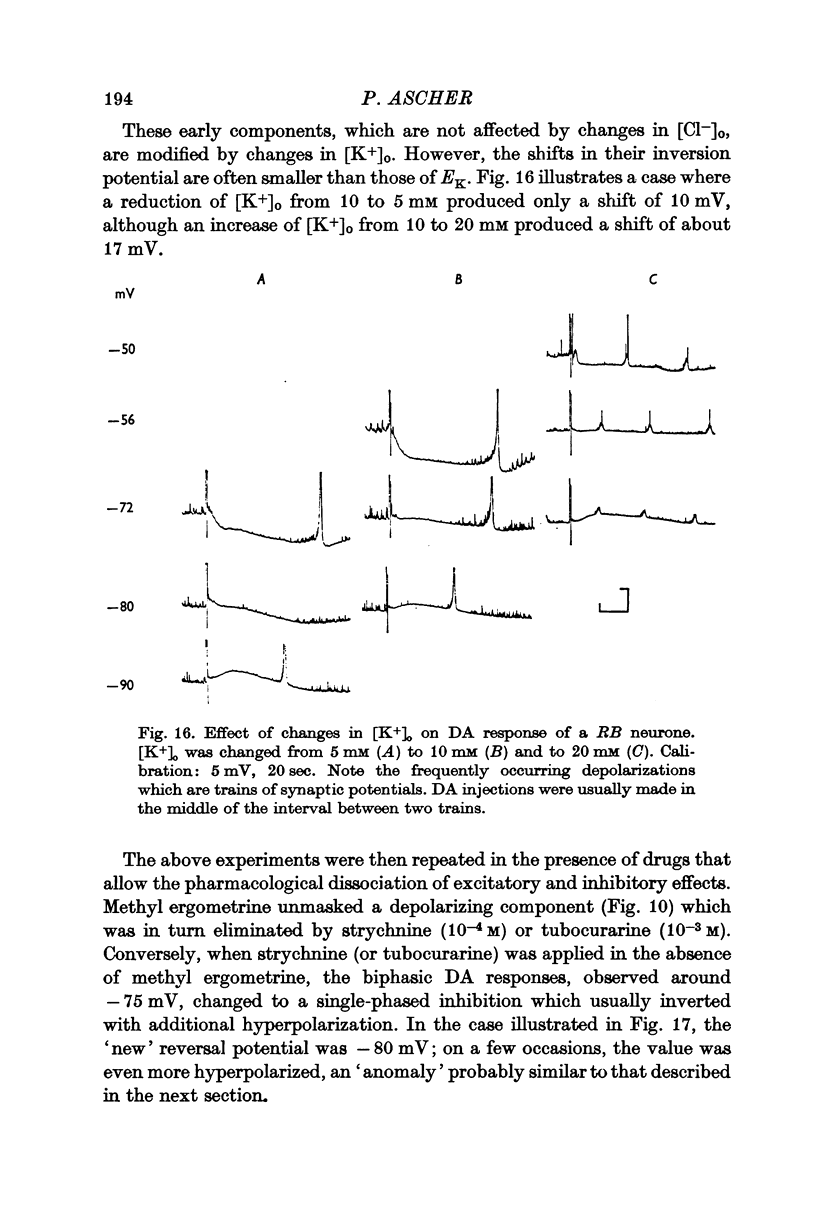
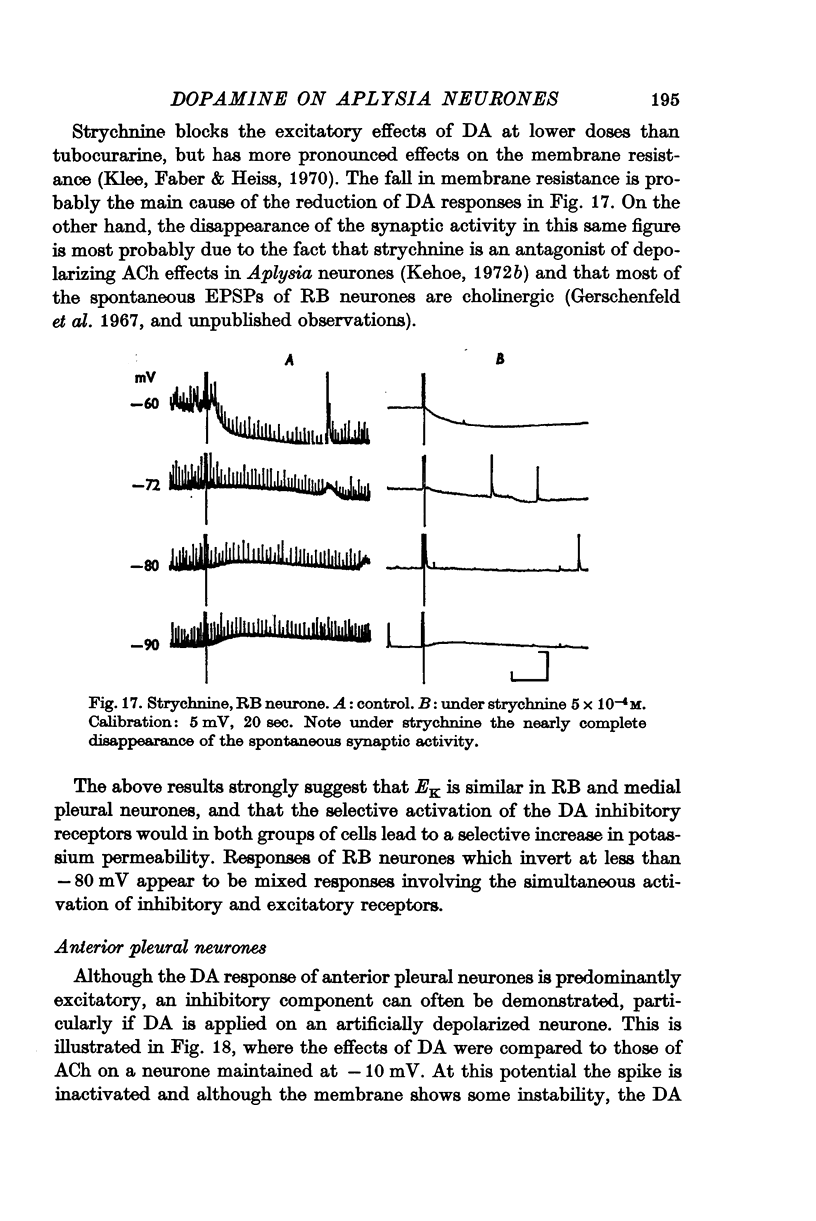
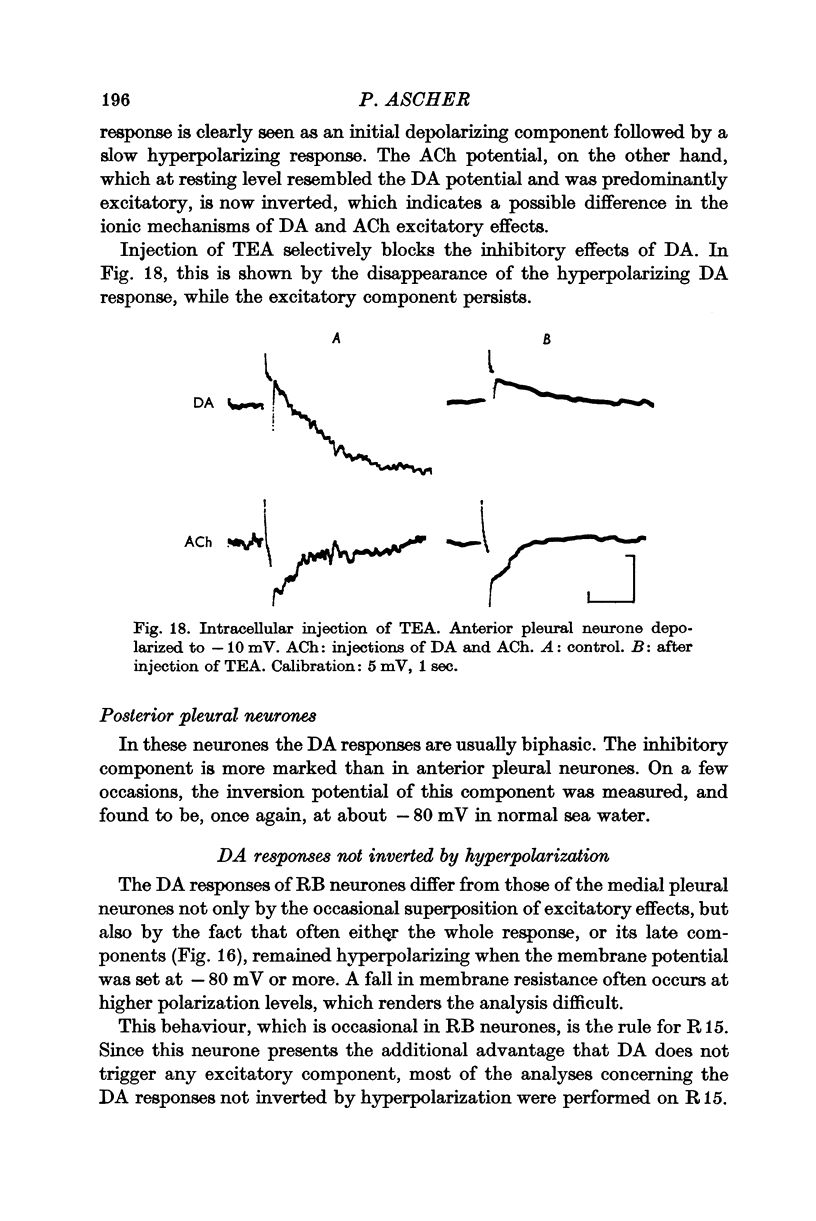
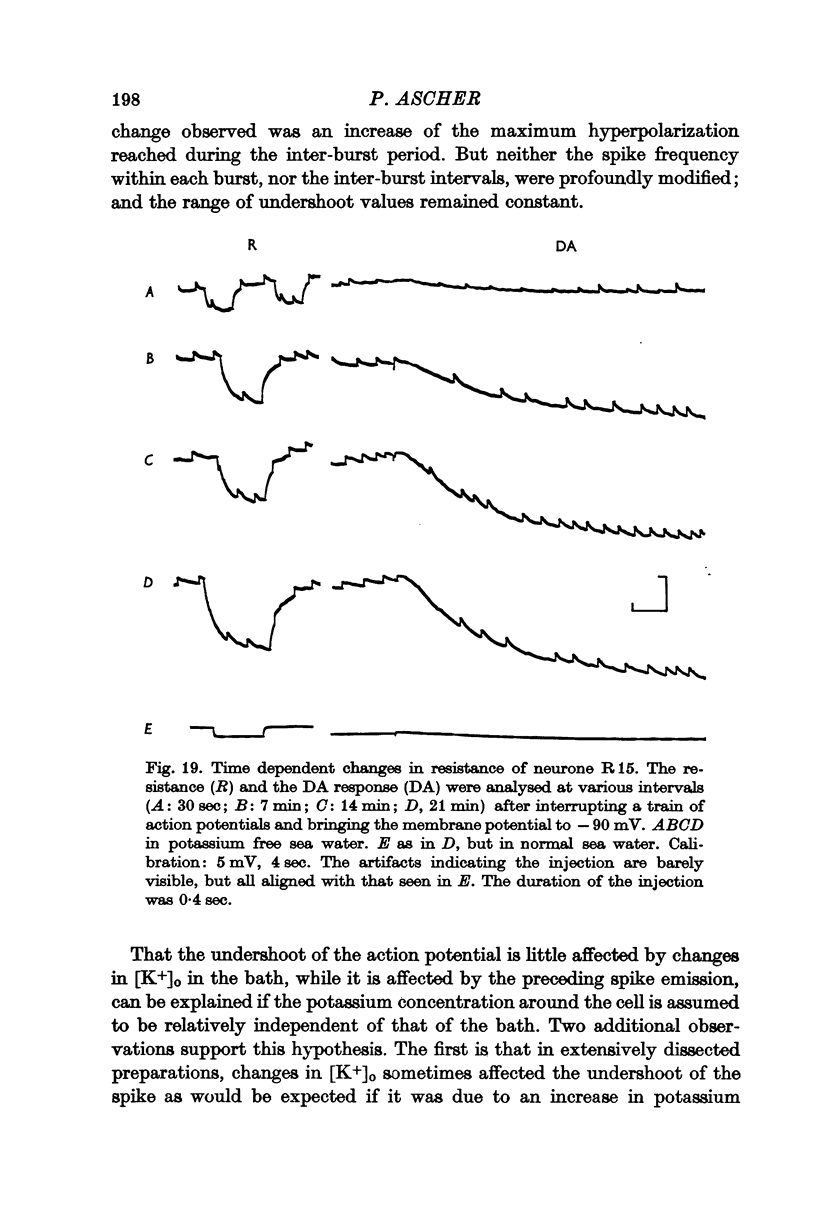
Abstract
