Abstract
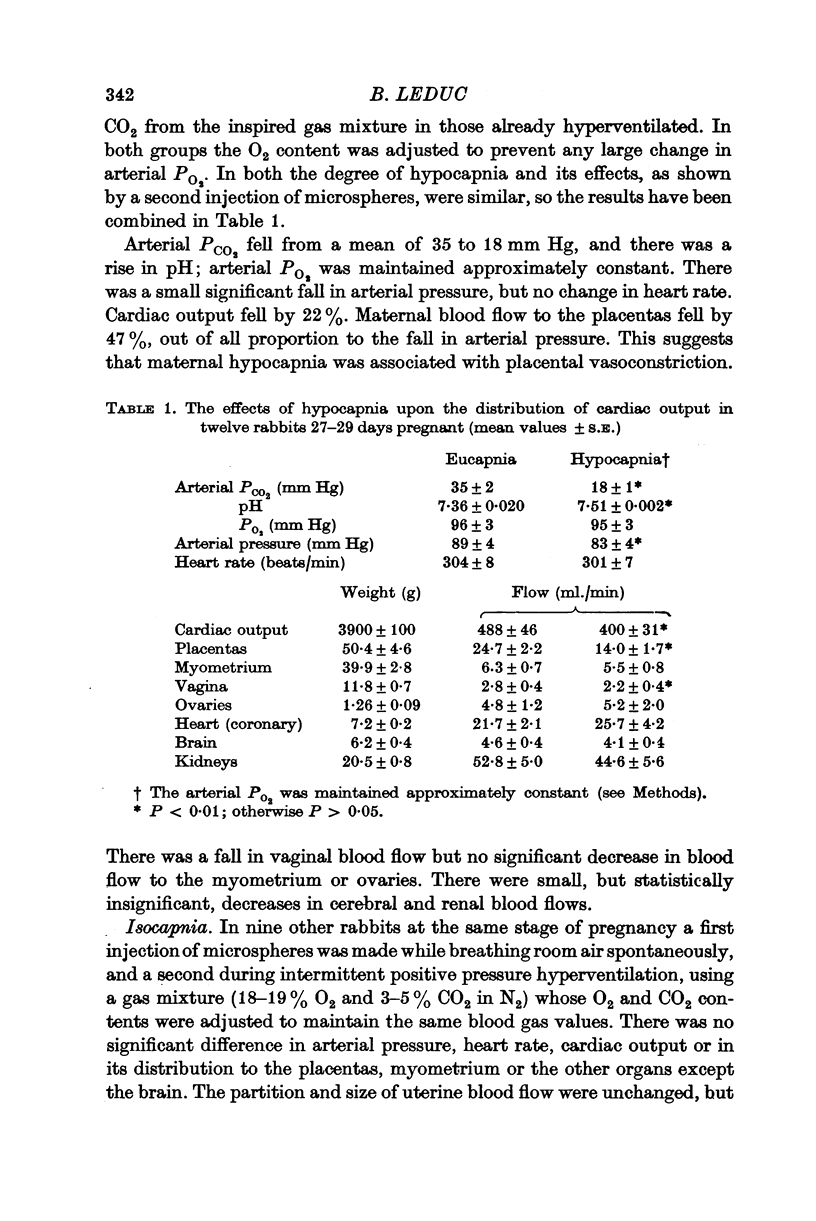
1. In anaesthetized pregnant rabbits near term, cardiac output and its distribution were measured by injection of isotope-labelled microspheres. Hypocapnia (mean arterial PCO2 18 mm Hg), induced by intermittent positive pressure hyperventilation, caused a 43% reduction in maternal placental blood flow, attributed mainly to vasoconstriction. Myometrial flow was not significantly changed.
2. Moderate hypercapnia (mean arterial PCO2 46 mm Hg) caused no change in placental flow, compared with observations made while breathing air spontaneously (PCO2 31 mm Hg).
