. J Physiol. 1972 Aug;225(1):173–209. doi: 10.1113/jphysiol.1972.sp009933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner J., Tauc L. Habituation at the synaptic level in Aplysia. Nature. 1966 Apr 2;210(5031):37–39. doi: 10.1038/210037a0. [DOI] [PubMed] [Google Scholar]
- ECCLES J., ECCLES R. M., ITO M. EFFECTS PRODUCED ON INHIBITORY POSTSYNAPTIC POTENTIALS BY THE COUPLED INJECTIONS OF CATIONS AND ANIONS INTO MOTONEURONS. Proc R Soc Lond B Biol Sci. 1964 May 19;160:197–210. doi: 10.1098/rspb.1964.0036. [DOI] [PubMed] [Google Scholar]
- Gardner D. Bilateral symmetry and interneuronal organization in the buccal ganglia of Aplysia. Science. 1971 Aug 6;173(3996):550–553. doi: 10.1126/science.173.3996.550. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- HUGHES G. M., TAUC L. AN ELECTROPHYSIOLOGICAL STUDY OF THE ANATOMICAL RELATIONS OF TWO GIANT NERVE CELLS IN APLYSIA DEPILANS. J Exp Biol. 1963 Sep;40:469–486. doi: 10.1242/jeb.40.3.469. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Frazier W. T., Waziri R., Coggeshall R. E. Direct and common connections among identified neurons in Aplysia. J Neurophysiol. 1967 Nov;30(6):1352–1376. doi: 10.1152/jn.1967.30.6.1352. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Anomalous rectification in the metacerebral giant cells and its consequences for synaptic transmission. J Physiol. 1966 Mar;183(2):287–304. doi: 10.1113/jphysiol.1966.sp007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. S., Ascher P. Re-evaluation of the synaptic activation of an electrogenic sodium pump. Nature. 1970 Feb 28;225(5235):820–823. doi: 10.1038/225820a0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. S. Suppression sélective par l'ion tétraéthylammonium d'une inhibition cholinergique résistant au curare. C R Acad Sci Hebd Seances Acad Sci D. 1969 Jan 6;268(1):111–114. [PubMed] [Google Scholar]
- Kehoe J. Ionic mechanisms of a two-component cholinergic inhibition in Aplysia neurones. J Physiol. 1972 Aug;225(1):85–114. doi: 10.1113/jphysiol.1972.sp009930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Single presynaptic neurone mediates a two component postsynaptic inhibition. Nature. 1969 Mar 1;221(5183):866–868. doi: 10.1038/221866a0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze D. L., Brown A. M. Internal potassium and chloride activities and the effects of acetylcholine on identifiable Aplysia neurones. Nat New Biol. 1971 Feb 24;229(8):229–231. doi: 10.1038/newbio229229a0. [DOI] [PubMed] [Google Scholar]
- Kusano K., Livengood D. R., Werman R. Correlation of transmitter release with membrane properties of the presynaptic fiber of the squid giant synapse. J Gen Physiol. 1967 Dec;50(11):2579–2601. doi: 10.1085/jgp.50.11.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan H., Tauc L. Acetylcholine receptors: topographic distribution and pharmacological properties of two receptor types on a single molluscan neurone. J Physiol. 1972 May;222(3):537–558. doi: 10.1113/jphysiol.1972.sp009813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsker H., Kandel E. R. Synaptic activation of an electrogenic sodium pump. Science. 1969 Feb 28;163(3870):931–935. doi: 10.1126/science.163.3870.931. [DOI] [PubMed] [Google Scholar]
- Wachtel H., Kandel E. R. Conversion of synaptic excitation to inhibition at a dual chemical synapse. J Neurophysiol. 1971 Jan;34(1):56–68. doi: 10.1152/jn.1971.34.1.56. [DOI] [PubMed] [Google Scholar]