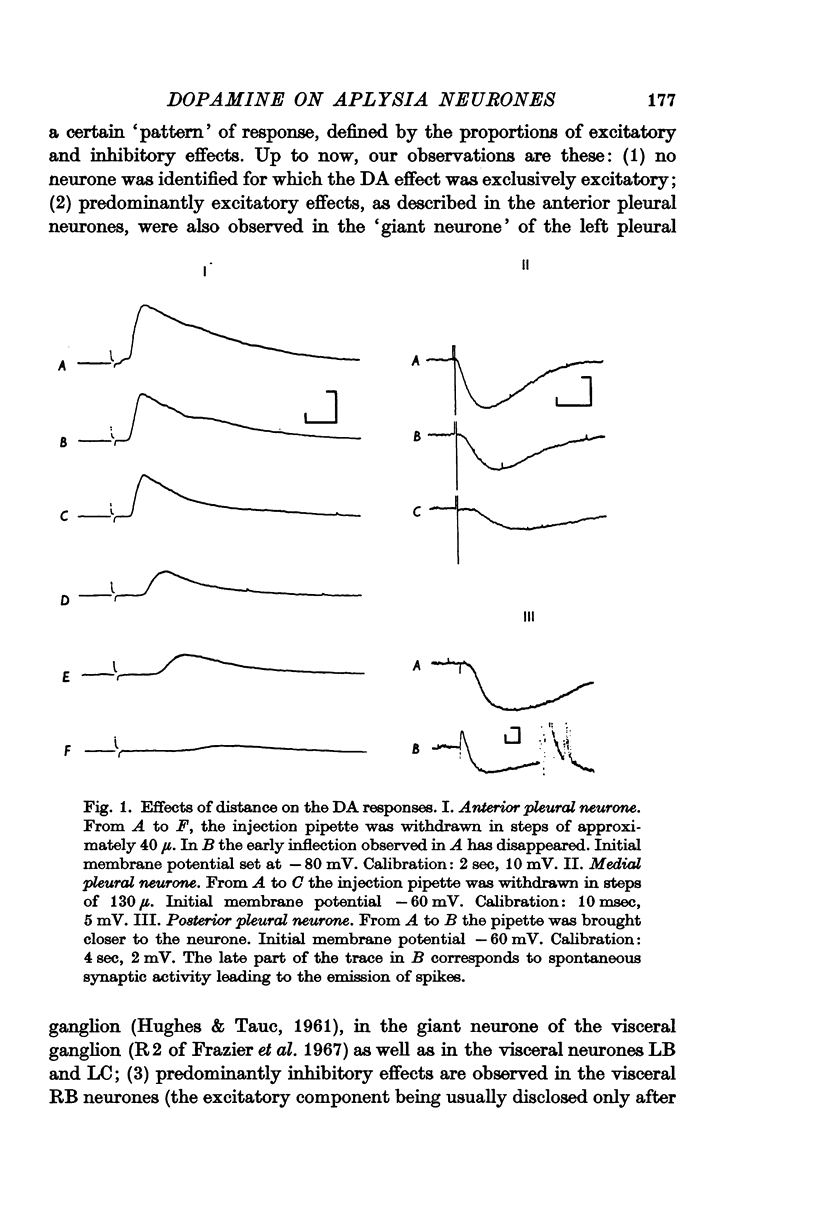
1. Electrophoretic application of dopamine (DA) on Aplysia neurones elicits both excitatory and inhibitory effects, which in many cases are observed in the