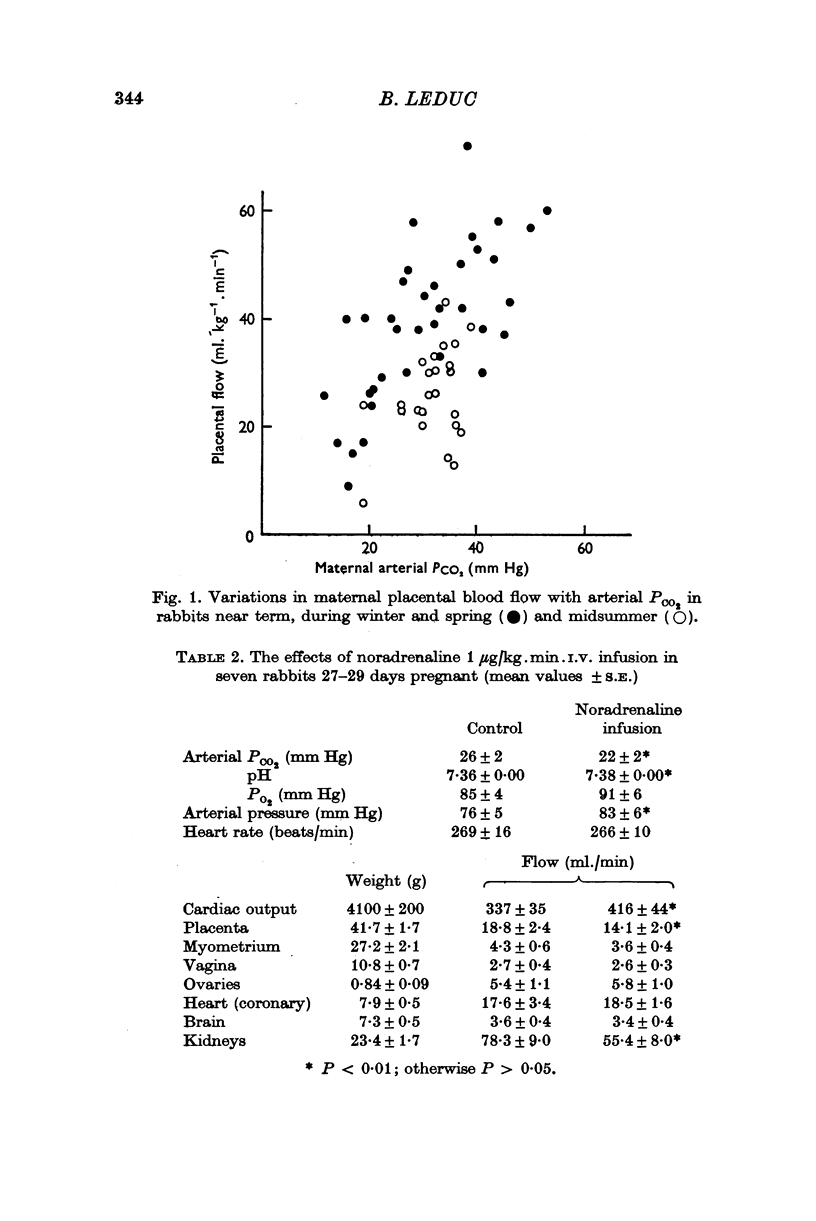
3. Intravenous infusions of adrenaline or noradrenaline 1 μg/kg. min caused maternal placental vasoconstriction.
4. During the especially warm summer of 1969, there was a mean 46% reduction in maternal placental blood flow in pregnant rabbits near term, breathing room air spontaneously with normal blood gas values and rectal temperatures. This was associated with an increase in the number of runts and dead foetuses.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C. Dual vasoconstrictor and vasodilator innervation of the uterine arterial supply in the guinea pig. Circ Res. 1968 Aug;23(2):279–289. doi: 10.1161/01.res.23.2.279. [DOI] [PubMed] [Google Scholar]
- DORNHORST A. C., YOUNG I. M. The action of adrenaline and noradrenaline on the placental and foetal circulations in the rabbit and guinea-pig. J Physiol. 1952 Oct;118(2):282–288. doi: 10.1113/jphysiol.1952.sp004793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S. L., Lewis B. V. Maternal placental and myometrial blood flow in the pregnant rabbit. J Physiol. 1969 Jun;202(2):471–481. doi: 10.1113/jphysiol.1969.sp008821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S. L. The partition of uterine blood flow in the pregnant rabbit. J Physiol. 1969 Oct;204(2):421–433. doi: 10.1113/jphysiol.1969.sp008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. J. Congenital defects in guinea pigs: fetal resorptions, abortions, and malformations following induced hyperthermia during early gestation. Teratology. 1969 Nov;2(4):313–328. doi: 10.1002/tera.1420020406. [DOI] [PubMed] [Google Scholar]
- Járai I. The redistribution of cardiac output on cold exposure in new-born rabbits. J Physiol. 1969 Jun;202(3):559–567. doi: 10.1113/jphysiol.1969.sp008827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc B. The effect of acute hypocapnia on maternal placental blood flow in rabbits. J Physiol. 1970 Sep;210(2):165P–166P. [PubMed] [Google Scholar]
- MACFARLANE W. V., PENNYCUIK P. R., THRIFT E. Resorption and loss of foetuses in rats living at 35degree C. J Physiol. 1957 Mar 11;135(3):451–459. doi: 10.1113/jphysiol.1957.sp005723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN J. D., YOUNG I. M. Influence of gestational age and hormones on experimental foetal bradycardia. J Physiol. 1960 Jun;152:1–13. doi: 10.1113/jphysiol.1960.sp006465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAH M. K. Reciprocal egg transplantations to study the embryo-uterine relationship in heat-induced failure of pregnancy in rabbits. Nature. 1956 Jun 16;177(4520):1134–1135. doi: 10.1038/1771134a0. [DOI] [PubMed] [Google Scholar]
- Shelton M., Huston J. E. Effects of high temperature stress during gestation on certain aspects of reproduction in the ewe. J Anim Sci. 1968 Jan;27(1):153–158. doi: 10.2527/jas1968.271153x. [DOI] [PubMed] [Google Scholar]
- YOUNG I. M. Some observations on the mechanism of adrenaline hyperpnoea. J Physiol. 1957 Aug 6;137(3):374–395. doi: 10.1113/jphysiol.1957.sp005820. [DOI] [PMC free article] [PubMed] [Google Scholar]