same neurone, and often result in a biphasic response.
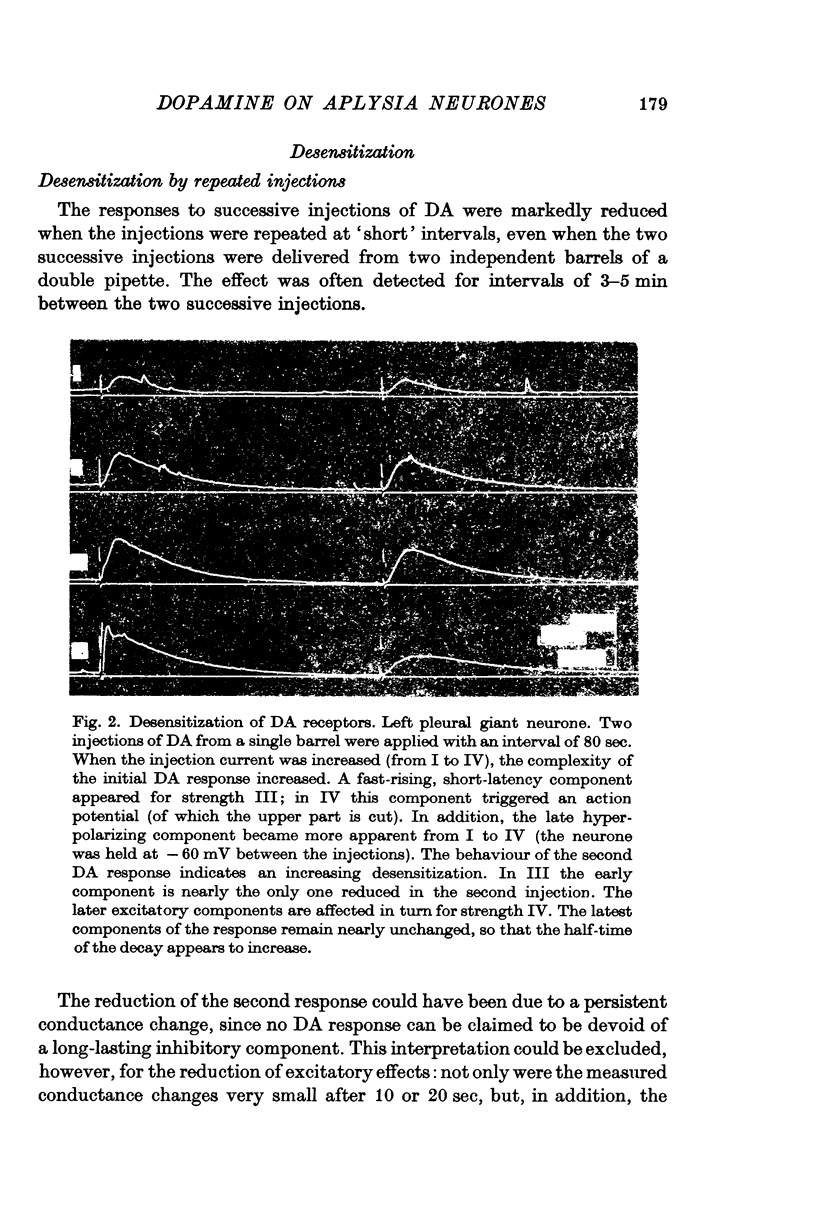
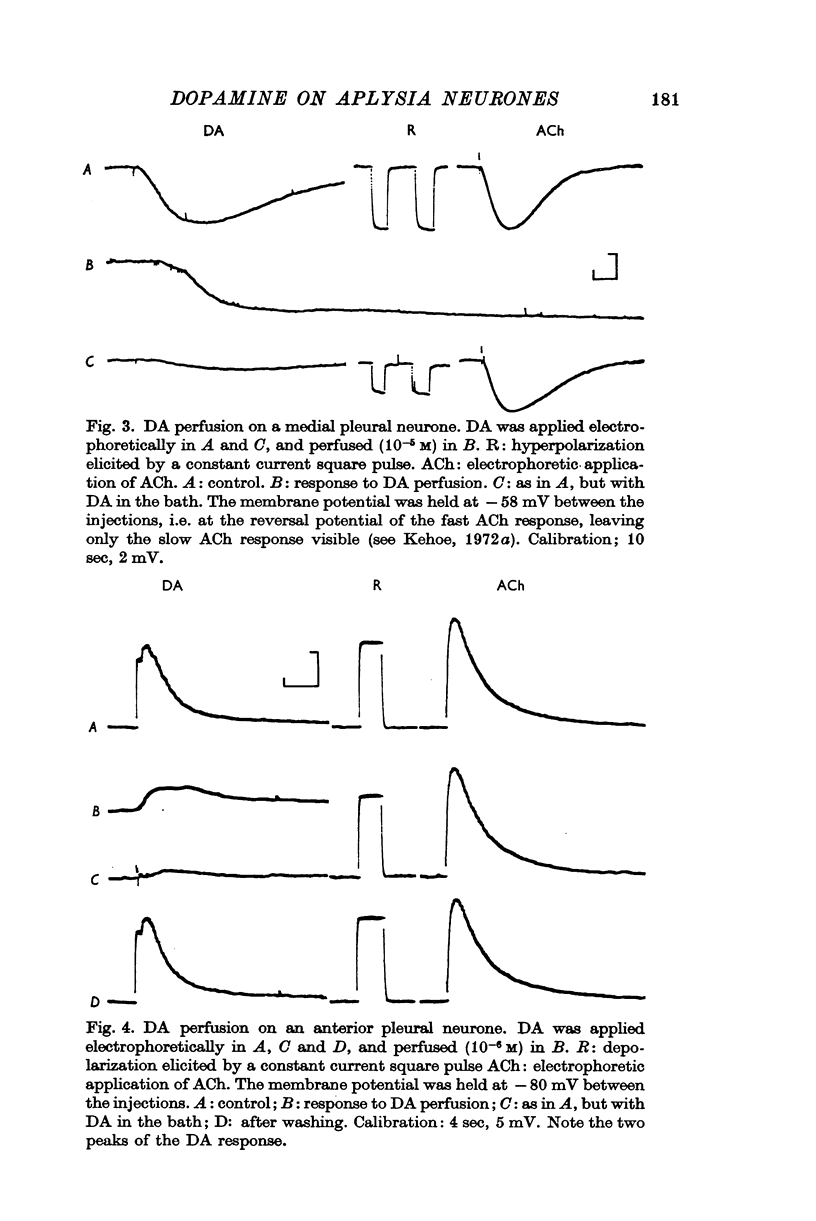
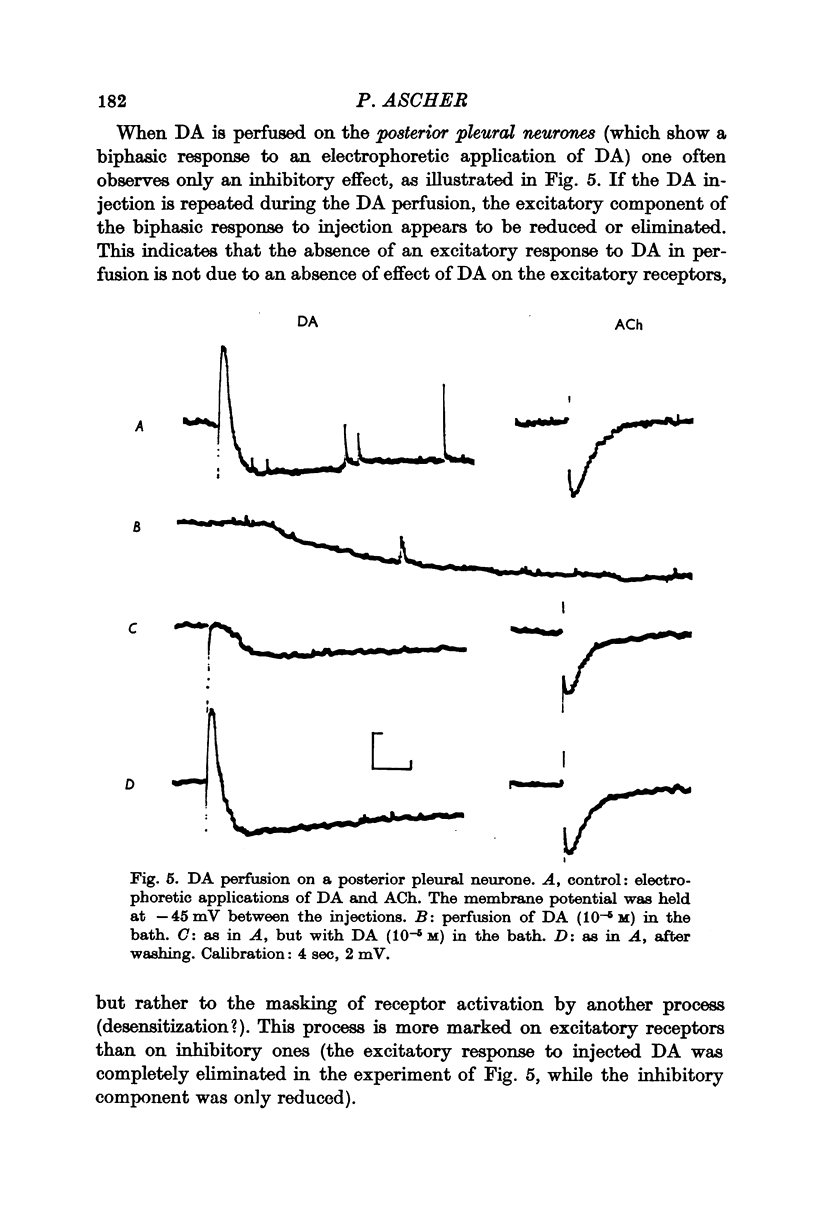
2. The DA receptors are localized predominantly on the axons. Desensitization, which occurs after repeated injections or with bath application of DA, is more marked for excitatory responses.
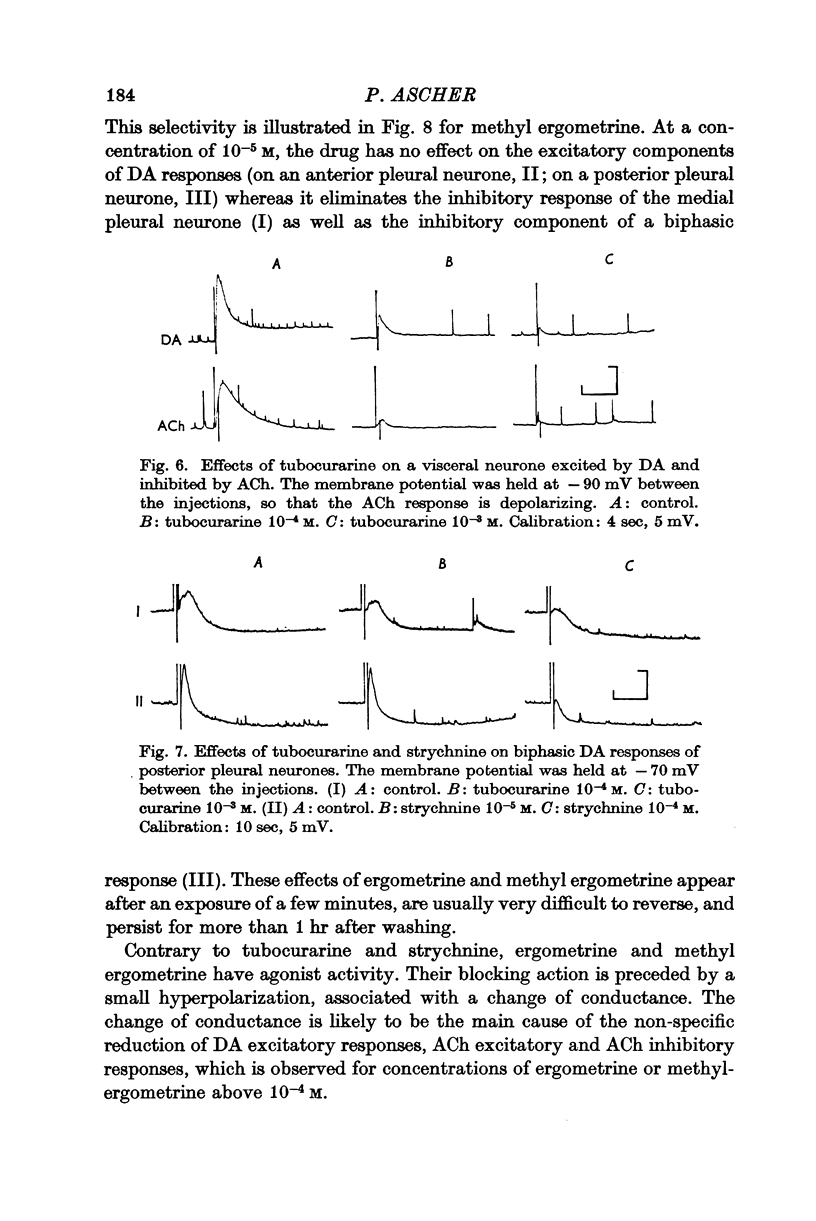
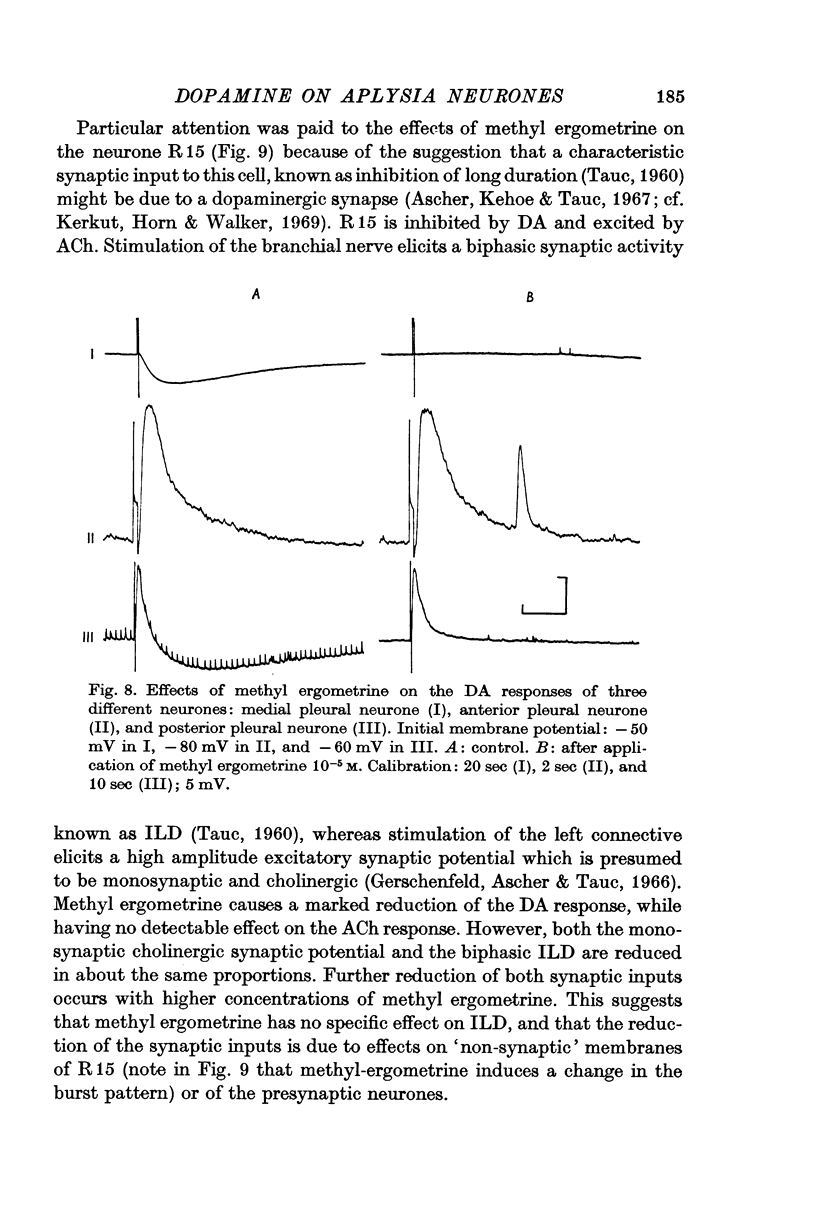
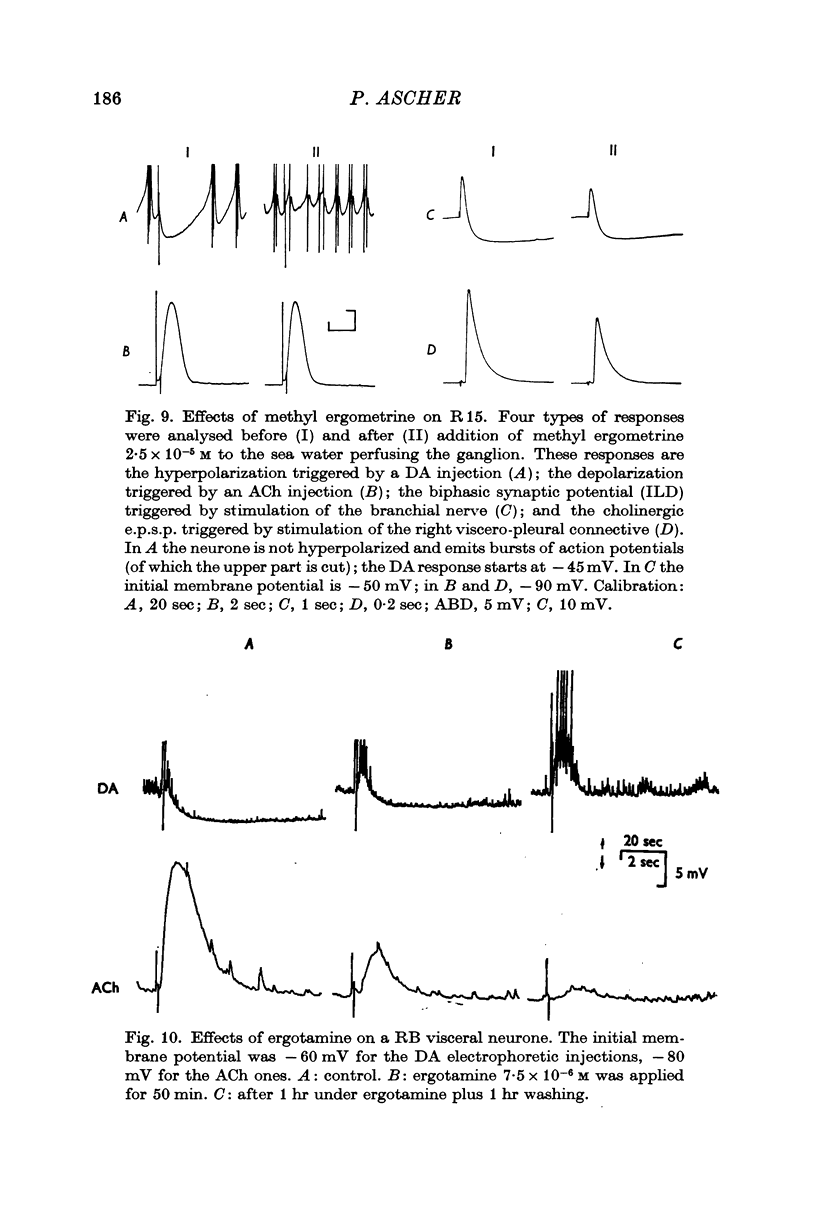
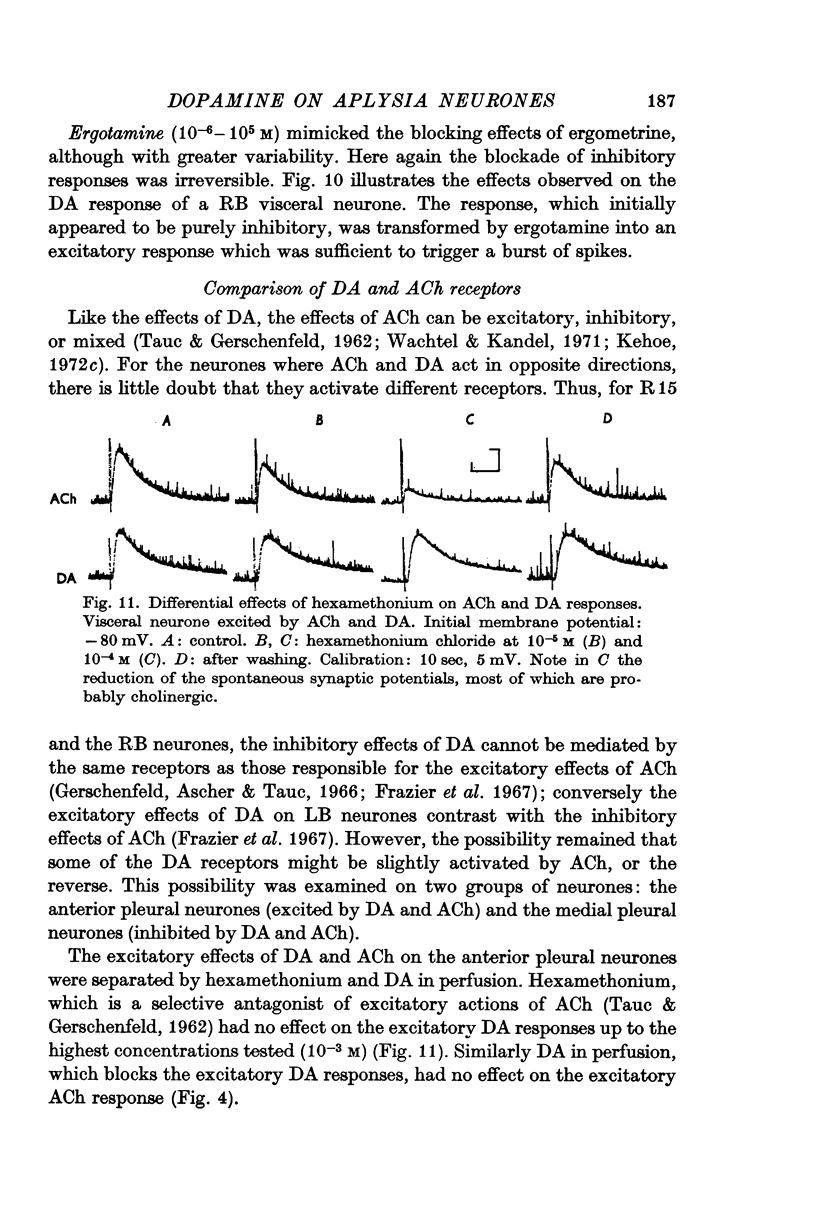
3. Tubocurarine and strychnine block the DA excitatory responses without affecting the inhibitory ones, which can be selectively blocked by ergot derivatives. It is concluded that the excitatory and inhibitory effects are mediated by two distinct receptors.
4. The two DA receptors can be pharmacologically separated from the three ACh receptors described in the same nervous system.
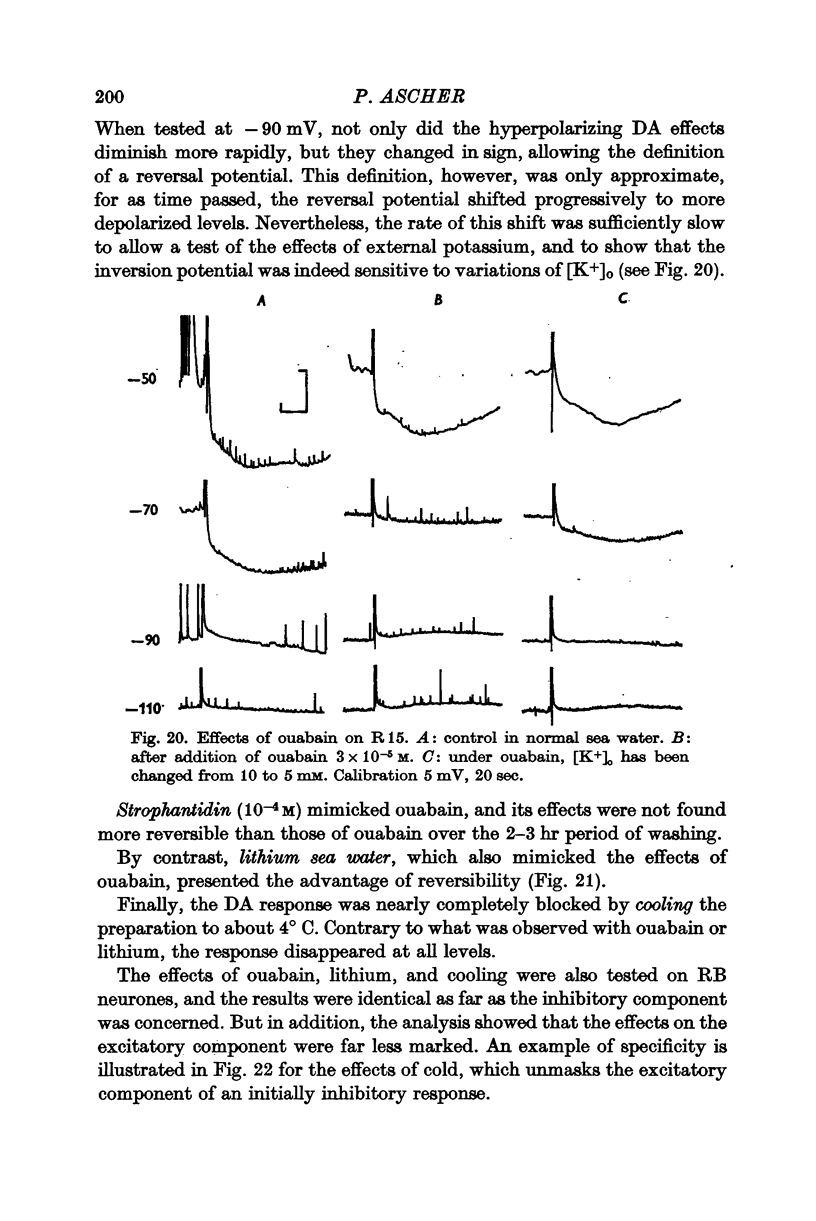
5. In some neurones the dopamine inhibitory responses can be inverted by artificial hyperpolarization of the membrane at the potassium equilibrium potential, EK, indicating that dopamine causes a selective increase in potassium permeability.
6. In other neurones the reversal potential of dopamine inhibitory responses is at a more depolarized level than EK, but can be brought to EK by pharmacological agents known to block the receptors mediating the excitatory effects of DA.
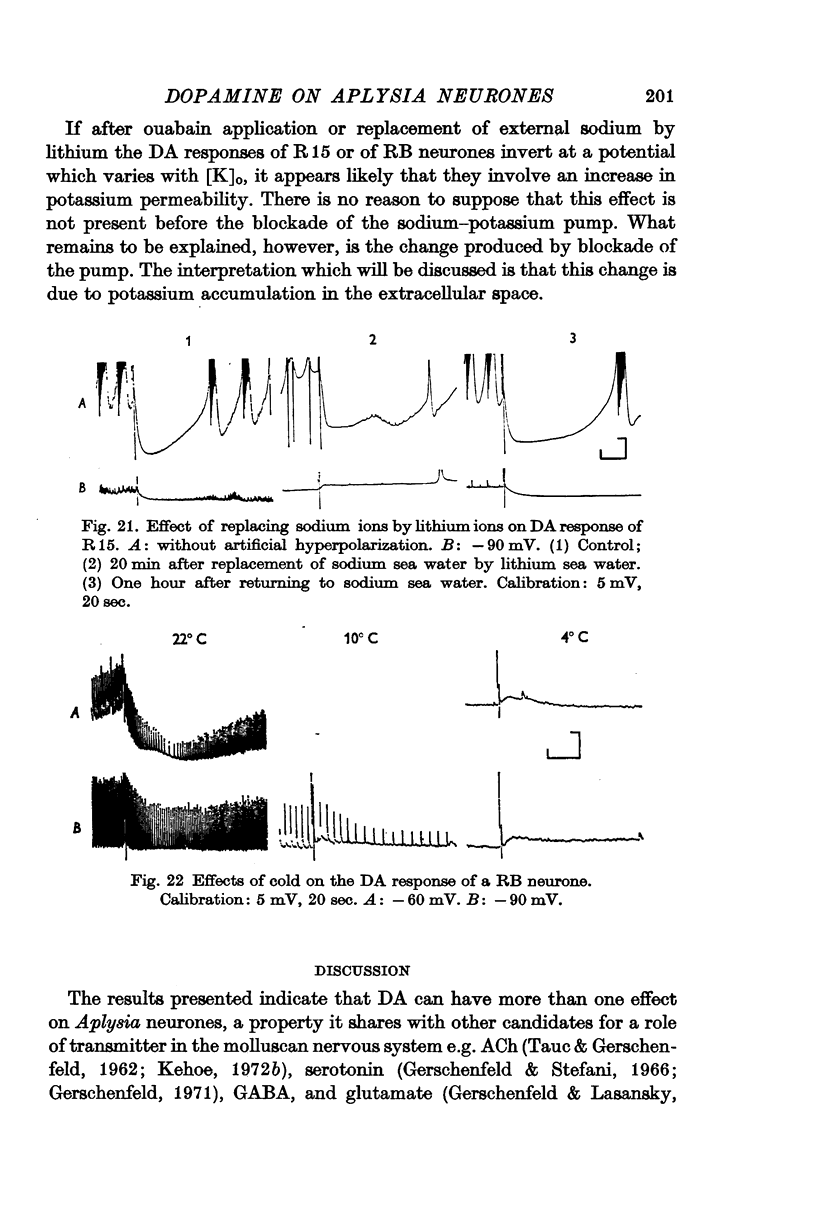
7. In still other neurones, the hyperpolarization induced by DA cannot be inverted in normal conditions, but a reversal can be induced by ouabain or by the substitution of external sodium by lithium. These results are discussed in terms of an hypothesis in which dopamine increases the potassium permeability of a limited region of the axonal membrane.
8. It is concluded that a selective increase in potassium permeability probably accounts for all dopamine inhibitory effects in the neurones studied.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving B. O. Differences between pacemaker and nonpacemaker neurons of Aplysia on voltage clamping. J Gen Physiol. 1969 Oct;54(4):512–531. doi: 10.1085/jgp.54.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Kehoe J. S., Tauc L. Effets d'injections électrophorétiques de dopamine sur les neurones d'aplysie. J Physiol (Paris) 1967;59(4 Suppl):331–332. [PubMed] [Google Scholar]
- BATHAM E. J. Infoldings of nerve fibre membranes in the opisthobranch mollusc Aplysia californica. J Biophys Biochem Cytol. 1961 Feb;9:490–492. doi: 10.1083/jcb.9.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGEN A. S., TERROUX K. G. On the negative inotropic effect in the cat's auricle. J Physiol. 1953 Jun 29;120(4):449–464. doi: 10.1113/jphysiol.1953.sp004910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURN J. H., GIBBONS W. R. THE EFFECT OF PHENOXYBENZAMINE AND OF TOLAZOLINE ON THE RESPONSE TO SYMPATHETIC STIMULATION. Br J Pharmacol Chemother. 1964 Jun;22:527–539. doi: 10.1111/j.1476-5381.1964.tb01707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall R. E. A light and electron microscope study of the abdominal ganglion of Aplysia californica. J Neurophysiol. 1967 Nov;30(6):1263–1287. doi: 10.1152/jn.1967.30.6.1263. [DOI] [PubMed] [Google Scholar]
- Dahl E., Falck B., von Mecklenburg C., Myhrberg H., Rosengren E. Neuronal localization of dopamine and 5-hydroxytryptamine in some mollusca. Z Zellforsch Mikrosk Anat. 1966;71(4):489–498. doi: 10.1007/BF00349609. [DOI] [PubMed] [Google Scholar]
- ECCLES J., ECCLES R. M., ITO M. EFFECTS PRODUCED ON INHIBITORY POSTSYNAPTIC POTENTIALS BY THE COUPLED INJECTIONS OF CATIONS AND ANIONS INTO MOTONEURONS. Proc R Soc Lond B Biol Sci. 1964 May 19;160:197–210. doi: 10.1098/rspb.1964.0036. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSCHENFELD H. M. A NON-CHOLINERGIC SYNAPTIC INHIBITION IN THE CENTRAL NERVOUS SYSTEM OF A MOLLUSC. Nature. 1964 Jul 25;203:415–416. doi: 10.1038/203415a0. [DOI] [PubMed] [Google Scholar]
- GERSCHENFELD H. M., LASANSKY A. ACTION OF GLUTAMIC ACID AND OTHER NATURALLY OCCURRING AMINO-ACIDS ON SNAIL CENTRAL NEURONS. Int J Neuropharmacol. 1964 Jul;3:301–314. doi: 10.1016/0028-3908(64)90022-x. [DOI] [PubMed] [Google Scholar]
- GERSCHENFELD H. M., TAUC L. DIFF'ERENTS ASPECTS DE LA PHARMACOLOGIE DES SYNAPSES DANS LE SYST'EME NERVEUX CENTRAL DES MOLLUSQUES. J Physiol (Paris) 1964 May-Jun;56:360–361. [PubMed] [Google Scholar]
- Gerschenfeld H. M., Ascher P., Tauc L. Two different excitatory transmitters acting on a single molluscan neurone. Nature. 1967 Jan 28;213(5074):358–359. doi: 10.1038/213358a0. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Stefani E. An electrophysiological study of 5-hydroxytryptamine receptors of neurones in the molluscan nervous system. J Physiol. 1966 Aug;185(3):684–700. doi: 10.1113/jphysiol.1966.sp008010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES G. M., TAUC L. The path of the giant cell axons in Aplysia depilans. Nature. 1961 Jul 22;191:404–405. doi: 10.1038/191404a0. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966 Jun;18(2):925–964. [PubMed] [Google Scholar]
- KERKUT G. A., WALKER R. J. The effects of drugs on the neurones of the snail Helix aspersa. Comp Biochem Physiol. 1961 Oct;3:143–160. doi: 10.1016/0010-406x(61)90051-2. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Frazier W. T., Waziri R., Coggeshall R. E. Direct and common connections among identified neurons in Aplysia. J Neurophysiol. 1967 Nov;30(6):1352–1376. doi: 10.1152/jn.1967.30.6.1352. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Anomalous rectification in the metacerebral giant cells and its consequences for synaptic transmission. J Physiol. 1966 Mar;183(2):287–304. doi: 10.1113/jphysiol.1966.sp007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. S., Ascher P. Re-evaluation of the synaptic activation of an electrogenic sodium pump. Nature. 1970 Feb 28;225(5235):820–823. doi: 10.1038/225820a0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Ionic mechanisms of a two-component cholinergic inhibition in Aplysia neurones. J Physiol. 1972 Aug;225(1):85–114. doi: 10.1113/jphysiol.1972.sp009930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Pharmacological characteristics and ionic bases of a 2 component postsynaptic inhibition. Nature. 1967 Sep 30;215(5109):1503–1505. doi: 10.1038/2151503b0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. The physiological role of three acetylcholine receptors in synaptic transmission in Aplysia. J Physiol. 1972 Aug;225(1):147–172. doi: 10.1113/jphysiol.1972.sp009932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkut G. A., Horn N., Walker R. J. Long-lasting synaptic inhibition and its transmitter in the snail Helix aspersa. Comp Biochem Physiol. 1969 Sep 15;30(6):1061–1074. doi: 10.1016/0010-406x(69)91044-5. [DOI] [PubMed] [Google Scholar]
- MILEDI R. Junctional and extra-junctional acetylcholine receptors in skeletal muscle fibres. J Physiol. 1960 Apr;151:24–30. [PMC free article] [PubMed] [Google Scholar]
- Otsuka M., Kravitz E. A., Potter D. D. Physiological and chemical architecture of a lobster ganglion with particular reference to gamma-aminobutyrate and glutamate. J Neurophysiol. 1967 Jul;30(4):725–752. doi: 10.1152/jn.1967.30.4.725. [DOI] [PubMed] [Google Scholar]
- Ozeki M., Freeman A. R., Grundfest H. The membrane components of crustacean neuromuscular systems. II. Analysis of interactions among the electrogenic components. J Gen Physiol. 1966 Jul;49(6):1335–1349. doi: 10.1085/jgp.0491335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsker H., Kandel E. R. Synaptic activation of an electrogenic sodium pump. Science. 1969 Feb 28;163(3870):931–935. doi: 10.1126/science.163.3870.931. [DOI] [PubMed] [Google Scholar]
- SWEENEY D. Dopamine: its occurrence in molluscan ganglia. Science. 1963 Mar 15;139(3559):1051–1051. doi: 10.1126/science.139.3559.1051. [DOI] [PubMed] [Google Scholar]
- Speckmann E. J., Caspers H., Sokolov W. Aktivittsänderungen spinaler neurone während und nach einer Asphyxie. Pflugers Arch. 1970;319(2):122–138. doi: 10.1007/BF00592491. [DOI] [PubMed] [Google Scholar]
- Stefani E., Gerschenfeld H. M. Comparative study of acetylcholine and 5-hydroxytryptamine receptors on single snail neurons. J Neurophysiol. 1969 Jan;32(1):64–74. doi: 10.1152/jn.1969.32.1.64. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. LOCALIZED ACTION OF GAMMA-AMINOBUTYRIC ACID ON THE CRAYFISH MUSCLE. J Physiol. 1965 Mar;177:225–238. doi: 10.1113/jphysiol.1965.sp007588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. A cholinergic mechanism of inhibitory synaptic transmission in a molluscan nervous system. J Neurophysiol. 1962 Mar;25:236–262. doi: 10.1152/jn.1962.25.2.236. [DOI] [PubMed] [Google Scholar]
- TRAUTWEIN W., DUDEL J. Zum Mechanismus der Membranwirkung des Acetylcholin an der Herzmuskelfaser. Pflugers Arch. 1958;266(3):324–334. doi: 10.1007/BF00416781. [DOI] [PubMed] [Google Scholar]
- Tauc L. Transmission in invertebrate and vertebrate ganglia. Physiol Rev. 1967 Jul;47(3):521–593. doi: 10.1152/physrev.1967.47.3.521. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel H., Kandel E. R. Conversion of synaptic excitation to inhibition at a dual chemical synapse. J Neurophysiol. 1971 Jan;34(1):56–68. doi: 10.1152/jn.1971.34.1.56. [DOI] [PubMed] [Google Scholar]
- Walker R. J., Ralph K. L., Woodruff G. N., Kerkut G. A. Evidence for a dopamine inhibitory post-synaptic potential in the brain of Helix aspersa. Comp Gen Pharmacol. 1971 Mar;2(5):15–26. doi: 10.1016/0010-4035(71)90063-2. [DOI] [PubMed] [Google Scholar]
- Walker R. J., Woodruff G. N., Glaizner B., Sedden C. B., Kerkut G. A. The pharmacology of Helix dopamine receptor of specific neurones in the snail, Helix aspersa. Comp Biochem Physiol. 1968 Feb;24(2):455–469. doi: 10.1016/0010-406x(68)90997-3. [DOI] [PubMed] [Google Scholar]
- Waziri R., Kandel E. R. Organization of inhibition in abdominal ganglion of Aplysia. 3. Interneurons mediating inhibition. J Neurophysiol. 1969 Jul;32(4):520–539. doi: 10.1152/jn.1969.32.4.520. [DOI] [PubMed] [Google Scholar]


